REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
Official Publication of the Brazilian Society of Anesthesiologywww.sba.com.br
SCIENTIFIC
ARTICLE
Comparative
study
between
fast
and
slow
induction
of
propofol
given
by
target-controlled
infusion:
expected
propofol
concentration
at
the
effect
site.
Randomized
controlled
trial
夽
Ricardo
Francisco
Simoni
a,b,c,d,∗,
Luiz
Eduardo
de
Paula
Gomes
Miziara
c,d,
Luis
Otávio
Esteves
b,c,d,
Diógenes
de
Oliveira
Silva
d,e,f,
Cristina
Alves
Ribeiro
b,d,
Mariana
Oki
Smith
b,d,
Leonardo
Ferreira
de
Paula
b,d,
Luis
Henrique
Cangiani
b,c,daDepartmentofPharmacology,UniversidadeEstadualdeCampinas(Unicamp),Campinas,SP,Brazil
bCentrodeEnsinoeTreinamentodaSociedadeBrasileiradeAnestesiologia(CET/SBA),CentroMédicodeCampinas,Campinas,
SP,Brazil
cFundac¸ãoCentroMédicodeCampinas,Campinas,SP,Brazil
dSociedadeBrasileiradeAnestesiologia,Brazil
eCentrodeMedicinadoAparelhoDigestivodeSantaCatarina,SC,Brazil
fHospitalSãoFranciscodeAssisdeSantoAmarodaImperatriz,SantoAmarodaImperatriz,SC,Brazil
Received2May2013;accepted15July2013 Availableonline28November2014
KEYWORDS Anesthetics; Intravenous; Propofol; Pharmacology; Anesthetic techniques; General; Intravenous
Abstract
Backgroundandobjective: Studieshaveshownthattherateofpropofolinfusionmayinfluence the predicted propofol concentration atthe effectsite (Es). The aimof thisstudy was to evaluate the Es predicted by the Marshpharmacokinetic model (ke00.26min−1) inloss of
consciousnessduringfastorslowinduction.
Method: Thestudyincluded28patientsrandomlydividedintotwoequalgroups.Inslow induc-tiongroup(S),target-controlledinfusion(TCI)ofpropofolwithplasma,Marshpharmacokinetic model(ke00.26min−1)withtargetconcentration(Tc)at
2.0-gmL−1wereadministered.When
thepredictedpropofolconcentrationattheeffectsite(Es)reachedhalfofEsvalue,Eswas increasedtopreviousEs+1gmL−1,successively,untillossofconsciousness.Inrapidinduction
group(R),patientswereinducedwithTCIofpropofolwithplasma(6.0gmL−1)ateffectsite,
andwaiteduntillossofconsciousness.
夽
StudydevelopedatCET/SBAofInstitutoPenidoBurniereCentroMédicodeCampinas.
∗Correspondingauthor.
E-mail:ricaboss@gmail.com(R.F.Simoni).
http://dx.doi.org/10.1016/j.bjane.2013.07.015
Results:Inrapidinductiongroup,Tcforlossofconsciousnesswassignificantlylowercompared toslowinductiongroup(1.67±0.76and2.50±0.56gmL−1,respectively,p=0.004).
Conclusion:Thepredictedpropofolconcentrationattheeffectsiteforlossofconsciousnessis differentforrapidinductionandslowinduction,evenwiththesamepharmacokineticmodel ofpropofolandthesamebalanceconstantbetweenplasmaandeffectsite.
©2014SociedadeBrasileiradeAnestesiologia.PublishedbyElsevier EditoraLtda.Allrights reserved.
PALAVRAS-CHAVE Anestésicos; Venoso; Propofol; Farmacologia; Técnicasanestésicas; Geral;
Venosa
Estudocomparativoentreinduc¸ãorápidaelentadepropofoleminfusão
alvo-controlada:concentrac¸ãodepropofolprevistanolocaldeac¸ão.Ensaioclínico aleatório
Resumo
Justificativaeobjetivo:Estudosmostraramqueataxadeinfusãodepropofolpodeinfluenciar naconcentrac¸ãoprevistadepropofolnolocaldeac¸ão(Ce).Oobjetivodesteestudofoiavaliar aCeprevistapelomodelofarmacocinéticodeMarsh(ke00,26min−1)naperdadaconsciência
duranteinduc¸ãorápidaoulenta.
Método: Participaramdeste estudo 28 pacientes, divididos aleatoriamente em dois grupos iguais.Nogrupoinduc¸ãolenta(L),foraminduzidoscompropofoleminfusãoalvo-controlada (IAC)plasmática,modelofarmacocinéticodeMarsh(ke00,26min−1),comconcentrac¸ãoalvo
(Ca)em2,0g.ml−1.Quandoaconcentrac¸ãodepropofolprevistanolocaldeac¸ão(Ce)atingia
metadedovalordaCa,aumentava-seaCaparaCaanterior+1g.ml−1.Assimsucessivamente
atéomomentodaperdadaconsciênciadopaciente.Nogrupoinduc¸ãorápida(R),ospacientes foraminduzidoscompropofolemIACplasmáticacomCaem6,0g.ml−1eaguardava-seaperda
daconsciênciadopaciente.
Resultados: Nogrupoinduc¸ãorápida,aCenaperdadaconsciênciafoisignificativamentemais baixa em relac¸ãoao grupo deinduc¸ãolenta (1,67±0,76e 2,50±0,56g.ml−1,
respectiva-mente,p=0,004).
Conclusão:A concentrac¸ão previstade propofol nolocal de ac¸ãodurante aperda da con-sciência édiferentenuma induc¸ãorápida enumainduc¸ão lenta,atécomomesmomodelo farmacocinéticodepropofoleamesmaconstantedeequilíbrioentreoplasmaeolocalde ac¸ão.
©2014SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Todosos direitosreservados.
Introduction
Recently several studies have shown a good correlation between the predicted propofol concentration at the effect site (Es) by Marsh pharmacokinetic model (ke0 0.26min−1)andsedationdegree,bispectralindex(BIS) val-ues,entropy,evokedpotentialindex,andlossandrecovery ofconsciousness.1---5
Becauseof thisgoodcorrelation with
pharmacodynam-ics,someauthorssuggested thatthetargetconcentration
ofpropofolshouldbetitratedduringmaintenanceof
anes-thesiabasedonEsreachedinlossofconsciousness.3,4,6
However, other studies show that the rate of infusion
ofpropofolmayinfluencethebalancebetweentheplasma
concentrationandtheconcentrationattheeffectsite;that is,inthefirst-ordermathematicalconstantcalledKe0.7,8
The main objective of this study was to evaluate
the Es predicted by the Marsh pharmacokinetic model
(ke0 0.26min−1) on loss of consciousness during rapid
or slow induction of patients undergoing laparoscopic
cholecystectomy under total intravenous anesthesia with
propofol and remifentanil. Es was also evaluated during
anesthesiamaintenanceandrecovery.
Thehypothesistobetestedisthat,evenusingthesame
pharmacokineticmodelandthesameequilibriumconstant
between plasma and effect site, the effect site in rapid
inductionisdifferentfromthatinslowinductionduringloss
ofconsciousnesses.
Method
AfterapprovalbytheResearchEthicsCommitteeand
receiv-ingthewritteninformedconsent,28patients,agedbetween
18and65years,ofbothsexes,ASAphysicalstatus1and2,
and undergoing laparoscopic cholecystectomy under total
intravenousanesthesiawithpropofolandremifentanil,were
enrolledinthisrandomizedclinicaltrial.
The sample size was based on a previous pilot study.
Table1 Age,weight,heightandsexofpatientspergroup. Group Age(years) Weight(kg) Height(cm) Sex
(m/f)
S 43.1±11.8 70.7±16.9 167.1±9.3 5/9 R 46.8±12.0 76.5±8.64 166.2±8.8 6/8
S,slowinductiongroup;R,rapidinductiongroup;p>0.05.
betweenthepropofolconcentrationsprovidedontheeffect siteduringlossofconsciousnesswithrapidandslowinfusion was67%,thepower analysiswithalphaof 1%andbetaof 5% showedthat11 patients wouldberequired per group. Threemorepatientspergroupwereaddedtocompensate forpossiblelossesduringtheclinicaltrial.
No patientreceived premedication and allwere moni-toredwithelectrocardiogram(DIIandV1),pulseoximetry, non-invasivemeanarterialbloodpressure(MAP),bispectral index(BIS),andend-tidalCO2aftertrachealintubation.
Patientswererandomlyallocatedintotwoequalgroups throughadefinedsequencebycomputer.Theslowinduction group (Group S) received propofol by plasma target-controlledinfusion(TCI),Marshpharmacokineticmodel(ke0 0.26min−1),withtargetconcentration(Tc)of2.0
gmL−1.
When the predicted propofol concentration at the effect site(Es)reached halfthevalueofTc,Tcwasincreasedto previousTc+1gmL−1,andsoonuntilthepatient’slossof
consciousness(lossofverbalresponseandeye-blinkreflex). Therapidinductiongroup(GroupR)receivedplasma propo-folviaTCIwith6gmL−1Tcandwaiteduntilpatient’sloss
ofconsciousness.
In both groups, after loss of consciousness, TCI remifentanil was initiated to an effect site of 5gmL−1 (Minto’s pharmacokinetic model), rocuronium 0.6mgkg−1 wasadministered,andaftertwominutestrachealintubation wasperformed.
During the intraoperative period, Tc of propofol was adjusted to maintainBIS between 35 and 50,while Tcof remifentanilwasadjustedtomaintainMAPbetween±20% oftheinitialMAP.
Aftersurgery,bothinfusionswereturnedoff.
Es of propofol was recorded at the time of loss and recoveryofconsciousness(BIS=70)andeveryminuteofthe intraoperativeperiod.
All patients received dipyrone30mgkg−1 and ketopro-fen1.5mgkg−1forpostoperativeanalgesiaandmethadone 0.1mgkg−1asrescueanalgesicinthePost-AnesthesiaCare Unit.
For infusion management and data collection, the Anestfusor®software,coupledtotwoPilot2syringepumps (Fresenius-Kabi)andBIS,wasused.
Forstatistical analysisof parametricdata, Student’st -testwasusedandthedifferencewasconsideredsignificant whenpvalueswere<0.05.
Results
Therewasno significantdifferencebetween demographic variablesofthetwogroups(p>0.05)(Table1).
InductiontimeinGroupSwashighercomparedtoGroup
R,4.54and1.46min,respectively(p<0.001).Therewasno
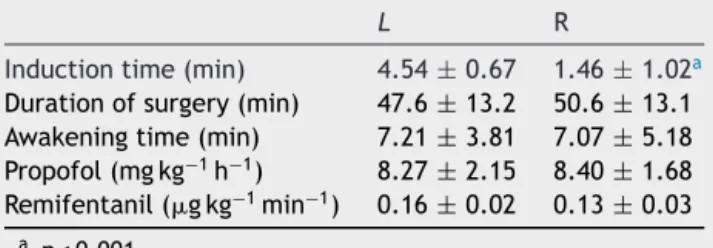
Table2 Induction,durationofsurgery,awakeningtimes, andpropofolandremifentanilconsumption.
L R
Inductiontime(min) 4.54±0.67 1.46±1.02a
Durationofsurgery(min) 47.6±13.2 50.6±13.1 Awakeningtime(min) 7.21±3.81 7.07±5.18 Propofol(mgkg−1h−1) 8.27±2.15 8.40±1.68
Remifentanil(gkg−1min−1) 0.16±0.02 0.13±0.03
a p<0.001.
significant differenceinsurgery andawakening timesand
consumptionofpropofolandremifentanilbetweenthetwo
groups(p>0.05)(Table2).
Thepredictedpropofoleffect-siteconcentration(Es)in
loss of consciousness was higherin Group S compared to
Group R, 2.50 and 1.67gmL−1 respectively (p=0.004).
Eswassignificantly differentat loss andrecovery of
con-sciousnessin Group S, 2.5 and 1.60gmL−1, respectively
(p<0.001). InGroup R,Eswaslowerat lossof conscious-nesscomparedtointraoperativeEs,1.67and2.52gmL−1,
respectively (p=0.002). There was no significant
differ-ence between groups regarding Es values’ intraoperative
andatrecoveryofconsciousness(p>0.05).Therewasalso
nosignificant differenceinEs duringloss andrecovery of
consciousnessinGroupR(p>0.05)(Fig.1).
Discussion
ThemaindifferencefoundinthisstudywastheEspredicted bytheMarshpharmacokineticmodel(ke00.26min−1)during
lossofconsciousnessbetweenrapidandslowinduction.
Althoughthesamepharmacokineticmodelandthesame
equilibrium constant plasma/effect-site (Ke0) have been
used, Es at loss of consciousness was significantly lower
in Group R comparedto Group S, 1.67 and 2.50gmL−1,
respectively. This difference was also found by other
authors.8
Studies have shown that the pharmacokinetic models
ofpropofol usedin target-controlledinfusion systems are
poorlyaccurateforearlypredictionofpropofolactualblood
concentrationafterbolusorrapidinfusion,whenmaximum
LOC S LOV R IO S IO R ROC S ROC R 1.0
1.5 2.0 2.5 3.0 3.5 4.0
Data
a
Figure1 Effect-site concentration ofpropofol (mcgmL−1).
effect is observed.7,9---11 These conventional nipple
multi-compartmentalpharmacokinetic models assumethatdrug
mixingincentralcompartmentoccursimmediatelyandthe
mixtureimmediatelyappearsinthearterialcirculation.In
fact,thereisadelaybetweenthedrugadministrationand
itsappearanceinarterialblood.Thishasbeenreportedin
severalstudies.7,12,13
Among other factors, this delay depends on the
pul-monaryextractionof propofolduringthefirst pass.14,15 In
Marshpharmacokinetic model, thisinitial error is evident
duringthefirstfiveminutes,whichmakesthemodelnotso
preciseforthesefirstminutes.7
Byassuminganinstantaneousmixtureafterabolus
injec-tion,thetraditionalpharmacokinetic modelsoverestimate
thecentralvolume.Becausethebolusdosedependsonthe
size of the central compartment, its overestimation may
resultinalargebolus,whichmayexceedthetarget concen-trationwheneverthetargetisincreased.14,15
Because of this poor predictability of pharmacokinetic
model in the first minutes after a bolus injection, some
authorshaveshown thattherateof propofolinfusioncan
influence the equilibrium constant between plasma and
effectsite.7Apparently,theplasma/effect-siteequilibrium
constantisfaster forbolus administrationthanfor slower
infusions.This mayanswerwhydifferentke0arefoundin
theliterature,evenwhenusingthesamepharmacokinetic
model.7
Maybetherearephysiologicalreasonsforthesedifferent
valuesofke0obtainedthroughbolusandslowerinfusions.
Onestudyshowedthatpropofolreducescerebralbloodflow
inadose-dependentmanner.16So,whenusingabolus
injec-tion to extract ke0, the achieved high concentrations of
propofolmayreducecerebralbloodflow.Ontheotherhand,
whenslowerinfusionsareused,thispropofoleffecton
cere-bralbloodflowshouldbereduced.
With the use of a conventional continuous infusion
scheme,the valuesfor propofolt½ ke0 inliterature vary
between 2.3and 3.5min.17---19 The Marshpharmacokinetic
model,whichispresentinthefirsttarget-controlled
infu-sionsystemcommerciallyavailable(Diprifusor),wasbased
ondatafromaslowinfusionandisassociatedwithat½ ke0
of2.65min.17
As an option to reduce this initial error of the
propo-fol pharmacokinetic models,some authors have proposed
toincorporate intothe Schnidermodel different ke0
val-uesfor different infusion rates.7 Ifthe maximum infusion
rateremains between 300 and 900mLh−1, t½ ke0should
beabout2.2min(ke0=0.32min−1).However,iftheinfusion issimilartoabolusinjection,ashortert½ ke0of 1.2min
shouldbeused.
For other pharmacokinetic models of propofol as the
Marsh,forexample,ifthepump iscapableofdeliveringa
bolusinductioninaminuteorless,thetimemustbe
imple-mentedtomaximumeffectof1.5min.7Withtheseoptions,
thismodelpredictedeffectconcentrationismoreaccurate
overtime.
Some authors have assessed more appropriate
phar-macokinetic models for this initial kinetic phase and the
correlation with possible covariates such as age, weight,
and infusion rate.20 In these more sophisticated models,
itwasdemonstrated thatthe use ofa singleke0 value is
appropriate and can be applied to the target controlled
infusion systems, which use syringe pumps with infusion
ratebetween10and160mgkg−1h−1.Therefore,forthese
studies,pharmacodynamicsisnotinfluencedbytherateof
propofolinfusion.20,21
ThemeanvaluesofEsinrapidandslowinductiongroups
were similar during the intraoperative and recovery of
consciousness times, 2.52±0.43 and 2.52±0.76gmL−1,
respectively, and 1.63±0.42 and 1.60±0.58gmL−1,
respectively.
As shown in some studies, Es of propofol for loss
and recovery of consciousness aresimilarwhen using the
Marsh pharmacokinetic model (ke0 0.26min−1).3,4
There-fore,someauthorssuggestedthatthetargetconcentration
ofpropofolshouldbetitratedduringmaintenanceof
anes-thesiabasedonEsduringlossofconsciousness.3,4,6Themain
objectivewouldbetoreducethepossibilityofpatient awak-eningduringsurgery.Itisworthnotingthatthisisonlyvalid
whenanalgesiaiscompletethroughouttheprocedure.
Todate,theliteratureonthesubjectdoesnotallow say-ingthattheactualpropofolconcentrationattheeffectsite issimilaratlossandrecoveryofconsciousnessorthatitis reallydifferent.
Recently,astudyshowedthatregardlessofthe
pharma-cokineticmodelofpropofolused(Schnider:ke00.45min−1
and time to peak effect 1.7min; Marsh: ke0 1.21min−1
andtimetopeakeffect1.7min;or Marsh:ke0 0.26min−1
and time to peakeffect of 4.5min), the predicted value
of propofolat the effectsiteduring lossof consciousness
afterabolusinjectionshouldnotbeusedasreferencevalue
fortitrationofhypnosisduringmaintenanceofanesthesia,
astheeffectconcentrationofpropofolpredictedbythese
modelsduringlossofconsciousnessisverydifferent(4.40,
3.55and1.28gmL−1,respectively).8
Inthisstudy,therapidandslowinductiongroupsshowed
similarEsatrecoveryofconsciousness.However,Esatloss
andrecoveryofconsciousnesswassimilaronlyintherapid
inductiongroup(Fig.1).
Basedonthepresentedresults,wecanconcludethatin
casesofrapidinductionwithMarshmodel(ke00.26min−1), Esatlossandrecoveryofconsciousnessissimilar(1.63and 1.60gmL−1,respectively).However,Esduringthe
intraop-erativeperiodshouldbeabout50%higher.
Incasesofslowinduction,thetargetmaintenancedose
maybesimilartotheEsduringlossofconsciousness.This
result was expected, as the ke0 used in this study was
derived fromslow infusion data.17 Consequently, the
pre-dicted effect-site concentration of propofol over time is
moreprecise.
Althoughthegoalwastoevaluatethepredicted
effect-siteconcentration ofpropofol,themain limitationof this
studywasnotmeasuringtheplasmaconcentrationof
propo-folatdifferenttimes.
Another aspect to be considered is that the use of
patientsofbothsexesmayhaveincreasedthestudybias,as
sexisanimportantvariableinpropofolpharmacokinetics.22
However, there was no significant difference between
groupsinthenumberofpatientsofbothgenders.
Conclusion
induction, even withthe same pharmacokinetic model of
propofolandthesameequilibriumconstantbetweenplasma
andeffectsite.Recognizingthisdifferenceiscrucialto
per-formatotalintravenousanesthesiawithtarget-controlled
infusionofpropofolsafelyforthepatient.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Gajraj RJ, Doi M, Mantzaridis H. Comparison of bispectral EEG analysis and auditory evoked potentials for monitoring depthofanaesthesiaduringpropofolanaesthesia.BrJAnaesth. 1999;82:672---8.
2.Barakat AR, Sutcliffe N, Schwab M. Effect site concen-tration during propofol TCI sedation: a comparison of sedationscorewithtwopharmacokineticmodels.Anaesthesia. 2007;62:661---6.
3.IwakiriH,NishiharaN,NagataO.Individualeffect-site concen-trationsofpropofolaresimilaratlossofconsciousnessandat awakening.AnesthAnalg.2005;100:107---10.
4.SimoniRF,EstevesLO,MiziaraLEPG,etal.Avaliac¸ãoclínicade duaske0nomesmomodelofarmacocinéticodepropofol:estudo daperdaerecuperac¸ãodaconsciência.RevBrasAnestesiol. 2011;61:397---408.
5.IannuzziM, Iannuzzi E, Rossi F, et al. Relationship between bispectral index, electroencephalografic state entropy, and effect-siteEC50forpropofolatdifferentclinicalendpoints.Br JAnaesth.2005;94:613---6.
6.LysakowskyC,EliaN,CzarnetzkiC,etal.Bispectraland spec-tralentropyindicesatpropofol-inducedlossofconsciousness inyoungandelderlypatients.BrJAnaesth.2009;103:387---93.
7.StruysMMRF,CoppensMJ,NeveND,etal.Influenceof admin-istration rate on propofol plasma-effect site equilibration. Anesthesiology.2007;107:386---96.
8.SepulvedaPO,CortinezLI,RecartA,etal.Predictiveabilityof propofoleffect-siteconcentrationsduringfastandslowinfusion rates.ActaAnaesthesiolScand.2010;54:447---52.
9.SchniderTW,MintoCF,ShaferSL,etal.Theinfluenceofagein propofolpharmacodynamics.Anesthesiology.1999;90:1502---16.
10.Kasama T, MoritaK, IkedaT,et al. Comparisonofpredicted inductiondosewithpredeterminedphysiologiccharacteristics of patients and with pharmacokinetic models incorpo-rating those characteristics as covariates. Anesthesiology. 2003;98:299---305.
11.SchuttlerJ,IhmsenH.Populationpharmacokineticsofpropofol: amulticenterstudy.Anesthesiology.2000;92:727---38.
12.KrejcieTC,HenthornTK,NiemannCU,etal.Recirculatory phar-macokineticsmodelsofmakersofblood,extracellularfluidand totalbodywateradministeredconcomitantly.JPharmacolExp Ther.1996;278:1050---7.
13.UptonRN,GrantC,MartinezAM,etal.Recirculatorymodelof fentanyl dispositionwiththebrainas thetargetorgan. BrJ Anaesth.2004;93:687---97.
14.Avram MJ, Krejcie TC. Using front-end kinetics to opti-mizetarget-controlleddruginfusion.Anesthesiology.2003;99: 1078---86.
15.HenthornTK, Krejcie TC, AvramMJ.Earlydrug distribution: agenerallyneglectedaspectofpharmacokineticsofparticular relevancetointravenouslyadministeredanesthesicagents.Clin PharmacolTher.2008;84:18---22.
16.Ludbrook GL, Visco E, Lam AM. Propofol: relation between brainconcentrations,electroencephalogram, middlecerebral arterybloodflowvelocity,andcerebraloxygenextraction dur-inginductionofanesthesia.Anesthesiology.2002;97:1363---70.
17.SchwildenH,StoeckelH,SchuttlerJ.Closed-loopfeedback con-trol of propofol anaesthesiaby quantitative EEG analysisin humans.BrJAnaesth.1989;62:290---6.
18.BillardV,GambusPL,ChamounN,etal.Acomparisonof spec-traledge,deltapower,andbispectralindexasEEGmeasuresof alfentanil,propofol,andmidazolamdrugeffect.ClinPharmacol Ther.1997;61:45---58.
19.WhiteM,SchenkelsMJ,EngbersFH,etal.Effect-sitemodelling of propofol using auditory evoked potentials. Br J Anaesth. 1999;82:333---9.
20.MasuiK,KiraM,KasamaT,etal.Earlyphasepharmacokinetics butnotpharmacodynamicsareinfluencedbypropofolinfusuion rate.Anesthesiology.2009;111:805---17.
21.DoufasAG,BakhshandehM,BjorkstenAR,etal.Inductionspeed isnotadeterminantofpropofolpharmacodynamics. Anesthe-siology.2004;101:1112---21.