w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Heliopsis
longipes:
anti-arthritic
activity
evaluated
in
a
Freund’s
adjuvant-induced
model
in
rodents
Carolina
Escobedo-Martínez
a,∗,
Silvia
Laura
Guzmán-Gutiérrez
b,
María
de
los
Milagros
Hernández-Méndez
a,
Julia
Cassani
c,
Alfonso
Trujillo-Valdivia
a,
Luis
Manuel
Orozco-Castellanos
a,
Raúl
G.
Enríquez
daDepartamentodeFarmacia,DivisióndeCienciasNaturalesyExactas,UniversidaddeGuanajuato,Guanajuato,Mexico
bCatedráticaCONACyT,DepartamentodeInmunología,InstitutodeInvestigacionesBiomédicas,UniversidadNacionalAutónomadeMéxico,México,DF,Mexico
cDepartamentodeSistemasBiológicos,UniversidadAutónomaMetropolitanaUnidadXochimilco,México,DF,Mexico
dInstitutodeQuímica,UniversidadNacionalAutónomadeMéxico,México,DF,Mexico
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received26June2016 Accepted5September2016 Availableonline19October2016
Keywords: Heliopsislongipes Affinin Anti-arthritic
CompleteFreundadjuvant Spilanthol
a
b
s
t
r
a
c
t
Thisstudyassessestheanti-arthriticeffectoftheaffinin-enriched(spilanthol,mainalkamide)hexane extractfromtherootsofHeliopsislongipes(A.Gray)S.F.Blake,Asteraceae,onaFreundadjuvant-induced arthritismodelinrodents.Theextractwasorallyadministeredatadoseof2,6.6,or20mg/kg;asignificant edema-inhibitoryactivityintheacuteandchronicphaseswasobservedwithadoseof2and20mg/kg, respectively.Theextractshowedhigheranti-inflammatoryandanti-arthriticeffectsthanthereference drugphenylbutazone(80mg/kg).Moreover,theextractpreventedtheoccurrenceofsecondarylesions associatedtothispharmacologicalmodel.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Rheumatoidarthritis(RA) isanidiopathicauto-immune
dis-ease,characterizedbysymmetricalsynovitis inlargeand small jointsthatmayleadtoprogressivearticulardamageanddisability (Mendoza-Vázquezetal.,2013).Analgesicandanti-inflammatory drugs,includingsteroids,areusedtosuppressthesymptoms.New therapies, like those targeting the tumor necrosis factor alpha (infliximab)ortheanti-CD20therapy(rituximab),whichinhibit theunderlyingimmuneprocess,havebeenproposed.However,all thesedrugshaveseveralundesiredeffects.Recentresearchaimsto discoverlong-actinganti-inflammatorydrugswithminimalside effects(Ekambarametal.,2010).Anumberofanimalmodelsare usedtoevaluatepotentiallyusefulcompoundsagainstRA,andthe choiceofaparticularmodeldependsontheimmunological prop-ertiesbeing observedin the model and theirrelationwiththe
humandisease.Amongtheavailablemodelsiscollagen-induced
oradjuvant-induced(e.g.,Freund’sadjuvant)arthritisinrodents, spontaneousarthritisinducedbyTNF-␣orgeneticmodels,likethe
transgenicanimalmodel.WhilenoRAmodelcanbeconsidered
∗ Correspondingauthor.
E-mail:c.escobedo@ugto.mx(C.Escobedo-Martínez).
ideal,Freund’sadjuvant-inducedratarthritissharesseveraltraits withhumanarthritis,anditssensitivitytoevaluateanti-arthritic agentswasthebasisforitschoiceasthemodeltoassessthe activ-ityoftheHeliopsislongipes(A.Gray)S.F.Blake,Asteraceae,hexane extract(Ekambarametal.,2010;TanushreeandSaikat,2013).
RAprevalenceindevelopedcountriesis0.5–2%,withanannual
incidenceof12–1200per 100,000inhabitants.Thefemale:male
ratiois 2–3:1, and thepeak age range ofonset is 30–55 years old,butit couldoccuratanyage.In Mexico,RAaffects 1.6%of generalpopulation,beingthemainreasonforconsultationatthe
Rheumatology Service (Mendoza-Vázquez et al., 2013; Guía de
PrácticaClínica,DiagnósticoyTratamientodeArtritisReumatoide delAdulto,México:SecretaríadeSalud,2009).
H.longipesisaperennialherb,endemicinaregioncomprising partsofSierradeAlvarezandSierraGorda,nearthetripleborderof SanLuisPotosi,GuanajuatoandQueretaro,inCentralMexico.The commonnamesforthisplantarechilcuague,pelitre,goldenroot, andAztecroot(Cilia-Lópezetal.,2008).Theparalyzingandtoxic actionagainstfliesandotherinsectsofH.longipesrootextracts wasdiscoveredassimilarasthatofpyrethrinsduringWorldWar II(Acreeetal.,1945).
Fortunately,chilcuaguedidnotbecomeextinctafterthe expor-tationboom,eventhoughitswildpopulationwasseverelyreduced. Todate,landplotssolelydedicatedtothecultivationofthisspecies,
http://dx.doi.org/10.1016/j.bjp.2016.09.003
disseminatedeitherbyseedorbycutting,canbefoundbetween GuanajuatoandQueretaro;therootisfullydevelopedafter2–3 years (García-Chávez et al., 2004).It is the most economically importantspecies initsgenus;itsrootis usedintheregionas a cookingcondiment, andit is added toalcoholic beveragesto improvetaste.Intraditionalmedicine,chilcuaguerootisusedto treatrespiratorydiseases,asananesthetictotreattoothand mus-cularaches,totreatbuccallesions,andasananti-parasitic(Cilia López,2007).SeveralalkamideshavebeenisolatedfromH.longipes, butaffinin,alsoknownasspilanthol((2E,6Z,8E)-N -isobutyl-2,6,8-decatrienamide),wasidentifiedasthemainalkamideintheplant (Molina-Torresetal.,1996;García-Chávezetal.,2004;Hernández etal.,2009).Theoilysubstancecausesintenselipnumbnessand tinglingwithina fewminutesafterplacedin themouth,and it alsostimulatessalivation(Correaetal.,1971).H.longipesisalso
reportedtoproduceanalgesia andanti-inflammation in human
dentalandoralpathologies(Colvardetal.,2006).Additionally,it hasbeenreportedthatH.longipesdichloromethaneextractshowed analgesicactivity,asdemonstratedinvitrobyreleasingof gamma-aminobutyricacid(GABA)inmousebrainslices;theincreasein GABArelease in a regionof cerebral cortex leads tosustained analgesia,favoringadescendentinhibitionofnociceptivespinal neurons(Ríosetal.,2007).
With regard to anti-inflammatory activity, Hernández et al.
(2009)evaluatedH.longipesethanolicextractandpurifiedaffinin
on mouse ear edema induced by either arachidonic acid (AA)
orphorbol12-myristate13-acetate(PMA),demonstrating a sig-nificantanti-inflammatoryeffect.DE50 valuesof 0.8mg/earand 1.2mg/earwereobtained,respectively,intheAA-inducededema
model; nimesulide (1mg/ear) was used as reference drug. In
thePMA-inducededemamodel,theethanolicextractandaffinin showedadose-dependentanti-inflammatoryeffectwithDE50 val-uesof2mg/earand 1.3mg/ear,respectively, usingindometacin (3mg/ear)asreferencedrug.
Ontheotherhand,Cilia-Lópezetal.(2010)evaluatedthe anal-gesicactivityofH.longipesethanolicextractandaffininandtheir effectonthecentralnervoussystemonamousemodel;bothaffinin (1mg/kg)andH.longipesrootethanolicextract(10mg/kg)showed ananalgesicactionsimilartoketorolac(6mg/kg),asassessedby paininductionwithaceticacidandthermalstimulation(hotplate); also,H.longipesextractandaffininshowedastimulanteffecton thecentralnervoussystemcomparabletocaffeine,asmeasuredby theIrwintest.Cari ˜no-Cortésetal.(2010)evaluatedthecytotoxic potentialof H.longipesethanolic extract byrecording the vari-abilityinthecountofmicronucleatedpolychromaticerythrocytes inducedby theextract and theratioof polychromatic erythro-cyteswithrespecttonormochromaticerythrocytes;nosignificant cytotoxiceffectwasobserved.However,brainhistopathological studiesshowednecroticchangesinthegraymatter,describedas
polioencephalomalaciaandneurophagy,atadoseof1000mg/kg
of ethanolic extract. No damage or histopathological change
wasobserved in other organs (liver, heart, kidney, spleen, and lung).
AmongtherecentstudiesonH.longipesistheassessmentof ethanolicextractandaffininaspotentialanti-mutagenicand anti-carcinogenicagents.Theanti-mutagenicpropertiesofaffininwere evaluatedbyaddingittoseveralmutagens,eitherwithorwithout S9metabolicactivation,inSalmonellatyphimurium(TA98,TA100, and TA102 strains) cultures. Affinin significantly decreased the
pointmutationsinducedby2-aminoanthracene(2AA)(40%)and
decreasedtheDNAoxidativedamageinducedbynorfloxacin(NOR) (37–50%).Additionally,itshowedantioxidantpropertiescapable
of reducing the rate of 2AA- and NOR-induced mutation in S.
typhimuriumTA98andTA102,respectively,whichcouldbe rele-vanttotreatsomepainsymptomsrelatedtoanti-radicalactivity (Arriga-Albaetal.,2013).
Materialsandmethods
Plantmaterial
Heliopsislongipes(A.Gray).S.F.Blake,Asteraceae,rootswere col-lectedonFebruary16,2014,2:14:59pm,withintheboundariesof alandplotinBeltrancommunity(N21◦16.427′,W100◦0.2672′)at 1780mabovesealevel,Xichumunicipality,inGuanajuato,Mexico. Afterproperidentification,fiveplantspecimensweredepositedin theIsidroPalaciosherbarium(SLPM),attheUniversidadAutónoma deSanLuis(No.048150).
Extraction
Dry,groundH.longipesroots(1285g)weremaceratedforthree
consecutive times: First component extraction was performed
with 2.5 l of hexane for 2h; then, therecovered extract solu-tion(1750ml)wasevaporated,yieldingadarkyellow,viscousoil (26.386g,labeledasHl-A).Asecondmacerationwasperformedon thesameplantmaterialafterthefirstextraction,adding1.250lof hexaneandleftfor24h;extractsolution(1l)wasrecovered,and afterevaporationityielded12.29gofviscousoil,labeledasHl-B.A thirdandlastmacerationwasperformedasdescribedabove,using 1.5lofhexanefor4h,recoveringanextractsolution(900ml)and yielding3.469gofviscousoil,labeledasHl-C.Eachmacerationwas monitoredbythin-layerchromatography.TheHl-Asampleyielded 26.386gofaffinin(1),witha95%purity.
HPLCandNMRanalysis
Highperformanceliquidchromatography(HPLC)analysisofthe
Hl-AsamplewasperformedinaWaters1525BinaryHPLCpump
equipment,controlledbyBreezesoftware.A15cm×4.6mm,C18 Kromasil100-5columnwasused,coupledtoadiodearraydetector
(maximumabsorbancewassetto230nm).Awater-acetonitrile
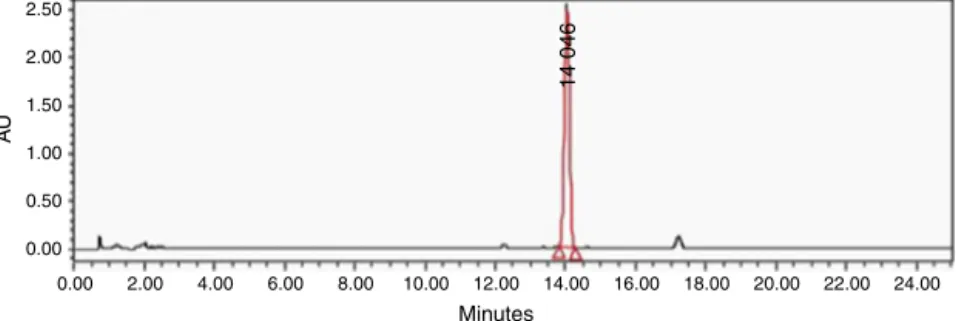
gradientfrom60:40to30:70in25minwasusedforelution,ata flowrateof1ml/min(Fig.1).
Magneticnuclearresonance(NMR)measurementsforthe
Hl-Asample wereacquiredin anAgilent600MHz,DD2,Oneprobe
equipmentfor1Hy13CinCDCl
3solution,usingtetramethylsilane asinternalstandard(Tables1and2).Chemicalshiftreadingswere comparedwiththosereportedintheliterature(Correaetal.,1971).
Pharmacologicalassay
Animals
All experiments were performed in accordance with
eth-ical standards for experimental pain research in animals
(Zimmermann,1983)andtheMexicanOfficialStandard for
ani-malcareandhandling(NOM-062-ZOO-1999).Theexperimental
protocol(CIBIUG-P01-2016) wasapproved and overseenby the
Institutional Ethics Committee for Care and Use of Laboratory
Animals of the Universidad de Guanajuato. Inbred Wistar rats
(250–300g) and Balb/c mice(20–25g), obtainedfrom the
Nat-uraland ExactScience Vivariumat UniversidaddeGuanajuato,
were used. Animals were housedin groups of six, under
con-trolled temperature (23±2◦C) and humidity (55±10%), with
2.50
2.00
1.50
AU
14 046
1.00
0.50
0.00
0.00 2.00 4.00 6.00 8.00 10.00 12.00
Minutes
14.00 16.00 18.00 20.00 22.00 24.00
Fig.1.ChromatogramoftheHl-Asampleaffinin(1),injectedataconcentrationof1mg/mlandreadatawavelengthof230nm.
Table1
1HNMRspectraldataofHl-Acomponent(affinin)(600MHz).a
Protona Chemical
shift
Multiplicity Area Coupling constant(Hz)
CH3-3′andCH3-4′ 0.92 d 6 J=6.8
CH3-10 1.77 d 3 J=7.2
H-2′ 1.80 m 1 J=6.0
CH2-4andCH2-5 2.24,2.32 dc 4 J=18.0,6.0
CH2-1′ 3.13 t 2 J=6.9
H-6 5.26 dt 1 J=12.0,6.0
H-9 5.69 dc 1 J=13.7,6.7
H-2 5.86 d 1 J=15.0
H-7 5.96 t 1 J=11.0
H-8 6.28 ddbroadsignal 1 J=18.0,12.0
H-3 6.82 dt 1 J=15.2,6.7
N-H 6.0 Broadsignal 1
aDatarecordedinCDCl
3.Chemicalshifts(ı)areexpressedinppmwithrespectto
tetramethylsilane(TMS).Couplingpatternsarelabeledas:d,doublet;dd,doubletof doublets;dt,doubletoftriplets;t,triplet;dc,doubletofquartets;m,multiplet.All assignmentswerebasedonthefollowingexperiments:1H-1D,13C-1D,COSY
(corre-lationspectroscopy),HSQC(heteronuclearsinglequantumcoherence;couplingof
1H 13Ctoabond),andHMBC(heteronuclearmultiplebondcorrelation;coupling
of1H 13Cto2and3bonds).
Table2
13CNMRspectraldataofHl-Acomponent(affinin)(125.7MHz).a
Carbona Chemicalshift
C-10 18.30
C-3′andC-4′ 20.16
C-5 26.42
C-2′ 28.58
C-4 32.12
C-1′ 46.91
C-6 124.26
C-8 126.70
C-7 127.63
C-2 129.43
C-3 129.89
C-9 143.36
C-1 166.17
aDatarecordedinCDCl
3.Chemicalshifts(ı)areexpressedinppmwithrespectto
tetramethylsilane(TMS).Allassignmentswerebasedonthefollowingexperiments:
1H-1D,13C-1D,COSY,HSQC,andHMBC.
environment one week before the experiments. Animals were
allowedfoodandwateradlibitum.Immediatelyafterthe exper-iments,allanimalsweresacrificedinaCO2chamber.
Acutetoxicityassays
TheDL50 valueper oswasdeterminedas reportedbyLorke
(1983). Mice were treated with Hl-A doses of 10, 100, or
1000mg/kg. Animalswere maintained under closeobservation
overa14-dayperiod.Theweightoftheanimalswasmonitored
throughouttheexperiments,andthedeathorsurvivalofthe ani-malswasregistered.
Anti-inflammatoryactivity
Arthritissyndromewasinducedbyaplantarintradermic injec-tion(througha20-gaugeneedle)intherighthindpawof0.05mlof completeFreund’sadjuvant(SigmaAldrich).Thismethod,reported byNewbould(1963),hasbeenwidelyusedtodetectpotential anti-inflammatorydrugswithanti-arthriticactivity.Amongtheseveral adjuvanttypesavailable,themostcommonlyusedin experimen-talanimalsisFreund’sadjuvant;twovarietiesofFreund’sadjuvant areused:IncompleteFreundAdjuvant(IFA)andCompleteFreund Adjuvant(CFA).IFAconsistsinamixtureofsurfactantandmineral oil,usuallyArlacelandDrakeol;CFAalsocontainskilled mycobacte-ria,usuallyMycobacteriumbovisBCGataconcentrationof1mg/ml orless(Rojas-Espinosa,2006).
Edema degree was evaluated by the volume displacement
method,usingaPANLABdigitalplethysmometer.Volume displace-mentwasrecorded24hbeforeand8,24,48,72,and 96hafter CFAinjection.Tocompletetheassessmentofactivityon arthri-tisinduction,volumevariationsinthehindpawwererecorded up to 25 days after injection. Phenylbutazone (reference drug, SigmaAldrich,80mg/kg)ortheHl-Asample(inadoseof2,6.6, or20mg/kg)wereadministeredp.o.24hbeforeCFAinjectionand dailyfor14daysafter.Theseverityandprogressionofsecondary lesionsintestanimalswerealsocompared.
Thepercentageofedemainhibitionwascalculatedforall ani-malsin each treatmentgroup withrespecttoa vehicle-treated controlgroup.Differencesbetweencontrolandtreatmentgroups
were analyzed byTukey’s test. A p-value lower than 0.05 was
regardedasstatisticallysignificant.
Results
Acutetoxicityassays
TheDL50valuep.o.fortheHl-Asamplewas775mg/kg.
Anti-inflammatoryactivity
ProgressionofFreund’sadjuvant-inducedarthritisinrats
CFAinjectionintherighthindpawcausedinflammation,which peakedwithinthefirst8h.Then,inflammationslowlydecreased untilday6post-injection,had aslightincreaseafterday7,and showedasustainedincreaseuntiltheendoftheexperiment.At day16post-CFA-injectionanduntiltheendoftheperiodofstudy, inflamedlesions (secondarylesions) in the lefthind pawwere detected,aswellasdeformedfrontpawsandincreasingthickness inearsandtail(Fig.2).
Dosing
EachtestedconcentrationoftheHl-Asampleandof
Fig.2.Secondarylesionsobservedontheday22afterplantarinjectionofcompleteFreund’sadjuvantinaratfromthecontrol,untreatedgroup.
Table3
EffectofHl-AonFreund’sadjuvant-inducededemainrats.
Time(h) Sample Dose(mg/kg) %Inhibition±SEM
8 Phenylbutazone 80 46.5±6.8*
8 Hl-A 2 18.9±7.5
8 Hl-A 6.6 14.5±8.1
8 Hl-A 20 17.5±8.6
24 Phenylbutazone 80 47.8±5.6*
24 Hl-A 2 42.3±4.2*
24 Hl-A 6.6 29.7±11.3
24 Hl-A 20 31.7±10.3
*DatawereanalizedbyANOVAfollowedbyTuke’stest.p<0.05.
administered1daybeforeCFAinjectionintherighthindpawpad
(Newbould,1963).Frequentmeasurementsinthehindpawswith
aplethysmometershowedthatthetreatmentwith
phenylbuta-zoneandwiththetestedHl-Aconcentrations(2,6.6,or20mg/kg) inhibitedinflammationinthehindpawduringthedosingperiod. AsshowninFig.4,prophylactictreatmentwiththereferencedrug (phenylbutazone,80mg/kg)significantlyinhibitedthe inflamma-tory effect of CFA injection 8h after the stimulus, during the
acute inflammation phase. This was observed again 24h after
injection; additionally,administration p.o. of Hl-A at a dose of 2mg/kginhibitedtheprogressionofacute-phaseedemaby42.3% (F4,20=4.7)(Table3,Fig.3).
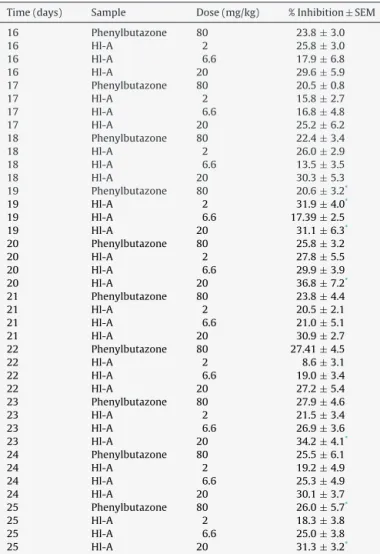
Duringinflammationchronicphase,significantvaluesof inhi-bitionpercentage after administrationp.o.of Hl-A at a doseof 20mg/kgwereobservedonlyondays19,20,23,and25afterCFA injection,withinhibitionpercentagesof31.1(F4,20=5.012),36.8 (F4,20=3.17),34.2(F4,20=3.56),and31.3(F4,20=4.30),respectively (Table4andFig.4).Ontheotherhand,Hl-Aadministrationatthe samedoseshowedahigherinhibitionpercentagethan phenylbu-tazoneinallchronic-phasedays.Secondarylesionsdidnotoccur duringthetreatmentperiodatallHl-Adoses(Fig.5),andratsfrom Hl-A-treatedgroupsshowedabetterjointmobility.
No significant difference was observed in corporal weight
betweenphenylbutazone-treatednorHl-A-treatedratgroupsat
anydosewithrespecttothecontrol,untreatedgroup.
Discussion
Anumberofanimal modelshave beenproposedtoevaluate
anti-inflammatoryactivity,liketheuseofsubstancessuchas for-malinofdextran,whichcauseacuteinflammationwheninjected
inrat hindpaws.However,thesemodelsrequireadministering
highdosesofanti-inflammatorydrugslikephenylbutazone,often neartoxicvalues,tosignificantlyinhibitinflammation(Domenjoz, 1960).Anothermethodisthesubcutaneousimplantofcotton pel-lets,amodelthatismoresensitivetosteroidanti-inflammatory drugsbutisrelativelyinsensitivetonon-steroidanti-inflammatory
1.2
1.0
0.8
∗
∗ ∗
0.6
0.4
Change in f
oot v
olume (ml)
0.2
0.0
8h 24h Treatment mg/kg
Vehicle Phen
yl 80
H.longipes 2H.longipes 6.6H.longipes 20 Vehicle
Phen yl 80
H.longipes 2H.longipes 6.6H.longipes 20
Fig.3.Acuteeffectofadministration(p.o.)ofphenylbutazone(80mg/kg)or
Heliop-sislongipes Hl-Asample(2, 6.6,and 20mg/kg)onFreund’sadjuvant-induced
inflammationinthehindpawoftherodentmodel(rat).Eachbarshowsthemeanof 5lectures±SEM.DatawereanalyzedbyANOVAfollowedbyTukey’stest.p<0.05.
drugslikephenylbutazone(Winderetal.,1962).Themethodused inthisstudyisbasedonasyndromemoresimilartorheumatoid arthritis,whichiscapableofdetectinganti-inflammatoryactivity inawiderangeofdrugtypesusefultotreatrheumatoidarthritisin humans(Pearsonetal.,1961;PearsonandWood,1963).
Phenylbutazone,previouslyusedinothermodels,wasasuitable referencedrugtoinhibitinflammationcausedbyCFAinjectionin rathindpaw;noweight-losswasobservedasreportedwithother drugs(Newbould,1963).Inourstudy,phenylbutazoneinhibited CFA-inducedinflammationby46.5%8hafterinjection(Table3), averysimilarresulttothatreportedbyAbadetal.(1996),who recordedaninhibitionrateof40%24hafterstimulus,increasing toover70%at72handdecreasingagainto40%at96h,remaining constantuntiltheday15.Inourstudy,thecontroldrugwas dis-continuedonthechronicphase,butinhibitionrateremainedina rangeof23–27%,indicatingalong-termeffect.Ontheotherhand, aninhibitionrangeof27.2–36.8%wasobservedintheHl-A-treated group(20mg/kg),higherthanthatofthephenylbutazone-treated animals.
The process underlying theoccurrence of secondary lesions
0.00 0.20 0.40 0.60 0.80 1.00 1.20 1.40
26 24 22 20 18 16 14
Change
in foot volume (ml)
Day
Vehi
cle
Phenyl
butazone 80 mg/kg
H. longipes 2 mg/kg
H. longipe
s 6.6 mg/k
g
Fig. 4.Effect administration (p.o.) of phenylbutazone (80mg/kg) or Heliopsis
longipesHl-Asample(2,6.6,and20mg/kg)onthelong-term,Freund’s
adjuvant-inducedinflammationphaseinthehindpawoftherodentmodel(rat).Eachpoint intime-courseshowsthemeanoffivelectures±SEM.DatawereanalyzedbyANOVA followedbyTukey’stest.p<0.05.
Table4
EffectofHl-AonFreund’sadjuvant-inducededemainrats.
Time(days) Sample Dose(mg/kg) %Inhibition±SEM
16 Phenylbutazone 80 23.8±3.0
16 Hl-A 2 25.8±3.0
16 Hl-A 6.6 17.9±6.8
16 Hl-A 20 29.6±5.9
17 Phenylbutazone 80 20.5±0.8
17 Hl-A 2 15.8±2.7
17 Hl-A 6.6 16.8±4.8
17 Hl-A 20 25.2±6.2
18 Phenylbutazone 80 22.4±3.4
18 Hl-A 2 26.0±2.9
18 Hl-A 6.6 13.5±3.5
18 Hl-A 20 30.3±5.3
19 Phenylbutazone 80 20.6±3.2*
19 Hl-A 2 31.9±4.0*
19 Hl-A 6.6 17.39±2.5
19 Hl-A 20 31.1±6.3*
20 Phenylbutazone 80 25.8±3.2
20 Hl-A 2 27.8±5.5
20 Hl-A 6.6 29.9±3.9
20 Hl-A 20 36.8±7.2*
21 Phenylbutazone 80 23.8±4.4
21 Hl-A 2 20.5±2.1
21 Hl-A 6.6 21.0±5.1
21 Hl-A 20 30.9±2.7
22 Phenylbutazone 80 27.41±4.5
22 Hl-A 2 8.6±3.1
22 Hl-A 6.6 19.0±3.4
22 Hl-A 20 27.2±5.4
23 Phenylbutazone 80 27.9±4.6
23 Hl-A 2 21.5±3.4
23 Hl-A 6.6 26.9±3.6
23 Hl-A 20 34.2±4.1*
24 Phenylbutazone 80 25.5±6.1
24 Hl-A 2 19.2±4.9
24 Hl-A 6.6 25.3±4.9
24 Hl-A 20 30.1±3.7
25 Phenylbutazone 80 26.0±5.7*
25 Hl-A 2 18.3±3.8
25 Hl-A 6.6 25.0±3.8
25 Hl-A 20 31.3±3.2*
* DatawereanalizedbyANOVAfollowedbyTuke’stest.p<0.05.
Fig.5. Statusoftherighthindpawofasubjectratonday22afterprophylactic treatmentwithHl-A(p.o.)atadoseof20mg/kg.
to beresult of a generalized immune response tothe
compo-nentsoftuberclebacilli,disseminatedafterlocaladministration
(WarsmanandSharp,1960).Neitherphenylbutazone-treatednor
Hl-A-treatedratsatanydose(2,6.6,or 20mg/kg)showed sec-ondarylesions,incontrastwithcontrol,untreatedanimals(Fig.5); thisindicatesthattreatmentsmodifytheimmuneresponse.
Ontheotherhand,thehighestextractdose(20mg/kg)wassetto muchlowervaluethantheDL50valuehereinreported(775mg/kg,
p.o.), which allows us to regard it as a low-toxicity substance (Lorke,1983).Extractaccumulationduetoadailyadministration for15daysfailedtocauseanytoxicitysignintheperiodofstudy. Therefore,hereinweprovideinformationsupportingthepotential anti-inflammatoryusefulness of H.longipes hexane extract (Hl-A),whichhasaffinin asitsmain constituentasshownbyHPLC andNMRanalysis.Thepotentialusefulnessasanti-inflammatory
agentoftheextract ata lowdose(2mg/kg)wasdemonstrated
in the acute-phase inflammation of the model, while its
anti-inflammatoryandanti-arthriticactivityathigherbutsecuredoses
(20mg/kg)wasdemonstratedinthechronic-phaseinflammation
ofthemodel,withnooccurrenceofsecondarylesions.
Conclusions
ThehexaneextractofH.longipes,ofwhichaffininisthemain constituent,haspotentialusefulnessasananti-arthriticagent.This studycontributestoprovideinformationsupportingthetraditional useofthisplantspecies.Itisnoteworthythattheavailable infor-mationaboutthisspecieswouldsufficetocreateamonographand
suggesttheproduction of aphytopharmaceutical, which would
helpencouragingastandardized,high-qualitycultivationofthis plantspeciesandimprovetheeconomyofpeasantsinSierraGorda, Mexico.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Authors’contributions
CEMdesignedthestudy,supervisedthelaboratoryanddrafted thepaper.SLGGanalyzeddataandcontributedtodraftthepaper. MMHMevaluatedtheAnti-arthriticactivity,runningthe
labora-torywork.JCcontributedtorecordNMRspectra.ATVandLMOC
contributedtocriticalreadingofthemanuscript.RGEcontributed tothechromatographicanalysisofplantmaterialandthe isola-tionandidentificationofaffinin.Alltheauthorshavereadthefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Thefundingof14-IJDPP-Q182-44,Convocatoria“Investigadores
Jóvenes2014”; CONCYTEG,401/2014,ConvocatoriaInstitucional
deApoyoalaInvestigaciónCientífica2014–UG;UGTO-PTC-382, ConvocatoriaApoyoalaIncorporacióndeNuevosPTC-PRODEP-14; and1088/2016,Convocatoria InstitucionaldeApoyoala Inves-tigaciónCientífica2016-2017–UG,isthankfullyacknowledged. ThankstoTax.JoséD.GarcíaPérez(UASL)forspecimen identifica-tion,andDr.MuraliVenkatBasavanagforNMRspectrarecording.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2016.09.003.
References
Abad,M.J.,Bermejo,P.,Carretero,E.,Martínez-Acitores,C.,Noruega,B.,Villar,A., 1996.AntiinflamatoryactivityofsomemedicinalplantextractsfromVenezuela. J.Ethnopharmacol.55,63–68.
Acree,F.,Jacobson,M.,Haller,H.L.,1945.Anamidepossessinginsecticidalproperties fromtherootsofErigeronaffinisDC.J.Org.Chem.10,236–242.
Arriga-Alba,M.,Ríos,Y.M.,Déciga-Campos,M.,2013.Antimutagenicpropertiesof affininisolatedfromHeliopsislongipesextract.Pharm.Biol.51,1035–1039. Cari ˜no-Cortés,R.,Gayosso-De-Lucio,J.A.,Ortíz,N.I.,Sánchez-Gutiérrez,M.,
García-Reyna,P.B.,Cilia-López,V.G.,Pérez-Hernández,N.,Moreno,E.,Ponce-Monter,H., 2010.Antinociceptive,genotoxicandhistopatologicalstudyofHeliopsislongipes S.F.Blakeinmice.J.Ethnoparmacol.130,216–221.
CiliaLópez,V.G.,(Tesisdedoctorado)2007.Biologíayutilizacióndelchilcuague (HeliopsislongipesS.F.Blake).UniversidadAutónomadeSanLuisPotosí,San LuisPotosí,S.L.P.México,pp.1–104.
Cilia-López,V.G.,Aguirre-Rivera,J.R.,Reyes-Agüero,J.A.,Juárez-Flores,B.I.,2008. EtnobotánicasdeHeliopsislongipes(asteraceae:Heliantheae).Bol.Soc.Bot. Méx-ico83,81–87.
Cilia-López,V.G.,Juárez-Flores,B.I.,Aguirre-Rivera,J.R.,Reyes-Agüero,J.A.,2010. AnalgesicactivityofHeliopsislongipesanditseffectonthenervoussystem. Pharm.Biol.48,195–200.
Colvard,M.D.,Cordel,G.A.,Villalobos,R.,Sancho,G.,Perkowits,K.M.,Michel,J.,2006. Surveryofmedicalethnobotanicalsfordentalandoralmedicineconditionsand pathologies.J.Ethnopharmacol.107,134–142.
Correa,J.,Roquet,S.,Díaz,E.,1971.MultipleNMRanalysisoftheaffinin.Org.Magn. Reson.3,1–5.
Domenjoz,R.,1960.Thepharmacologyofphenylbutazoneanalogues.Ann.N.Y.Acad. Sci.86,263–291.
Ekambaram, S., Selvan Perumal, S., Subramanian, V., 2010. Evaluation of antiarthritic activity ofStrychnos potatorum Linn seeds in Freund’s adju-vant induced arthritic rat model. BMC Complement. Altern. Med. 10, http://dx.doi.org/10.1186/1472-6882-10-56.
García-Chávez,A.,RamírezChávez,E.,Molina-Torres,J.,2004.ElgéneroHeliopsis (Heliantheae:Asteraceae)enMéxicoylasalcamidaspresentessensusraíces. ActaBot.Mex.69,115–131.
GuíadePrácticaClínica,2009.DiagnósticoyTratamientodeArtritisReumatoidedel Adulto,México:SecretariadeSalud.CENETEC-IMSS-195-08México,DF. Hernández,I.,Márquez,L.,Martínez,I.,Dieguez,R.,Delporte,C.,Prieto,S.,
Molina-Torres,J.,Garrido, G.,2009.Anti-inflammatoryeffectsof ethanolicextract andalkamides-derivedfromHeliopsislongipesroots.J.Ethnopharmacol.124, 649–652.
Mendoza-Vázquez,G.,Rocha-Mu ˜noz,A.D.,Guerra-Soto,A.J.,Ramírez-Villafa ˜na,M., González-Sánchez,A.G.,Gámez-Nava,J.I.,Nava,A.,2013.Artritisreumatoidey dislipidemias.ElResidente8,12–22.
Molina-Torres,J.,SalgadoGarciglia,R.,Ramírez-Chávez,DelRío,R.E.,1996.Purely olefinicalkamidesinHeliopsislongipesandAcmelia(Spilanthes)oppositifolia. Biochem.Syst.Ecol.24,43–47.
Newbould,B.B.,1963.Chemotherapyofarthritisinducedinratsbymicobacterial adyuvant.Br.J.Pharmacol.21,127–136.
Lorke,D.,1983.Anewapproachtopracticalacutetoxicitytesting.Arch.Toxicol.54, 275–287.
Pearson,C.M.,Waksman,B.H.,Sharp,J.T.,1961.Studiesofarthritisandotherlesions inducedinratsbyinjectionofmycobacterialadjuvant.V.Changesaffectingthe skinandmucousmembranes.Comparisonoftheexperimentalprocesswith humandisease.J.Exp.Med.113,485–509.
Pearson,C.M.,Wood,F.D.,1963.Studiesofarthritisandotherlesionsinducedinrats bytheinjectionofmycobacterialadjuvant.VII.Pathologicdetailsofthearthritis andspondylitis.Amer.J.Path42,73–95.
Ríos,M.Y.,Aguilar-Guadarrama, B.,Gutiérrez,M.C.,2007.Analgesicactivityof affinin,analkamidefromHeliopsislongipes(Compositae).J.Ethnopharmacol 110,364–367.
Rojas-Espinosa,O.,2006.Inmunología.Ed.PanamericanaMéxico,DF.,pp.100–101. Tanushree, R., Saikat, G., 2013. Animal models of rheumatoid arthritis: correlation and usefulness with human rheumatoid arthritis. IAJPR 3, 6131–61342.
Warsman,B.H.,Sharp,J.T.,1960.Immunologicnatureofarthritisinducedinratsby mycobacterialadjuvant.Fed.Proc.19,210.
Winder,C.V.,Wax,J.,Scotti,L.,Sherrer,R.A.,Jones,E.M.,Short,F.W.,1962. Antiinflam-matory,antipyreticandantinociceptivepropertiesofN-(2,3-xylyl)anthranilic acid(mefenamicacid).J.Pharmacol.exp.Ther.138,405–413.