w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Campomanesia
velutina
leaves
extracts
exert
hypouricemic
effects
through
inhibition
of
xanthine
oxidase
and
ameliorate
inflammatory
response
triggered
by
MSU
crystals
Marcela
C.P.M.
Araújo
a,
Zilma
S.
Ferraz-Filha
a,b,
Fernanda
C.
Ferrari
a,
Dênia
A.
Saúde-Guimarães
a,∗ aLaboratóriodePlantasMedicinais,EscoladeFarmácia,UniversidadeFederaldeOuroPreto,OuroPreto,MG,BrazilbDepartamentodeQuímica,InstitutoFederaldeMinasGerais,CampusOuroPreto,OuroPreto,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16February2016 Accepted11May2016 Availableonline25July2016
Keywords:
Campomanesiavelutina
Gout Hyperuricemia Inflammation Myricitrin Xanthineoxidase
a
b
s
t
r
a
c
t
Goutisadestructivearthritiswithahighprevalenceworldwide.However,theavailabletherapyisnot
abletoincreaselifequalityinmanypatients.Campomanesiavelutina(Cambess)O.Berg,Myrtaceae,is
usedinBrazilianfolkmedicinetotreatpain,inflammationandrheumatism.Theaimofthisstudywasto
evaluatethepotentialofethanolicandaqueousextractsfromC.velutinaleavestotreathyperuricemia
andinflammationingoutarthritismodel.Ethanolicextractofleavesandaqueousextractofleaveswere
invitroassayedonxanthineoxidaseinhibitoryeffectandinvivoonanexperimentalmodelof
oxonate-inducedhyperuricemiainmice,liverxanthineoxidaseinhibitionandmonosodiumuratecrystal-induced
pawedemamodel.Theextractsatbothtesteddoses(100and300mg/kg)reducedserumuratelevels.
Theywerealsoabletoinhibitxanthineoxidaseinvitroandinvivo,demonstratingthatthismightbethe
mechanismofactionunderlyingtheurate-loweringeffects.Inaddition,theextractsshowedsignificant
anti-inflammatoryactivityonmonosodiumuratecrystal-inducedpawedema,especiallyaqueousextract
(100and300mg/kg)thatreducededemaatallevaluatedtimes.Rutinandmyricitrinwereidentifiedin
ethanolicandinaqueousextracts.Inthisstudy,myricitrinwasabletoreduceserumuricacidlevelsand
inhibitliverxanthineoxidaseatthedoseof15mg/kg.Theanti-hyperuricemicactivityofrutinhasbeen
previouslyreported.Thus,rutinandmyricitrinseemtocontributetotheobservedeffectsofethanolic
andaqueousextracts.Theresultsdemonstratedtheabilityofaqueousandethanolicextractstolower
serumuratelevelsandtoreduceedemainducedbymonosodiumuratecrystals.Therefore,theymay
contributetothemanagementofgoutinthefuture.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Goutisaninflammatorydisorderthatariseswhen supersatu-rationofbodytissueswithurateoccurs,leadingtotheformation anddepositionofmonosodiumurate(MSU)crystalsinarticular andperiarticulartissues(RoddyandChoi,2014).Theprevalence of both hyperuricemia and gout has risen in the last decades andthereforetheburdenofgouthasincreased(Perez-Ruizetal., 2015).Clinicalmanifestationsofthisdiseaseincludeacutegouty arthritisflarescharacterizedbyseverepain,swelling,warmthand erythema.Ifhyperuricemiapersists,MSUcrystaldepositsfurther inducechronicinflammatoryresponsesthatmayleadtothe for-mationoftophaceousMSUcrystaldepositsinjointsandotherbody
∗ Correspondingauthor.
E-mail:saude@ef.ufop.br(D.A.Saúde-Guimarães).
tissues,chronicjointdamage,renalstoneformationwithpotential renalinsufficiencyandcardiovascularproblems(Perez-Ruizetal., 2015;RoddyandChoi,2014).
Thetherapiesfortreatinggout’spainandinflammationinclude nonsteroidal anti-inflammatories (NSAID), colchicine and oral corticosteroids (Edwards and So, 2014). However, MSU crystal depositsmustbeconsideredthemostimportanttargetforgout management.ByloweringMSUlevelsbelow6mg/dl,dissolution ofpathogenicMSUcrystalsisachievedanddisappearanceof clin-icalfeaturesofgoutcanbeobtained(Perez-Ruizetal.,2015).The urate-loweringtherapiesarebasedontheuseofallopurinoland probenecidsincede1960s.Recentstudieshaveshowedhow inad-equateisthetraditionalapproachtothisdestructivearthritis.After all,patientsdonotexperienceasignificantreductionofpainand intolerancetoallopurinolandprobenecidmeansthatthepatient wouldgountreated.Theseproblemshaveledtotherecognition that gout’s treatmentrequires better and more specific agents (EdwardsandSo,2014).
http://dx.doi.org/10.1016/j.bjp.2016.05.016
Plantshavebeenusedforcenturiestotreatnumerous patholog-icalconditionsanddiseasesandevennowadays,theystillprovide arichsourcefornewdrugdiscoveriesduetoatremendous chem-icaldiversity of compounds.Campomanesia species areused in Brazilianfolkmedicinetotreatawiderangeofclinicalconditions, includingtheirusetotreatrheumatism(Aliceetal.,1995;Cravo, 1994).Thetermrheumatismincludesawiderangeofdisorders markedby inflammation,degenerationand pain,affecting con-nectivetissuesstructures,speciallyjointsandrelatedstructures (Dorland’s MedicalDictionary, 2007).ThespecieCampomanesia velutina(Cambess)O.Berg,Myrtaceae,canbefoundintheBrazilian cerradobiomaandtherearereportsaboutitsusebythe popula-tionofitsoccurrencearea(DiasandLaureano,2009;Giraldiand Hanazaki,2010;Oliveiraetal.,2010).Previousstudieswiththis specieassesseditsanti-inflammatoryandantinociceptiveactivities invivoandleadtotheisolationoftheactiveconstituentmyricitrin fromtheethanolicextractofleaves(Micheletal.,2013).
Since Campomanesia species are used to treat rheumatism and previous studies demonstrated the anti-edematogenic and antinociceptiveactivityofthespecie,thisstudywasconductedin ordertoevaluatetheroleofC.velutinaingout,aknownand preva-lentrheumaticdisease.Thus,theaimofthisstudyistoevaluatethe biologicaleffectsofaqueousandethanolicextractsfromC.velutina leavesoverthehyperuricemiaandinflammationtriggeredbyMSU crystals.Theabilityofextractstoinhibitxanthineoxidase(XO)was alsoevaluatedbothinvitroandinvivo.Inaddition,itwas evalu-atedmyricitrinabilitytoinhibitXOandthusreduceuricacidlevels invivo.
Materialsandmethods
Chemicals
Xanthineoxidasefromcow’smilk,xanthine,potassiumoxonate, uric acid, allopurinol and indomethacin were purchased from Sigma–Aldrich(USA).Uricacidassaykitwaspurchasedfrom Bio-clin(Brazil).Ketamineand xylazinewereobtainedfromSespro IndustriaeComercioLtda(Brazil).Waterwaspurifiedusing Milli-Qapparatus fromMillipore(USA). Ethanol,dimethylsulphoxide (DMSO)andTween80wereofanalyticalgrade.HPLCsolventswere purchasedfromTedia(Brazil)andstandardswerepurchasedfrom Sigma–Aldrich(USA).
Plantmaterial
LeavesfromCampomanesiavelutina(Cambess.)O.Berg, Myr-taceae,werecollectedinLagoaSantacity,MinasGeraisstate,Brazil, inDecemberof2012,withpermissionofChicoMendesInstituteof BiodiversityConservation(InstitutoChicoMendesdeConservac¸ão daBiodiversidade–ICMBio/SistemadeAutorizac¸ãoeInformac¸ão emBiodiversidade–SISBIO),licenseno.17021-5.Theplant botan-icalidentificationwasrealizedbyDr.MarcosE.GuerraSobralfrom theDepartmentofNaturalSciences,FederalUniversityofSãoJoão Del-Rei(UniversidadeFederaldeSãoJoãoDel-Rei (UFSJ),Minas Gerais,Brazil.Avoucherspecimen(HUFSJ4637)wasdepositedat theherbariumofUFSJ.
Preparationofplantextracts
The leaveswere air-driedand powdered. Part of the leaves powder(540g)wasexhaustivelyextractedwithethanolatroom temperaturebypercolation.Solventwasremovedunderreduced pressure,at40◦C, yielding41gofthedriedethanolicextractof
leaves(EEL).Inordertoobtaintheaqueousextracts,450gofleaves powderwaspercolatedwith4.5lofwater.Thewaterwasremoved
bylyophilization, yielding19goftheaqueousextractof leaves (AEL).
CharacterizationoftheextractsbyHPLC-UV/DAD
HPLC-UV/DAD analysis were performed on a Waters Liquid Chromatography(modelAlliance2695)equippedwithavacuum degasser,aquaternarypump,anautosampler,adiodearray detec-tor(DADWaters2996)andreversedphaseC18column(Shimadzu ODS–250mm×4.6mm,5m).
Toassigncompoundstothepeaks,itwasusedtheretention timeandUVmaxofstandardselutedonthesameconditionsasthe
extracts.Thefollowingstandardswereused:oleanolicacid, chloro-genicacid,caffeicacid,galocatequin,quercetin,pinocembrin,rutin, kaempferol,crisinandmyricitrin.ToobtaintheHPLCprofiles,the UV-DADdetectorwassettorecordbetween200and400nmand UVchromatogramswererecordedat254nm.
Theextractsandthestandardsweresolubilizedinmethanolto yieldaconcentrationof5mg/mland1mg/ml,respectively.Then, theywerefilteredthrough a0.45mMillexsyringefilters.The volumeinjectedwas25l.EELwaselutedinasystemwith5% of methanoland 95% of water, taking60mintoreach100% of methanolandanother5mintoreturntotheinitialcondition.The flowratewaskeptconstantat1ml/min.AELwaselutedina sys-temwith100%ofwater,taking30mintoreach20%ofmethanol, another10mintoreach40%ofmethanoland15mintoreach100% ofmethanol.Thesystemwasreturnedtotheinitialconditionin 5min.Theflowratewaskeptconstantat0.8ml/min.Inbothcases, theseparationtemperaturewas25◦C.
InhibitionofXOactivityinvitro
Toevaluatetheeffectoftheextractsover XOactivity,itwas used the method described by Ferraz-Filha et al. (2006) with modifications.TheEELwasdissolvedin DMSO:Tween80:Water (1:1:8)and theAEL wasdissolvedin water. Theassay mixture consistedof500lofextractsolution,1125lof1/15Mphosphate buffer (pH 7.5) and 187.5l of enzyme solution (0.28units/ml inbuffer). Thereactionwasinitiated byadding1375lof xan-thinesubstrate solution(0.15mM in water).Theassay mixture wasincubatedat 25◦C and theabsorbance (295nm)was
mea-suredspectrophotometricallyeveryminutefor12minusingaCary 50BioSpectrophotometer(Varian–Australia).Ablank(0%ofXO inhibition)waspreparedwithouttheextractssolutions. Allopu-rinol wasusedasa positive control.XOinhibitory activitywas expressedasthepercentageofXOinhibitionintheassaymixture systemandcalculatedas:%inhibition=(1−testinclination/blank inclination)×100,where testinclinationisthelinearchangein absorbanceoftestmaterialperminuteandblankinclinationisthe linearchangeinabsorbanceofblankperminute.TocalculateIC50,
finalconcentrationsofextractswere10,20,30,40and50g/ml andfinalconcentrationsofallopurinolwere0.1,0.25,0.5,0.75and 1g/ml.Allassayswereperformedintriplicate.
Animals
Preparationofdrugsandtestsolutions
Allopurinol,indomethacin,myricitrin,Campomanesiavelutina extractsandpotassiumoxonatewerepreparedaccordingtothe averageweight ofeach experimentalgroup.Potassiumoxonate (250mg/kg) and MSU crystals (80mg/ml) were suspended in 0.9%sterilesaline.Allopurinol(10mg/kg),indomethacin(3mg/kg) andEEL (100and 300mg/kg)weresolubilized inDMSO:Tween 80:water(1:1:8).AEL(100and300mg/kg)wassolubilizedinwater andmyricitrin(15mg/kg)wassolubilizedinDMSO:water(5:95). Allsolutionsandsuspensionswerepreparedonthedayoftheir use.
Anti-hyperuricemiceffectsandinhibitionofliverXOresidual activityinoxonate-inducedhyperuricemicmice
Theanti-hyperuricemicactivityofmyricitrin,AELandEELwas evaluatedusinganexperimentalanimalmodelofhyperuricemia inducedbypotassiumoxonate,anuricaseinhibitor,aspreviously describedbyHalletal.(1990)andmodifiedbyothers(deSouza etal.,2012;Lemosetal.,2015).Animalsweredividedinto exper-imentalgroups(n=6)andfasted1hbeforedrugadministration. Potassiumoxonatewasadministratedintraperitoneallytoanimals inthefirstandthirddayoftheexperiment1hbeforeoral admin-istrationoftestsolutions.Miceofnormalcontrolgroupwerenot treatedwithpotassiumoxonate.Alltreatmentswereorally admin-isteredbygavageonceadayforthreeconsecutivedays.Miceof normalcontroland hyperuricemiccontrol groupsreceivedonly vehicle(DMSO:Tween80:wateror DMSO:Water).Animalsfrom treatedcontrolgroupreceivedallopurinol.Animalsofremaining groupsweretreatedwithmyricitrin,EELandAEL.Onthethirdday, 1hafterthelastoraltestadministration,micewereanesthetized withamixtureofketamineandxylasine(100and20mg/kg, respec-tively)andthebloodwascollectedthroughcardiacpuncture.The bloodwasallowedtoclotforapproximately45minatroom tem-peratureandthencentrifugedat3500×gfor10min.Serawere separatedandstoredat−20◦Cuntilassayforuricacid
quantifica-tion.Theliverwasremoved,washedin0.9%salineandstoredat −80◦C.
Uricacidassay
Serum uric acid concentration was spectrophotometrically (VarianCary50BioSpectrophotometer,Australia)determinedby enzymaticcolorimetricmethod(UOD-PAP)usingastandard diag-nostickit(Bioclin,Brazil)accordingtomanufacturer’sinstructions. Thistestisbasedonuricacidoxidationbyuricaseproducing allan-toinandhydrogenperoxidewhichisusedbyperoxidasetoproduce aredchromogenthroughthereactionof4-aminoantipyrinewith thehydroxyl-dichloro-benzenesulphonicacid(HDBS).Thecolor intensityisproportionaltotheconcentrationofuricacidinthe samplewithmaximumabsorptionat505nm.
LiverXOactivityassay
Enzymeextractionfromliverwascarriedoutaccordingto pre-viously described (Zhu et al., 2004; Haidari et al., 2008). Liver XOresidualactivitywasassayedspectrophotometricallyby mon-itoringuric acidformation fromxanthineaccording tomethod describedbyHalletal.(1990)andmodifiedbyothers(deSouza etal.,2012;Ferrarietal.,2016).Briefly,100lofliversfinal super-natant werepre-incubated for 15min at 37◦C with 5000l of
50mMphosphatebuffer(pH7.4)containing1mMofpotassium oxonate.Thepresenceofpotassiumoxonatepreventsthe oxida-tionofuricacidtoallantoin.Then,thereactionwasinitiatedby theadditionof 1200lof250mMxanthinesolution.The addi-tionof500lof0.6MHClsolutiontothereactionmediumafter
0and 30minstoppedthereaction.The reactionmixtureswere thencentrifugedat3000×gfor5min.Thesupernatantswere sep-aratedandtheabsorbancemeasuredat295nm(VarianCary50Bio Spectrophotometer,Australia).Theamountofuricacidformedwas quantifiedbythedifferenceofabsorbancefrom30and0minusing auricacidcalibrationcurve.XOresidualactivitywasexpressed as nanomolesof uric acidformed perminute per milligram of protein.Proteinconcentrationwasdetermined spectrophotomet-ricallyusingmethoddescribedbyBradford(1976).
Monosodiumuratecrystal-inducedinflammationinmice
Theanti-inflammatoryactivityofCampomanesiavelutinaleaves extractswasevaluatedonanexperimentalmodelofgout accord-ingtopreviouslydescribedby(RassolandVaralakshimi,2006)and modifiedbyothers(deSouzaetal.,2012;Lemosetal.,2015;Ferrari etal.,2016).Animalsweredividedintosevenexperimentalgroups (n=9)andfasted1hbeforedrugadministration.Inflammationwas inducedonthefirstdayoftheexperimentbyintradermal injec-tionof50lofMSUcrystalsuspensionintothemicerighthind paw.MSUcrystalswerepreparedaccordingtopreviouslydescribed method(RassolandVaralakshimi,2006).AllanimalsreceivedMSU injection,exceptthosefromnormalcontrolgroup(group1),which wereadministeredonlysaline.Alltreatmentswereorally admin-isteredbygavage1hbeforeMSUinjectiononthefirstdayand repeateddaily,atthesametime,forthreemoredays.Animalsfrom group1and2weretreatedwiththevehicleandservedasnormal controlgroupandMSU-inducedcontrolgroup,respectively.Mice ofgroup3weretreatedwiththestandardanti-inflammatorydrug indomethacin.Animalsofremaining groups(4–7) weretreated withEELandAEL(100and300mg/kg).Pawthicknesswas mea-suredwithacaliperrule(150mm–6in.,Vonder,China)beforeand 4,24,48and72hafterMSUinjection.Inflammatoryswellingwas expressedaspercentageofthicknessvariation.
Statisticalanalysis
ExperimentaldatawasanalyzedusingGraphPadPrism5.0 Soft-ware(Inc.,SanDiego,CA,USA). IC50 values werecalculated by
linearregressionofplotsonanXYgraphofinhibitionversus con-centration values, assuming a 95% confidence interval. Results frominvivoexperimentswerepresentedasmeanvalues±S.E.M. One-way analysis of variance (ANOVA) was used followed by Newman–Keuls’ multiple comparison test. p values<0.05 were consideredstatisticallysignificant.
Resultsanddiscussion
Brazilian traditional medicineuses Campomanesia speciesto treatpain,inflammationandrheumatism.Rheumatismisageneric termusedtodescribevariousclinicalconditionsthataffect mus-cles,bonesorarticulationscausingreductionorlossofmobility,as arthritis,gout,arthrosis,amongothers.
0.08
0.06
1
26 747 peak 1 28 028 peak 2
28 885 rutin 262.3
260.0 362.9
352.9
27 902 Myricitrin
44 583 rutin 46 183 Myricitrin
43 726 peak 1
260.0 351.7
261.1 352.9
254.0
255.2 355.3
354.1 45 527 peak 2
245.0
255.2
nm nm
nm 356.5
AU
AU
AU
AU
AU
AU
355.3 Ethanolic extract of leaves
(EEL)
Aqueous extract of leaves (AEL) 2
1
2 0.04
AU
AU
0.02
0.00
0.30
0.25
0.20
0.15
0.10
0.05
0.00
0.00 5.00 10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 50.00 55.00 60.00 nm
AU
AU
0.00 10.00 20.00 30.00
Minutes
Minutes
40.00 50.00 60.00
Fig.1.HPLCchromatogramat254nmofEELandAELofCampomanesiavelutinawithextractedUVspectrumofidentifiedandstandardssubstancesmyricitrinandrutin.
intendedtotreatgout(Ahmadetal.,2008),andinthiscontext, naturalproductsareapotentialsourceofnewagents(Kongetal., 2002).
Previous studies with Campomanesia velutina demonstrated that the specie possess antinociceptive and anti-inflammatory properties(Micheletal.,2013).Theaimofthisstudywasto eval-uate the ability of the specie tolower serum urate levels and investigatethemechanismsunderlyingthiseffect.Inaddition,was investigateditsabilitytoactovertheinflammatoryprocess trig-geredbyMSUcrystals.Thisway,theresultsobtainedmayhelp developanalternativetherapytotreatgout,sincethetraditional approachtothisdiseasehaslowefficacyandvarioussideeffects andrestrictions.
Flavonoids are naturally occurring plant compounds with antioxidant, anti-inflammatory and XO inhibitory properties (Nagao et al.,1999; Tunget al.,2015).Furthermore,their con-sumptionhasbeenassociatedwiththeprotectiveeffectsofcertain dietsandherbsagainstsomeofthecomplicationsofhyperuricemia andgout,suchascardiovasculardiseaseanddiabetes(Sampson etal.,2002).Astudyalsodemonstratedtheabilityoftheflavonoid quercetintopreventkidneyinjuryassociatedwithhyperuricemia (Wang et al., 2012). Asflavonoids possess important biological effectsininflammationandhyperuricemia,HPLCanalysiswas car-riedoutinordertoverifythepresenceofsomecommonflavonoids andcorrelatedphenolicsubstances.AELandEELprofileshowed atretention timeof approximately43and 30min,respectively, thepresence ofsubstances that absorbenergy at twodifferent wavelengths,likeflavonoids.Theextractionofthechromatograms at254nmand thecomparisonof retentiontime and UVmax of
thepeakswithstandardsrevealedthepresence oftwo distinct flavonoidsatthoseretentiontimes(Fig.1).Thepresenceofthe flavonoidmyricitrinwasconfirmedin EELandfoundinAEL. In addition,anotherflavonoid,rutin,wasfoundinEELandAEL.
Myricitrinandrutinhavebeenreportedtopossessawiderange ofbiologicalactivitiesandmanyoftheseactivitiesarerelatedto thebiologicaleffectsinvestigatedinthisstudy.Inpreviousstudies, myricetin-3-O-rhamnoside(myricitrin)wasabletomodulatethe releaseand/orproductionofNO,TNF-␣andIL-10onmacrophages
(Ferreiraetal.,2013;Micheletal.,2013).Invivo,myricitrinhas beenreportedasanitricoxide(NO)andproteinkinaseCinhibitor thatexertsantinociceptiveeffect(Meottietal.,2006)anditsoral administrationreducedTNF-␣andCOX-2expressioninmicelivers (Domitrovi ´cetal.,2015).Myricitrinalsoshowedinhibitoryeffects againstTNF-␣productioninRAW264.7macrophages(Shimosaki etal.,2011)andirreversiblyinactivatedmyeloperoxidaseactivity, closelyrelatedtotheprogressionofchronicinflammatorydiseases (Meottietal.,2011).
Rutin is a typical flavonoid with several biological effects demonstrated in vitroand in vivoincluding antioxidative, anti-inflammatory, anticancer,antidiabetic,antimicrobial,antifungal, anti-allergic,amongothers.Mostoftheseactivitiesareattributed tothepotentantioxidantpropertyofrutin,particularlyasafree radicalscavenger(Chua,2013).RutincaninhibitXODinvitro(Chen etal.,2011)andathree-dayoralpretreatmentwithrutinproduced adose-dependentdecreaseonserumuratelevelsinhyperuricemic miceandtheseeffects werepartlydue toinhibitionofXDH/XO activitiesinmouseliver(Zhuetal.,2004).Rutinalsohavethe abil-itytoinhibitNOproductioninducedbyLPS(Shenetal.,2002)and theanti-inflammatoryactivityofrutinwasfoundtobebeneficial forthetreatmentofrheumatoidarthritisandosteoarthritis(Umar etal.,2012).
XOistheenzymeresponsiblefortheconversionof hypoxan-thineinto xanthineand ofxanthine intouric acid.Assays with this enzyme are used to test compounds that may inhibit the enzymeandthus beusefultothetreatmentofgoutandothers diseasesrelatedtoXO(Haidarietal.,2008).Twodifferentextracts obtainedfromCampomanesiavelutinaleaves(EELandAEL)were assayedforXOinhibitoryactivityinvitro.Theresultsareshown inTable1.Atfinalconcentrationof100g/ml,thetwoextracts producedaninhibitiongreaterthan60%overXO.TheIC50values
weredeterminedforbothofthemsincetheextractspresentedan inhibitionbiggerthan25%at100g/ml.EELshowedanIC50value
of35.63g/ml.ForAEL,theIC50was47.33g/ml.AllopurinolIC50
was0.3287g/ml.AccordingtoSchmeda-Hirschmannetal.(1996), compoundswithIC50valueslowerthan50g/mlshouldbefurther
Table1
Xanthineoxidaseinhibitoryactivityofethanolicandaqueousextractsfrom Campo-manesiavelutinaleaves.
Extract Inhibitionat100g/ml (%±S.E.M.)
IC50g/ml
(Confidenceinterval–95%)
EEL 66.43±3.17 35.63(30.14to42.84)
AEL 66.82±1.19 47.33(45.06to49.89)
Allopurinol – 0.33(−0.5981to0.6105)
EEL,ethanolicextractofleaves;AEL,aqueousextractofleaves.
theactivityobservedinvitrowouldproduceimportantbiological effects.
Inmostmammals,uricaseisanenzymethatcatalysesthe con-versionofuricacidintoallantoin,thus,serumuricacidlevelsis typicallylow.However,humanandotherprimateslosttheability toexpressuricaseandtheresultishigherserumuricacidlevels. Potassiumoxonateis themostuseduricaseinhibitorin animal
modelsof hyperuricemia, since it is a low-costcompound and
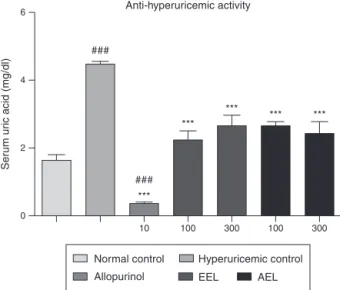
producesarapideffect.Therefore,thehyperuricemicmodel pro-ducedbyoxonate isthemostsuitabletothepreliminarystudy ofnewdrugs(Kongetal.,2002).Thus,toassaythehypouricemic activityofCampomanesiavelutina,hyperuricemicoxonate-induced animalsweretreatedforthreedayswithEELandAELat100and 300mg/kg.Fig.2showstheresults.
Treatmentwith uricase inhibitor potassium oxonate signifi-cantlyincreased serum urate levelswhen compared to normal group,showing that the model waseffective to induce hyper-uricemia.A three-daytreatmentwiththetwo extracts,at both doses(100and300mg/kg)significantlyreducedserumuratelevels comparedtohyperuricemiccontrolgroup.Asobservedoninvitro assays,extractswerealsoabletoinhibitXOresidualactivityinvivo. AsshowninTable2,treatmentwithEELandAELforthreedays wasabletoinhibitliverXOresidualactivityinhyperuricemicmice atbothdoseswhencomparedtocontrolgroup.Sincethe reduc-tionofserumuricacidlevelswasfollowedbyXOinhibition,these resultsindicatethattheanti-hyperuricemicactivityoftheextracts isstronglyrelatedtoXOinhibitionandthismightbethe mecha-nismofaction.
Statistical analysis revealed that there were no differences betweenthedosesconcerninguricacidlevelsandXOinhibition, Thisisprobablybecausethemaximumresponsewasreachedwith
10 6
4
2
Ser
um ur
ic acid (mg/dl)
Anti-hyperuricemic activity
0
100 300 100 300
###
### ***
*** ***
*** ***
Normal control Hyperuricemic control
Allopurinol EEL AEL
Fig.2.Anti-hyperuricemiceffectsofethanolicandaqueousextractsfrom Campo-manesiavelutinaleavesinmicetreatedwithpotassiumoxonate.Datarepresents means±S.E.M.ofsixanimals.One-wayANOVAfollowedbyNewman–Keuls’ multi-plecomparisontestwasusedforstatisticalsignificance.***p<0.001comparedwith hyperuricemiccontrolgroup.###p<0.001comparedwithnormalcontrolgroup.
Table2
EffectsofethanolicandaqueousextractsfromCampomanesiavelutinaleavesonliver xanthineoxidaseresidualactivityafterathree-dayoraladministration.
Treatment Dose(mg/kg) XOD(nm/min/mgprotein) %Inhibition
Vehicle – 14.86±0.802 –
Allopurinol 10 4.21±0.429a 71.67
EEL 100 7.76±1.452a 47.78
300 7.49±0.496a 49.59
AEL 100 5.89±0.569a 60.36
300 5.28±0.345a 64.47
EEL,ethanolicextractofleaves;AEL,aqueousextractofleaves;nm,nanomolesof uricacid.
Data represents mean±S.E.M. of six animals. One-way ANOVA followed by Newman–Keuls’multiplecomparisontestwasusedforstatisticalsignificance.
ap<0.001comparedtohyperuricemiccontrolgroup.
thedoseof100mg/kg.Thisway,anincreaseinthedosedidnot produceabetterresponse.Inaddition,resultsofuricacidandXO inhibitionexhibitednostatisticaldifferencesbetweenAELandEEL. Thisprobablymeansthattheactivecompoundsarepresent on bothextracts.Infact,myricitrinandrutinwerefoundinAELand EEL.Previousstudieswithrutindemonstratedtheabilityofthis flavonoidtodecreaseserum uratelevelsinhyperuricemicmice
Anti-hyperuricemic activity
XO residual ctivity ###
***
***
***
*** ##
###
Dose (mg/kg)
Dose (mg/kg) 6
4
2
0
15
10
5
0
10 15
10 15
Ser
u
m ur
ic acid (mg/dl)
nm/min/mg protein
Normal control
Hyperuricemic control
Allopurinol
Myricitrin
Hyperuricemic control Allopurinol Myricitrin 15 mg/kg
4thhour
0 50 100 150
Dose (mg/kg)
Negative control Vehicle Indometacin AEL EEL
*** ** ***
*** **
Paw swelling, %
0 50 100 150
Dose (mg/kg)
*** *** ***
Paw swelling, %
48th hour
0 50 100 150
Dose (mg/kg)
* * **
Paw swelling, %
72th hour 24th hour
0 50 100 150
MSU - + + + + + + MSU - + + + + + +
MSU - + + + + + + MSU - + + + + + +
3 100 300 100 300 3 100 300 100 300
3 100 300 100 300 Dose 3 100 300 100 300
(mg/kg)
* * *
Paw swelling, %
Fig.4.EffectsofaqueousandethanolicextractsfromtheleavesofCampomanesiavelutinaonMSUcrystal-inducedpawedemainmice.Datarepresentsmeans±S.E.M.of nineanimals.One-wayANOVAfollowedbyNewman–Keuls’multiplecomparisontestwasusedforstatisticalsignificance.*p<0.05,*p<0.01and***p<0.001comparedwith vehiclecontrolgroup.
withathree-dayoralpretreatment(Zhuetal.,2004)andtoinhibit XOinvivo(Zhuetal.,2004)andinvitro(Chenetal.,2011).Because thelackofstudiesabouttheanti-hyperuricemicactivityof myric-itrin,thisflavonoidwasfurtherinvestigatedandtheresultsshowed thatmyricitrinwasabletosignificantlyreduceserumuricacid lev-elsandtoinhibitXOresidualactivityafterathree-daytreatment atthedoseof15mg/kg(Fig.3).Thus,itisreasonabletoassume thatmyricitrinandrutinarerelatedtotheeffectsofAELandEEL overserumuricacidlevelsandXOresidualactivity.However,other compoundscanalsocontributetotheobservedeffect,sincethefull compositionoftheextractsisnotknown.
Furthermore,theresultsofuricacidlevelsandXOinhibition fromanimalstreatedwiththeextractswerenotsignificantly dif-ferentofthoseobservedonnormalgroup.Allopurinol(10mg/kg) reducedserumuratelevelsofhyperuricemicmicetovalueslower thanthatfoundinnormalgroup(Fig.2)andinhibited71.67%of XOactivity(Table2).However,thefactthattheextractsdidnot producesuchreductioncanbeconsideredanadvantage.Despite elevatedserumuricacidlevelscantriggergoutandothermetabolic disorders,theantioxidantactivityofuricacid,particularlyitsability toinhibitDNAdamage,iswelldocumented(Stinefeltetal.,2005). Therefore,anexcessivedecreaseinuricacidlevelsmayevenbe harmful(Haidarietal.,2008).Thus,astheextractsreducedserum uratetothesamelevelsobservedinnormalanimals,apossible therapywiththemcouldleadtofewersideeffects.
PreviousstudieswithEEL demonstratedits abilitytoinhibit edemaformationafteracarrageenaninjection(Micheletal.,2013). However,therewerenodataconcerningtheaqueousextractor theroleofthespecieoveragout-likeinflammation.Therefore,AEL andEELweretestedabouttheirabilitytopreventedema forma-tiontriggeredbyMSUcrystals.MSUcrystalinjectionstartsalocal inflammatoryreactionwithsymptomssimilartothoseobserved clinically in gout, suggesting that this model is able topredict clinicalefficacyofnew agents(Gettingetal., 2002).MSU crys-talsstimulateinnateimmunesystemthroughtheproductionand
releaseofseveralinflammatorymediators,suchaskinins, inter-leukinsandTNF-␣.Someofthesemediatorsarechemotacticsand amplifytheinflammatoryresponseleadingtoneutrophil infiltra-tion followed bythe release of oxygenfree radicals, lysosomal enzymes,prostaglandin-E2,leukotrienesandinterleukin-1.Ifnot treated,theinflammationcanleadtostructuraldamage(Martinon etal.,2006).
MSU crystals injection caused a significant increase in paw thicknesswhencomparedtonegativecontrol(saline administra-tion). Indomethacin (3mg/kg) promoted a significantreduction onpawswellingobservedduringtheentireexperiment.Thetwo extractsatbothdosesreducededemaformationat4thhourafter MSUinjection,butonlyAEL(100and300mg/kg)wasableto main-taintheactivitythroughouttheexperiment.EELlimitedonlyinitial inflammatoryresponse,evaluated4hafterMSU-crystalinjection (Fig.4).TheseresultsindicatethatEELactsonlyontheinitialphase oftheinflammatoryprocessinitiatedbyMSUcrystalswhileAELcan actbothintheinitialandinthelatephasesoftheinflammatory pro-cess.Myricitrinandrutincanbeinvolvedintheanti-inflammatory activityoftheextractstoo.Aspreviouslydetailed,severalstudies demonstratedtheabilityoftheseflavonoidstoinhibitthe produc-tionand/orthereleaseofinflammatorymediators.
validtopointoutthattheseeffectswereobservedatthesamedoses andafteranoraltreatment,indicatingthattheactivesubstances arewellabsorbedin theintestinaltract.Thisway,aqueousand ethanolicextractsfromCampomanesiavelutinaleavescanactover crucialpointsofgoutmanagement:decreaseuricacidserum lev-elsbyinhibitingxanthineoxidaseactivityandreducepawedema inducedbyMSU.Therefore,theseextractsareapromising alter-nativetotreatgoutandcouldbeusedforthedevelopmentofan herbalmedicineorasasourceofnewmoleculestotreatthis dele-teriousdiseaseandthuscontributetoincreasethearmamentarium toachieveaproperlymanagementofgoutandagoodlifequality forthosepatients.However,morestudiesarenecessarytoestablish allthesubstancesintheextractsthatareresponsibleforthe activ-ity,identifyandproposethemolecularmechanismsunderthese effects.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
MCPMA(PhDstudent) contributedinrunningthelaboratory work,analysisofthedata anddraftedthepaper. ZSFFand FCF contributedtothedevelopment,implementationandrealization oftheassays.DASG raisedthenecessaryfundsfor work devel-opment,designedthestudy,supervisedthelaboratoryworkand contributedtocriticalreadingofthemanuscript.Alltheauthors readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Thisstudy wascarried outatUFOP. Theprojectwasfunded by FAPEMIG (grant APQ-01318-12 and APQ-00956-13), Rede TOXIFAR/FAPEMIG (Rede Mineira de Ensaios Toxicológicos e Farmacológicos/Fundac¸ão de Amparo à Pesquisa do Estado de Minas Gerais), grant CBB-RED-00008-14. PhD scholarship was providedbyCAPES.
References
Ahmad,N.S.,Farman,M.,Najmi,M.H.,Mian,K.B.,Hasan,A.,2008. Pharmacolog-icalbasisforuseofPistaciaintegerrimaleavesinhyperuricemiaandgout.J. Ethnopharmacol.117,478–482.
Alice,C.B.,Siqueira,N.C.S.,Mentz,L.A.,BrasileSilva,G.A.A.,José,K.F.D.,1995.Plantas Medicinaisdeusopopular:AtlasFarmacognóstico.ULBRA,Canoas.
Bradford,M.M.,1976.Arapidandsensitivemethodforthequantificationof micro-gramquantitiesofproteinutilizingtheprincipleofprotein-dyebinding.Anal. Chem.72,248–254.
Chen, L., Yin, H., Lan, Z., Ma, S., Zhang, C., Yang, Z., Li, P., Lin, B., 2011.
Anti-hyperuricemicandnephroprotectiveeffectsofSmilaxchinaL.J. Ethnophar-macol.135,399–405.
Chua,L.S.,2013.Areviewonplant-basedrutinextractionmethodsandits pharma-cologicalactivities.J.Ethnopharmacol.150,805–817.
Cravo,A.B.,1994.Frutaseervasquecuram:panacéiavegetal.Hemus,SãoPaulo.
deSouza,M.R.,dePaula,C.A.,Resende,M.L.P.,Grabe-Guimarães,A.,deSouzaFilho, J.D.,Saúde-Guimarães,D.A.,2012.PharmacologicalbasisforuseofLychnophora trichocarpha in gouty arthritis: anti-hyperuricemic and anti-inflammatory effectsofitsextract,fractionandconstituents.J.Ethnopharmacol.142,845–850.
Dias,J.E.,Laureano,L.C.,2009.FarmacopéiaPopulardoCerrado.Articulac¸ãoPacari, Goiás.
Domitrovi ´c,R.,Rashed,K.,Cvijanovi ´c,O.,Vladimir-Kneˇzevi ´c,S., ˇSkoda,M.,Viˇsic,A., 2015.Myricitrinexhibitsantioxidant,anti-inflammatoryandantifibrotic activ-ityincarbontetrachloride-intoxicatedmice.Chem.Biol.Int.230,21–29.
Dorland’s Medical Dictionary for Health Consumers, 2007. http://medical-dictionary.thefreedictionary.com/rheumatism(accessedApril2016). Edwards,N.L.,So,A.,2014.Emergingtherapiesforgout.Rheum.Dis.Clin.N.Am.40,
375–387.
Ferraz-Filha,Z.S.,Vitolo,I.F.,Fietto,L.G.,Lombardi,J.A.,Saúde-Guimarães,D.A.,2006.
XanthineoxidaseinhibitoryactivityofLychnophoraspeciesfromBrazil(Arnica). J.Ethnopharmacol.107,79–82.
Ferrari,F.C.,Lemos,R.C.L.,Ferraz-Filha,Z.S.,Barros,C.H.,Araújo,M.C.P.M., Saúde-Guimarães,D.A.,2016.EffectsofPimentapseudocaryophyllusextractsongout: anti-inflammatoryactivityandanti-hyperuricemiceffectthroughxanthine oxi-daseanduricosuricaction.J.Ethnopharmacol.180,37–42.
Ferreira,L.C.,Grabe-Guimarães,A.,Paula,C.A.,Michel,M.C.P.,Guimarães,R.G., Rezende,S.A.,SouzaFilho,J.D.,Saúde-Guimarães,D.A.,2013.Anti-inflammatory andantinociceptiveactivitiesofCampomanesiaadamantium.J.Ethnopharmacol. 145,100–108.
Getting,S.J.,Christian,H.C.,Flower,R.J.,Perretti,M.,2002.Activationofmelanocortin type3receptorasamolecularmechanismforadrenocorticotropichormone efficacyingoutyarthritis.ArthritisRheum.46,2765–2775.
Giraldi,M.,Hanazaki,N.,2010.Usoeconhecimentotradicionaldeplantasmedicinais noSertãodoRibeirão,Florianópolis,SC,Brasil.ActaBot.Bras.24,395–406.
Hall,I.H.,Scoville,J.P.,Reynolds,D.J.,Simlot,R.,Duncan,P.,1990.Substitutedcyclic imidesaspotentialanti-goutagents.LifeSci.46,1923–1927.
Haidari,F.,Rashidi,M.R.,Keshavarz,S.A.,Mahboob,S.A.,Eshraghian,M.R.,Shahi, M.M.,2008.Effectsofoniononserumuricacidlevelsandhepaticxanthine dehydrogenase/xanthineoxidaseactivitiesinhyperuricemicrats.Pak.J.Biol. Sci.11,1779–1784.
Kong, L.D., Zhou, J., Wen, Y.L., Li, J.M., Cheng, C.H.K., 2002. Aesculin pos-sesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenaseinhibitoryactivity.PlantaMed.68,175–178.
Lemos,R.C.L.,Ferrari,F.C.,deSouza,M.R.,Pereira,B.M.S.,dePaula,C.A., Saúde-Guimarães, D.A., 2015. Effects of extracts of leaves from Sparattosperma leucanthumonhyperuricemia andgouty arthritis.J. Ethnopharmacol. 161, 194–199.
Martinon,F.,Pétrilli,V.,Mayor,A.,Tardivel,A.,Tschopp,J.,2006.Gout-associated uricacidcrystalsactivatetheNALP3inflammasome.Nature440,237–241.
Meotti,F.C.,Luiz,A.P.,Pizzolatti,M.G.,Kassuya,C.A.,Calixto,J.B.,Santos,A.R.,2006.
Analysisoftheantinociceptiveeffectoftheflavonoidmyricitrin:evidencefora roleofthel-arginine–nitricoxideandproteinkinaseCpathways.J.Pharmacol. Exp.Ther.316,789–796.
Meotti,F.C.,Senthilmohan,R.,Harwood,D.T.,Missau,F.C.,Pizzolatti,M.G.,Kettle, A.J.,2011.Myricitrinasasubstrateandinhibitorofmyeloperoxidase: implica-tionsforthepharmacologicaleffectsofflavonoids.FreeRadicalBiol.Med.44, 109–120.
Michel,M.C.P.,Guimarães,A.G.,Paula,C.A.,Rezende,S.A.,Sobral,M.E.G.,Guimarães, D.A.S.,2013.ExtractsfromtheleavesofCampomanesiavelutinainhibits produc-tionofLPS/INF-␥inducedinflammatorymediatorsinJ774A.1cellsandexerts antiinflammatoryandantinociceptiveeffectsinvivo.Rev.Bras.Farmacogn.23, 927–936.
Nagao,A.,Seki,M.,Kobayashi,H.,1999.Inhibitionofxanthineoxidasebyflavonoids. Biosci.Biotechnol.Biochem.63,1787–1790.
Oliveira,F.C.S.,Barros,R.F.M.,MoitaNeto,J.M.,2010.Plantasmedicinaisutilizadas emcomunidadesruraisdeOeiras,semiáridopiauiense.Rev.Bras.PlantaMed. 12,282–301.
Perez-Ruiz,F.,Dalbeth,N.,Bardin,T.,2015.Areviewofuricacid,crystaldeposition diseaseandgout.Adv.Ther.32,31–41.
Rassol,M.,Varalakshimi,P.,2006.SuppressiveeffectofWithaniasomniferaroot pow-deronexperimentalgoutyarthritis:aninvivoandinvitrostudy.Chem.Biol. Interact.164,174–180.
Roddy,E.,Choi,H.K.,2014.Epidemiologyofgout.Rheum.Dis.Clin.N.Am.40, 155–175.
Sampson,L.,Rimm,E.,Hollman,P.C.,deVries,J.H.,Katan,M.B.,2002.Flavonoland flavoneintakesinUShealthprofessionals.J.Am.Diet.Assoc.102,1414–1420.
Shen,S.C.,Lee,W.R.,Lin,H.Y.,Huang,H.C.,Ko,C.H.,Yang,L.L.,Chen,Y.C.,2002.Invitro andinvivoinhibitoryactivitiesofrutin,wogoninandquercetinon lipopolysac-charideinducednitricoxideandprostaglandinE2production.Eur.J.Pharmacol. 446,187–194.
Shimosaki,S.,Tsurunaga,Y.,Itamura,H.,Nakamura,M.,2011.Anti-allergiceffectof theflavonoidmyricitrinfromMyricarubraleafextractsinvitroandinvivo.Nat. Prod.Res.25,374–380.
Schmeda-Hirschmann,G.,Zuniga,J.,Dutra-Behrens,M.,Habermehl,G.,1996. Xan-thineoxidaseinhibitoryactivityofflavonoidsandtanninsfromHexachlamys edulis(Myrtaceae).Phytother.Res.10,260–262.
Stinefelt,B.,Leonard,S.S.,Blemings,K.P.,Shi,X.,Klandorf,H.,2005.Freeradical scavenging,DNAprotection,andinhibitionoflipidperoxidationmediatedby uricacid.Ann.Clin.Lab.Sci.35,37–45.
oldhamii Maxim. leaf extracts reduce serum uric acid levels in potas-siumoxonate-inducedhyperuricemicmice.BMCComplement.Altern.Med.,
http://dx.doi.org/10.1186/s12906-015-0950-7.
Umar,S.,Mishra,N.K.,Pal,K.,Sajad,M.,NehaAnsari,M.M.,Ahmad,S.,Katiyar,C.K., Khan,H.A.,2012.Protectiveeffectofrutininattenuationofcollagen-induced arthritisinWistarratbyinhibitinginflammationandoxidativestress.IndianJ. Rheumat.7,191–198.
Wang, C., Pan, Y., Zhang, Q.Y., Wang, F.M., Kong, L.D., 2012. Quercetin and allopurinolamelioratekidneyinjuryinSTZ-treatedratswithregulationof
renalNLRP3inflammasomeactivationandlipidaccumulation.PLoSONE7, e38285.
Yao,X.,Ding,Z.,Xia,Y.,Wei,Z.,Luo,Y.,Feleder,C.,Dai,Y.,2012.Inhibitionof monosodiumuratecrystal-inducedinflammationbyscopoletinandunderlying mechanisms.Int.Immunopharmacol.14,454–462.