w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
In
vitro
and
in
vivo
evaluation
of
efficacy
and
safety
of
photoprotective
formulations
containing
antioxidant
extracts
Maria
Cristina
P.P.
Reis
Mansur
a,∗,
Suzana
Guimarães
Leitão
a,
Cristal
Cerqueira-Coutinho
a,
Alane
Beatriz
Vermelho
b,
Ronald
S.
Silva
c,
Octávio
A.F.
Presgrave
c,
Álvaro
A.C.
Leitão
d,
Gilda
G.
Leitão
e,
Eduardo
Ricci-Júnior
a,
Elisabete
P.
Santos
aaFaculdadedeFarmácia,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
bBioinovar-Biocatálise,BioprodutoseBioenergia,InstitutodeMicrobiologiaPaulodeGóes,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
cInstitutoNacionaldeControledeQualidadeemSaúde,Fundac¸ãoOswaldoCruz,RiodeJaneiro,RJ,Brazil
dInstitutodeBiofísicaCarlosChagasFilho,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
eInstitutodePesquisaemProdutosNaturais,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6September2015 Accepted30November2015 Availableonline1February2016
Keywords:
Leafextracts Cosmeticformulation Photoprotection Efficacyandsafety Evaluationmethods
a
b
s
t
r
a
c
t
Chronicexposuretosolarradiationcouldcontributetoprematureskinagingandskincancer.Skin presentsitsownantioxidantdefense,howeverwhendefensesareoutofbalance,reactiveoxygenspecies coulddamagebiologicalstructures.Inthepresentwork,anoil-in-waterphotoprotectiveemulsionwas developedandBauhiniamicrostachyavar.massambabensisVaz,Fabaceae,extractsat1%(obtainedby extractionwithdifferentsolvents)wereaddedtothisemulsion.Invitroandinvivoefficacyandsafetyof theformulationswereevaluated.SpectrophotometricmethodsandinvivoColipatestwereperformed toevaluatedefficacyoftheformulations,throughsunprotectionfactor(SPF)determinationandUVA protectionfactorassessment.TotheinvitrosafetyassessmentHET-CAM,CAM-TBSandRedBloodCell testswereperformed.Resultsshowedthatbothextractscontributedtoahigherinvivophotoprotection (SPF18)whencomparedtotheformulationwithoutextract(SPF13),thisresultcouldbeattributedtothe antioxidantactivityoftheplantextractsthatactbycapturingreactiveoxygenspecies.Concerningsafety, allformulationswereconsiderednon-irritantaccordingtoinvitrotests.Formulationscontainingextracts couldbeconsideredefficientandsafeforcosmeticusesincetheypresentedhighersunprotectionfactor andpassedthetoxicitytests.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Skinistheoutercoveringofhumanbodyconferringprotection againstultraviolet(UV)radiation(Bouwstraetal.,2007;Bolzinger etal.,2012).However,chronicexposuretoUVradiationleadsto manysideeffectstotheskin,suchasprematureaging,skin can-cerand reduction ofimmune response capability.These health problemsaredirectlyrelatedtotheformationofreactiveoxygen species(ROS)byUVradiation(JainandJain,2010;Gilbertetal., 2013).
Evenpresentingantioxidantdefensemechanisms,skincould beaffectedbyROS;whendefensemechanismsareoutofbalance, oxidativestresscoulddamagecellularmembranes,proteins, car-bohydrates and nucleicacids promoting theiroxidation (Finkel
∗ Correspondingauthor.
E-mail:mariacristinareis@micro.ufrj.br(M.C.P.P.ReisMansur).
andHolbrook,2000;Gálvez,2010).Ontheotherhand,ROSplay invivopositivefunctionsrelatedtoenergyproduction, phagocyto-sis,cellgrowthregulationandintercellularsignaling(Gutteridge andHalliwell,2000;Rouanetetal.,2010).
Inorganic and organic sunscreens are added to photopro-tective formulations since they act protecting the skin against UV radiation; however, recentlythere hasbeen much research abouttheuseofantioxidantsextractedfromplants.Thesenatural antioxidantsusuallycomefromadietrichinfruitsandvegetables or theyarecarried increams and topicallyapplied(Podda and Grundmann-Kollmann, 2001). Plant extracts with antioxidant propertiesraisegreatinterestinthephytocosmeticfieldasthey presentmoleculesthatcouldinactivateROSrestoringskin home-ostasisthuspreventingerythemaandprematureagingoftheskin (Calderon-Montanoetal.,2011;Mansuretal.,2012).Barradasand coworkers(2014)developednanoemulsionscontainingplantoil, sweetfenneloil,tobeappliedtopically;theresearchersverified thatsweetfenneloilpresentedantioxidantproperties,probably
http://dx.doi.org/10.1016/j.bjp.2015.11.006
dueto thepresenceof flavonoidsand terpenoidsthat promote highradicalscavengingactivity.
Inapreviousworkfromourgroup,itwasstudiedthe antiox-idanteffectof differentplant extractsfromthe genusBauhinia (Mansur etal., 2012).B. microstachya var. massambabensisVaz, Fabaceae,isrestrictedtoaridzonesanditisfoundonlyinRiode JaneiroState,Brazil.
Ourstudy hasdemonstrated a higherantioxidant activityof BauhinialeafextractwhencomparedtotheGingkobilobastandard extract(EGb761)andTrolox®(avitaminEwater-solubleanalog). Bauhiniaplantextractspresenthighamountsofflavonoid glyco-sides,includinggalloylderivatives,aswellasmethylgallateand gallicacid-likesubstancesthatareacknowledgedaspotent antiox-idantsthusbeingasourceforthestudyofdifferentpharmacologic activitiesanddidnotshowphototoxicityaccordingtotheMinimal InhibitoryConcentrationMethod(MIC),wherenozoneof inhibi-tioninthegrowthofintheSaccharomycescerevisiaewasverified (Mansuretal.,2012).
Themainsubstancesandtheirmolecularstructuresidentified in the phytochemical fractionation of Bauhinia ethyl acetate extract are the flavonoids kaempferol-3-O-rhamnoside (1) and astragalin-2′′,6′′-di-O-digallate (2) (Mansur et al., 2012). Other
authorsalsoidentifiedtheseflavonoidsin Bauhiniaactiveplant extracts(Menezesetal.,2004;Silvaetal.,2007).
Flavonoidsgenerallyoccurinplantsasglycosylatedderivatives thatparticipateinphotosynthesis(Pietta,2000).Thesephenolic compoundsconferprotectiontotheplantssincetheycaptureROS, protectingthemfromoxidation,whichisgeneratedbyUVradiation fromthesun.Forthisreason,thesecompoundscouldbetopically appliedonhumanswiththesameintentionofcapturingROS,thus inhibitinglipidperoxidation,whichisresponsibleforskinaging andskincancer(Zuanazziand Montanha,2004; Laguerreetal., 2007).
In recent years, natural substances have been increasingly incorporated into dermocosmetic formulations; it is a world-widetendencytoaddvaluetoproductsespeciallybecauseofthe greatcommercialappealandincreasedacceptancebycustomers. Besides,thedevelopmentofphotoprotectiveformulationsusing lesssyntheticsunscreensisoneoftheobjectivesinphotoprotection research(GilaberteandGonzález,2010;Hayesetal.,2011). How-ever,thesafetyoftheseformulasmustbeevaluatedbeforetheir availabilitytocustomers;thustheirritantpotentialof photopro-tectiveformulationsshouldbeassessedthroughinvitroandinvivo teststoguaranteethepresenceofsaferproductsinthemarket, reducingriskstocostumers(Anvisa,2012).
Manyinvitrotestsareavailabletoevaluatetoxicityofcosmetic products;someofwhicharedestinedtoevaluateoculartoxicity, e.g.Hen’sEggChorioallantoicMembrane(HET-CAM)test, Chorioal-lantoicMembrane-TrypanBlueStaining(CAM-TBS)test,RedBlood
Cell(RBC)testandBovineCornealOpacityandPermeability(BCOP) test(Anvisa,2012).Thesetestsaimtoevaluatethesafetyoffacial cosmeticproducts, since theproductcouldbeeasilyin contact withtheeyemucousmembranegeneratingirritation.Moreover, toxicitytestscouldbealsoperformedtoevaluateprimary cuta-neous irritation, which is necessary when it comes to topical formulations.
The aim of the present study was to develop photopro-tectiveoil-in-water (O/W) emulsionscontainingthesunscreens benzophenone-3 (BZF-3), octylmethoxycinnamate (OMC) and octocrylene(OCT)and Bauhiniamicrostachya var. massambaben-sisVazleafextracts–inwaterandacetone(WAc)andinethanol treatedwithactivatedcarbon(EtOH-AC)–andevaluatetheinvivo efficacyandsafetyoftheseformulations.
Materialsandmethods
Chemicals
Allreagentswereofanalyticalgrade(Sigma,Merck).Sunscreens usedintheformulations:BZF-3,purchasedfromGalena(Brazil); OMC,purchasedfromSpectrum(Brazil)andOCT,purchasedfrom DEG(Brazil).
Plantleafextracts
Bauhinia microstachya var. massambabensis Vaz, Fabaceae, leaveswerecollectedfromthebotanicalgardenoftheDepartment ofBotanyandPharmacognosy(FederalUniversityofRiodeJaneiro) andtheleafextractswereobtainedaccordingtotheManagement CouncilforBrazililanGeneticPatrimony(CGEN),resolutionsn◦28
andn◦29,2007.Thespecimenwasdepositedunderthenumber
30813intheHerbariumoftheBiologyInstituteoftheFederal Uni-versityofRiodeJaneiro,aCGEN-accreditedHerbariumRFA.Extracts wereobtainedaccordingto Mansurand coworkers(2012)– in waterandacetone(WAc)andinethanolsubmittedtotreatment withactivatedcarbon toprovidea less coloredethanol extract (EtOH-AC).Theseextractswerechosensincetheyaremore suit-abletocosmeticapplicationthantheotherextractsobtainedby thegroupinapreviouswork(Mansuretal.,2012).Sunscreensused intheformulations:BZF-3,purchasedfromGalena(Brazil);OMC, purchasedfromSpectrum(Brazil)andOCT,purchasedfromDEG (Brazil).
Developmentoftheformulations
Table1
CompositionoftheformulationsA,B,C,DandE.
Ingredients Formulations
A B C D E
Oilphase(wt%)
BZF-3 5 5 5 – –
OCT 5 5 5 – –
OMC 5 5 5 – –
Cetostearylalcoholethoxylate 3 3 3 3 3
Stearicacid 8 8 8 8 8
Isoctylstearate 7 7 7 7 7
Glycerylmonostearate 3 3 3 3 3
Propylparaben 0.1 0.1 0.1 0.1 0.1
Dimethiconecopolyol 9.5 9.5 9.5 9.5 9.5
Cyclomethicone 11 11 11 11 11
Aqueousphase(wt%)
Aminomethylpropanol95% 0.3 0.3 0.3 0.3 0.3
Glycerin 5 5 5 5 5
Imidazolidinylurea 0.2 0.2 0.2 0.2 0.2
Methylparaben 0.1 0.1 0.1 0.1 0.1
Hydroxypropylstarchphosphate 1 1 1 1 1
EtOH-ACextract – 1grams – 1grams –
WAcextract – – 1grams – 1grams
Purifiedwater Qsa100wt% Qsa100wt% Qsa100wt% Qsa100wt% Qsa100wt%
aQsf:quantitysufficient.
themixtureofthethreesunscreens, BZF-3,OMCand OCT (for-mulationA);withthesamemixtureofsunscreensand1wt%of EtOH-ACextract(formulation B)and withthesame mixtureof sunscreens and 1wt% of WAc extract (formulation C). In addi-tion, it was alsoprepared O/W emulsions containing only one extract,withoutsunscreens:formulationDwith1wt%of EtOH-ACextractandformulationEwith1wt%ofWAcextracttoverify iftheBauhiniaextractsalone(withoutsunscreens)presentedsun protection(invitroSPFassessment).
Thestandard O/Wemulsion(withoutsunscreensorextracts) wasatypicalwhite-coloredformulationwithpHof6.0thatwas produced by the classical emulsificationmethod where the oil phaseisheatedandpouredintotheaqueousphaseatthesame temperature (around 70◦C) under slow stirring until complete
homogenization; the emulsifier enable the interaction of both phases.ToformulationA,BandC,sunscreensweresolubilizedin theoilphasebeforethemixturewiththeaqueousphaseandto for-mulationsB,C,DandEtherespectiveextractswereaddedafterthe phases’mixture,underslowstirringwhenthetemperaturewasat 40◦Ctopreventtheextracts’degradationbyheat.
InvitroSPFandUVAProtectionFactor(UVA-PF)assessment
Invitro SPF evaluation isusually performed toestimatethe invivoSPF.TotheinvitroSPFassessmentformulationsA,B,C,D andEwereevaluated.
Sampleswere diluted in ethanol at a final concentration of 2l/mlandanalyzedbyUVspectrophotometry(JascoV-630)from 290 to 320nm, with intervals of 5nm, according to Mansur’s method(1986)(Mansuretal.,1986).Mansur’smethodissimple andeasilyreproducible,theSPFdeterminationwhichisthe cor-relationbetweentheerythemogeniceffect(EE)andtheradiation intensityateachwavelength(I)(Table2)andareadjusted accord-ingto Eq.(1).Wherethe correctionfactor (CF)is 10, EE()is theerythemogeniceffectofradiationonwavelength,I()isthe intensityofsolarlightwithwavelengthandabs()isthe sam-ple(2l/mlinethanol)spectrophotometricabsorbancevalueat wavelength.
SpectrophotometricSPF=CF
320
290EE()I()abs() (1)
Table2
Correlationbetweentheerythemogeniceffect(EE)andtheradiationintensityat eachwavelength(I)(Mansuretal.,1986).
(nm) EE()×I()
290 0.0150
295 0.0817
300 0.2874
305 0.3278
310 0.1864
315 0.0839
320 0.0180
TotheinvitroUVA-PFassessmentitwasusedaUVtransmittance analyzer(Labsphere® UV-2000S)andquartzplateswithanarea
of25cm2.TheplateswerecoveredbyTransporeTMtapeonone
surfaceandanamountof50mg(2mg/cm2)ofeachformulation
was appliedwitha micropipette onthis surface and manually spread with circular movements in order toobtain a homoge-neousfilm.Glycerinwasusedasreferencefor100%oftransmission (Labsphere,2008).Afterdryingfor15minindarkchamber, sam-pleswereanalyzed.Bothassayswereperformedintriplicateand themean±standarddeviation(SD)wasassessed.
Photostabilityassay
FormulationsA,BandCwereevaluatedinthephotostability assay. The assay was performed using a solar simulator (Oriel 91192-1000), a UV spectrophotometry(Jasco V-630)and a UV transmittance analyzer (Labsphere® UV-2000S). To the SPF assessment (Mansur’smethod), the amountof 250mg of each formulationwasappliedto30mmdiameterPetridisheswith1ml ofethanolformingahomogeneousfilm.Theplatesdriedinadark chamber for 60min toallowethanol toevaporate.For UVA-PF assessment,thesampleswereappliedinquartzplatescoveredby TransporeTMtape.AllplatesandPetridisheswereirradiatedfor
90mininthesolarsimulatorwithradiationintensityof315J/m2/s
(UVA)and3.35J/m2/s(UVB).ThreeplatesandthreePetridishes
(UVA-PF).Thetestwasperformedintriplicateandthemean±SD
wasassessed.
InvivoSPFassessment
TotheinvivoSPFassessment formulationsA,BandC were evaluated.ThisassaywascarriedoutaccordingtotheBrazilian EthicsCommitteeforClinicalTrials(number:120/10/2011).The methodologyemployedwasbasedontheInternationalSun Pro-tectionFactorTestMethoddevelopedbyColipaandpublishedasa guidelinein2006(Colipa,2006).
TheSPFvalueisdefinedastheratiobetweentheultraviolet energyrequiredtoproduceaminimalerythemaldose(MED)on protectedskinandtheultravioletenergy requiredtoproducea MEDonunprotectedskinaccordingtoEq.(2).
SPF=MEDMEDofofunprotectedprotectedskinskin (2)
Tenwomenbetweentheagesof18and42yearswithskin pho-totypesI,IIorIII(Fitzpatrick,1975)wereselectedasvolunteers afterbeinginformedaboutthestudyprotocolsandagreedto par-ticipategivingtheirwrittenconsent.Thebackofeachvolunteer was exposed to ultraviolet radiation using a multiport (model 601)ultravioletsolarsimulator.Theexposuretimewaschanged accordingtoskinphototype.OntheseconddaytheMED(without sunscreen)wasassessedforeachvolunteerandthenthe formula-tionsB,CandDwereappliedintheamountof2mg/cm2toother
areasonthebackofthevolunteer.Aftertheapplication,the prod-uctswerelefttodryfor15minbeforebeingirradiated.Areasof 5×6cmwereirradiatedinsixpointswithincreasingUVradiation
doses.TheSPFforeachformulationwasassessedasamean±SDof
theSPFvaluesobtainedforthevolunteers.
Safetyofthephotoprotectiveformulationsandextracts:irritant potentialevaluation
FormulationsA,BandCwereevaluatedregardingtheirsafety (Anvisa,2012).Inaddition,toverifythesafetyofthefreeextracts (withoutbeingincorporatedintotheO/Wemulsion),theywere vehiculatedat1wt%inphosphatebuffer(PB)pH7.4.Threedifferent methodswereperformed:HET-CAMtest,CAM-TBStestandRBC test.
Hen’sEggChorioallantoicMembrane(HET-CAM)test
Chickenembryoshavebeenwidelyusedasanalternativetothe invivoocularirritationtest.Forthistest,freshfertileLeghorneggs weighing50–60gwereplacedinanautomaticrotationincubator andkeptat37.5±0.5◦Cwithrelativehumidityof62.5±7.5%
dur-ing10days.Onthetenthday,theeggshellaroundtheairchamber wasremovedusinganodontologicalsaw,exposingtheshell mem-branethatwasmoistenedwithsalinesolution0.9%.Withtheaidof tweezerstheshellmembranewasremovedexposingthe chorioal-lantoicmembrane(CAM).Visualanalysiswasperformedtoverify iftheCAMwassuitabletothetestthen300lofeach formula-tionwasplacedontheCAMsurface.After20s,theformulation wasremovedwithsalinesolution.TheCAMwasobservedundera magnifyingglassfor5mintodeterminetheincidenceofanyirritant effectsintheCAMbloodvessels(hyperemia,hemorrhageor coagu-lation)(LuepkeandKemper,1986;WorthandBalls,2001;Liebsch andSpielman,2002).Theirritanteffectswereclassifiedbyscores(1, 3,5,7,or9)accordingtothetimetheywereobserved:lessthan30s (hyperemia:5;hemorrhage:7;clotformation/opacity:9);between 30and120s(hyperemia:3;hemorrhage:5;clotformation/opacity: 7);orbetween120and300s(hyperemia:1;hemorrhage:3;clot
formation/opacity:5).Ifaneffectwasnotobservedafter5min,it wasscoredaszero.
Eachformulationwasclassifiedaccordingtothescoresmean valueoffoureggs:0–4.99correspondingtonon-irritant/slightly irritant(NOI/SLI);5.00–8.99correspondingtomoderatelyirritant (MOI);and9.00–21.00correspondingtoseverelyirritant(SEI).Asa negativecontrolfoureggsweresubmittedtothesameprocedure, butnoformulationwasadded.
ChorioallantoicMembrane-TrypanBlueStaining(CAM-TBS)test
CAM-TBS is a quantitativemethod forthe evaluation ofthe formulations’ toxicity (Invitox, 1996; Lagartoet al., 2006).The CAM-TBStestusestrypanblueasanindicatorofchorioallantoic membrane(CAM)injuryandshowsagoodcorrelationwiththein vivoDraizeeyeirritationtest(LiebschandSpielman,2002;Scott etal.,2010).ThemethodologyissimilartotheHET-CAMandafter theremovalofthecosmeticformulation, 500lof aphosphate
salinebufferand0.1%oftrypanbluestaining(TBS)wereaddedto theCAMinthearealimitedbya18mmdiametersiliconering.The excessofTBSwasrinsedoffwithdistilledwater,andtheCAMarea thatwaslimitedbythesiliconeringwasremovedwithscissors andputinto5mlofformamideandthenagitatedandcentrifuged. Theabsorbanceofthesupernatantwasmeasuredby spectropho-tometryat595nm.Thequantificationofthetrypanbluestaining thatenteredthecellscouldbecorrelatedtotheinjurycausedby theformulationtotheCAM.
Eachformulationwasclassifiedaccordingtothemeanvalueof foureggsbasedontheHET-CAMscores:0–4.99correspondingto NOI/SLI;5.00–8.99correspondingtoMOI;and9.00–21.00 corre-spondingtoSEI.Thescore(d)foreachformulationwasassessed usingEq.(3)(Lagartoetal.,2006).Asanegativecontrolfoureggs weresubmittedtothesameprocedure,butnoformulation was added.
TBSconcentration=d×10005 ×109 nmol (3)
Redbloodcell(RBC)test
RBCtestenablesthequantificationandevaluationoftheside effectscaused bysurfactants addedtomanycosmeticproducts, suchasshampoo,showergelandemulsionsintheredbloodcells plasmatic membrane and consequentlythe hemoglobinrelease (hemolysis)andthehemoglobindenaturationindex,both quan-tifiedbyspectrophotometry.Therelationshipbetweenhemolysis andhemoglobinoxidationprovidesaparametertothe characteri-zationoftheinvitroirritanteffectsofthesurfactants(Alvesetal., 2008).
SheepRBCwereisolatedbygforce.Forhemolysisanalysis, solu-tionsof1mg/mlofeachformulationinPBSwereaddedtovialsin ordertocreatearangeofincreasingconcentrationstoenable lin-earregression.Theirvolumewasfilledupto975lwithPBSandit wasadded25lofRBCsuspensionwithawell-known concentra-tionofoxyhemoglobin.Theresultingsuspensionwasincubatedfor 10minatroomtemperatureunderslowstirring.After10minthe sampleswerecentrifugedat10,000×gfor1min.Theabsorbanceof thesupernatantwasdeterminedbyspectrophotometryat540nm (foroxyhemoglobin)andat575nm(fordeoxyhemoglobin).Fora 0%hemolysiscontrol,25lofRBCswasaddedto975lofPBS,and
Table3
InvitroassessmentoftheSPFandtheUVA-PFoftheformulationsA,B,C,DandEbeforeandafterirradiationforformulationsthatpresentedphotoprotectiveactivity.
Formulations SPFa SPFafterirradiationa UVA-PFa UVA-PFafterirradiationa
A 17.0±0.25 16.6±0.22 2.63±0.17 2.50±0.16
B 17.8±0.77 16.4±0.01 2.50±0.10 2.40±0.20
C 16.9±0.62 16.6±0.13 2.45±0.10 2.40±0.20
D 0.70±0.06 Theywerenotconsideredphotoprotective formulationssinceSPFintoolow E 0.68±0.02
aMean
±SD.
Statisticalanalysis
Experimentaldataarepresentedasthemean±SDwithatleast
threedeterminationsforindependentexperiments.Alldatawere analyzedbypairedandunpairedt-testsusingOrigin8.5.1 (Origin-Lab,USA)softwareandp<0.05wasconsideredtobestatistically significant.
Resultsanddiscussion
Developmentoftheformulations
TheformulationsA,B,C,DandEweresuccessfullyobtained.All formulationspresentedavisualaspectandtexturesuitabletobe appliedtopically.
InvitroassessmentofSPFandUVA-PFbeforeandafterirradiation (photostabilityassay)
Table3showstheresultsoftheinvitroSPFforformulationsA, B,C,DandE.
Formulationscontainingonly sunscreens(A) and sunscreens andextract(BandC)presentedinvitroSPFsuitableto photopro-tectiveproducts.FormulationsDandEdidnotpresentSPF,forthis reason theywerenot considered photoprotectiveformulations, thustheywerenotevaluatedintermsofUVA-PFand photosta-bility(Santosetal.,1999;Freitasetal.,2001;Monteiroetal.,2012; Motaetal.,2013).
EvennotcontributingwiththeinvitroSPF,bothextractspresent phenoliccompoundsthatcouldactpreventingUV-induceddamage byothermechanisms,e.g.capturingandinactivatingROS(Greul etal.,2002).
Theadditionof EtOH-ACandWAc Bauhiniaextractsdidnot influencesunscreens’photostabilitysincetherewasnostatistical differenceamonginvitroSPFandUVA-PF(p>0.05).Ontheother hand,extractsdidnotcontributetosunscreensphotodegradation whenexposedtoUVradiation.
Thephotodegradation of sunscreensgenerates ROSthat can damage skin structures (Butt and Christensen, 2000) and an antioxidant plant extract added to the formulation could act by capturing these reactive species and increasing sunscreens photostability (Jarzycka et al., 2013; Cerqueira-Coutinho et al., 2015).Cerqueira-Coutinhoandco-workers(2015)foundoutthat the addition of pomegranate extract to a photoprotective for-mulation increased sunscreens photostability, since the plant extract presented highantioxidant activity accordingto DPPH•
(2,2-diphenyl-1-picrylhydrazyl) assay(described as EC50– half maximaleffectiveconcentration).
Inourpreviousstudy(Mansuretal.,2012),DPPH•freeradical
assaywasperformedtoevaluatetheantioxidantpotentialofthe isolatedcompounds.DPPH• freeradicalassaydoesnottakeinto
accounttheformulation wherethecompound wasvehiculated. ThisasalimitationoftheDPPH•assaysincetheactivesubstance
mustbesolubilizedinasolvent,commonlyanorganicone.The
measurementsareperformedinaspectrophotometerandforthis reasonthesamplemustbecompletelysolubilized.Theother com-ponentspresentintheformulationsuchaswaxesandotheroily materialsthatconstitutetheemulsionwhere theextractswere vehiculated,couldinterfereinthemeasurementsandforthis rea-sonforthistest,theactiveisevaluatedalone(Blois,2002;Boonne andYotsawimonwat,2011;Faudaleetal.,2008;Oktayetal.,2003; Piccagliaand Marotti,2001; Rubertoet al.,2000; Salamaetal., 2013).The abilityto scavengeDPPH• radicalwasmeasuredby
the discoloration of thesolutions preparedin this experiment, using spectrophotometry. The greater the antioxidant activity thehighertheintensityofsolutiondiscoloration(Mensoretal., 2008).
InvivoSPFassessment
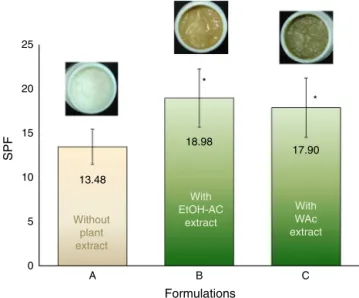
FormulationA,withoutplantextractpresentedthelowestSPF, 13.48±1.99while formulations Band C, both containing plant
extract,presentedSPFof18.98±3.30and17.90±3.35,respectively
(Fig.1).Nostatisticaldifferenceswereobtainedforcomparisons betweentheSPFofformulationsBandC(p>0.05).Ontheother hand,theSPFofformulationAwasstaticallylowerthantheSPFof formulationsBandC(p<0.05).
AllformulationspresentedasuitableSPFbuttheextracts con-tributedtotheenhancementofinvitroSPF.ThehigherinvivoSPFof formulationsBandC(withantioxidantextracts)whencompared toformulationA(withoutextract)couldbeattributedtothe scav-engingactivityoftheantioxidantmoleculespresentintheplant extractsthatactbycapturingROS.
AllformulationspresentedasuitableSPFbuttheextracts con-tributedtotheenhancementofinvivoSPF.ThehigherinvivoSPFof
13.48
18.98
17.90
0 5 10 15 20 25
C B
A Without
plant extract
With EtOH-AC
extract
With WAc extract
SPF
Formulations *
*
Table4
HET-CAM,CAM-TBSandRBCresultsforformulationsandpureextracts(1wt%inPBpH7.4)withtheirclassificationbasedontheirritanteffectsanddenaturationindex.
Formulations HET-CAM CAM-TBS RBC Classification
Scorea Scorea Lisys/denaturationindex
A 1.92±0.38 4.59±0.24 >100 NOI/SLI
B 2.13±0.88 4.53±0.42 >100 NOI/SLI
EtOH-AC1wt%inPB 2.25±1.06 3.09±0.38 >100 NOI/SLI
C 2.0±0.71 4.48±0.31 >100 NOI/SLI
WAc1wt%inPB 0 1.07±0.64 >100 NOI/SLI
aMean
±SD.
formulationsBandC(withantioxidantextracts)whencompared toformulationA(withoutextract)couldbeattributedtothe scav-engingactivityoftheantioxidantmoleculespresentintheplant extractsthatactbycapturingROS.Itissuggestedthatthesolvent usedintheextraction(WAcandEtOH-AC)didnotinfluenceonthe antioxidantactivity.
Theplantextractsevaluatedinthisworkdidnotabsorbinthe UVpartofthespectrum.However,theroleoftheplantextractin theformulationistoindirectlyenhanceinvivoSPF.ROSare pro-ducedbyUVradiationandareresponsibletotheskinerythema appearance,consequentlywhenROSareinactivatedbyantioxidant molecules,invivoSPFtendstoriseasitisassessedbasedonthe appearanceofskinerythema(GilaberteandGonzález,2010).For thisreasonitissuggestedthatthehigherphotoprotectivecapacity oftheformulationscontainingtheextractsrevealedthe antioxi-dantpotentialoftheextracts,alreadyconfirmedinourprevious work(Mansuretal.,2012).
Inaddition,oneofthegoalsofthephotoprotectionresearch istodecreasetheconcentrationofsyntheticsunscreensaddedin photoprotectiveformulationssincetheseorganicmoleculesinhigh concentrationscouldpromoteskinirritation(Morabitoetal.,2011; SambandanandRatner,2011;Chenetal.,2012;Jansenetal.,2013). Forthisreason,plantextractscouldbeaddedtophotoprotective formulationsenablingareductioninsunscreens’concentrationbut keepingthesameinvivoSPF.
Studiesrelatedtooral uptake ortopicalapplication ofplant derivativeshavingantioxidantcomponentsshowedthatthese sub-stancescouldprotect theskinagainstROS,avoiding premature skinagingandfreeradical-relateddiseases.Forexample,studies havedemonstratedthatregularapplicationofanemulsion con-taininghyperforinreducedfree radicalformationand stabilized thelipids that constitutethe skinbarrier (Meinkeet al., 2012, 2013;Haagetal.,2014).Intheinvitroexperiment,SPFresultsfor formulationsA,BandCwerenotstaticallydifferent;all formu-lationspresentedaSPFaround17.However,whencomparedto theinvitroexperiment,theinvivoSPFforformulationAwasthe lowestone(13.48±1.99).Itcouldoccursinceinvivotestingoften presentsbiologicalfactorsthathavetobetakenintoconsideration. Evenpresentingagoodinvitrocorrelation,onlytheinvivotest isacceptedtoregisteraphotoprotectiveformulationinahealth careregulatoryagencyforcommercialpurposes.InvitroSPFtests areusuallyperformedasascreeningtoselectthebest formula-tionsandthenthenextstepisinvivotesting(Vergnaninietal., 1999).
Hen’sEggChorioallantoicMembrane(HET-CAM)testand ChorioallantoicMembrane-TrypanBlueStaining(CAM-TBS)test
The results of HET-CAM and CAM-TBS tests are showed in Table4.Accordingtotheclassificationbyscores,forboth tests theformulations were classifiedas non-irritant/slightly irritant (NOI/SLI), suggesting that they are safe to be applied on the skin.
WAc extract vehiculated at 1wt% in phosphate buffer (PB) pH7.4showedthelowestscoreinHET-CAMandCAM-TBStests, indicatingthattheextractalone(withoutbeingvehiculatedina cosmeticformulation)isnotapotentialirritant.
Bothtestsarerelatedtoocularirritation,whichcanbe associ-atedtocosmeticapplicationontheface,neartotheocularmucous membrane.
HET-CAMand CAM-TBS classification requires a comparison withRBCassay,toconfirmthattheformulationisnottoxicsince theaimistoobtaindatarelatedtovascularization(HET-CAM)and cytotoxicity(RBC)toevaluateocularirritationpotential.Sinceskin irritationstartswithvascular alterations (Hyperemia)and HET-CAMalterationsisbaseduponvasculareffectsontheCAM,thenon observationoftheseeffectsmaysuggestthatformulationpossess alsoalowerskinirritationpotential.
Nascimentoandco-workers(2012)alsofoundthatsunscreens encapsulatedinpolymericnanocapsulesdidnotpresentirritant potential,whichcorroborateourresults.
Redbloodcell(RBC)test
TheresultstotheRBCtestareshowedinTable4.All formu-lationswere classifiedas non-irritant/slightly irritant(NOI/SLI), which confirmthepreviousin vitrosafety tests(HET-CAM and CAM-TBS).
Conclusions
Bauhiniamicrostachyavar.massambabensisleafextracts (EtOH-ACandWAc)weresuccessfullyincorporatedintoO/Wemulsions containing sunscreens. The photoprotective formulations con-taining extracts were photostable after irradiation in the solar simulator, thus the extracts did not contribute to sunscreen’s photodegradationwhenexposedtoUVradiation.Concerningthe efficacytests,allformulationspresentedasuitableSPFandboth plantextractscontributedtoahigherphotoprotectiveeffect,asan enhancementintheinvivoSPF,whentheextractswereaddedto theformulations.Itissuggestedthatplantextractsactedby cap-turingROS,thusminimizingerythemaandcollaboratingindirectly toinvivoSPFenhancement.Bothformulationscouldbeconsidered safeforcosmeticusesincetheypassedthetoxicitytests, demon-stratingthattheyarenotirritanttotheeyesandskin.
Authors’contributions
MCPPRM(MScstudent),contributedtothedevelopmentofthe formulations,efficacyandsafetytesting;CCCandABV,efficacy test-ing;RSS,andOAFP,safetytesting;AACL.Photostabilitystudies,SGL andGGL,plantcollection,dryingandpreparationofplantextracts; ERJandEPS,developmentoftheformulations.
Conflictsofinterest
Acknowledgements
TheauthorswishtoacknowledgetheBrazilianNationalCouncil forScientificandTechnologicalDevelopment(MCTI-CNPq), Car-losChagasFilhoFoundationforResearchSupportinRiodeJaneiro (FAPERJ)andtheCoordinationfortheImprovementofHigher Edu-cationPersonnel(CAPES)forthefinancialsupport.
References
Alves,E.N.,Presgrave,R.F.,Presgrave,O.A.,Sabagh,F.P.,Freitas,J.C.,Corrado,A.P., 2008.AreassessmentoftheinvitroRBChaemolysisassaywithdefibrinated sheepbloodforthedeterminationoftheocularirritationpotentialofcosmetic products:comparisonwiththeinvivodraizerabbittest.ATLA:Altern.Lab.Anim. 36,275–284.
Anvisa,2012.GuidelinefortheSafetyEvaluationofCosmeticProducts,2ed.Agência NacionaldeVigilânciaSanitária,Brasilia,DF,Brasil.
Barradas,T.N.,Campos,V.E.B.,Senna,J.P.,Coutinho,C.S.C.,Silva,K.G.H.,Mansur, C.R.E.,2014.Developmentandcharacterizationofpromisingo/w nanoemul-sionscontainingsweetfennelessentialoilandnon-ionicsurfactants.Colloids Surf.A1,1.
Blois,M.S.,2002.Antioxidantsdeterminationsbytheuseofstablefreeradical. Nature26,1199–1200.
Bolzinger,M.A.,Brianc¸on,S.,Pelletier,J.,Chevalier,Y.,2012.Penetrationofdrugs throughskin,acomplexrate-controllingmembrane.Curr.Opin.Colloid Inter-faceSci.17,156–165.
Boonne,P.,Yotsawimonwat,S.,2011.Anti-agingmicroemulsionsand nanoemul-sions.HPCToday5,42–46.
Bouwstra,J.A.,Gooris,G.S.,Ponec,M.,2007.Skinlipidorganization, composi-tion and barrier function. Int. Fed.Soc. CosmeticChemists Magazine 10, 297–307.
Butt,S.T.,Christensen,T.,2000.Toxicityandphototoxicityofchemicalsunfilters. Radiat.Prot.Dosim.91,283–286.
Calderon-Montano,J.M.,Burgos-Moron,E.,Perez-Guerrero,C.,Lopez-Lazaro,M.A., 2011.Reviewonthedietaryflavonoidkaempferol.Mini.Rev.Med.Chem.11, 298–344.
Cerqueira-Coutinho,C.,Santos,E.P.,Mansur,C.R.E.,2015.Developmentofa photo-protectiveandantioxidantnanoemulsioncontainingchitosanasanagentfor improvingskinretention.Eng.LifeSci.15,593–604.
Chen,L.C.,Hu,J.Y.,Wang,S.Q.,2012.Theroleofantioxidantsinphotoprotection:a criticalreview.J.Am.Acad.Dermatol.67,1013–1024.
Colipa,2006.Internationalsunprotectionfactortestmethod.In:TheEuropean Cosmetic.ToiletryandPerfumaryAssociation.
Faudale, M., Viladomat, F., Bastida, J., Poli, F., Codina, C., 2008. Antioxi-dant activity and phenolic composition of wild, edible, and medicinal fennel from different mediterranean countries. J. Agric. Food Chem. 56, 1912–1920.
Finkel,T.,Holbrook,N.J.,2000.Oxidants,oxidativestressandthebiologyofageing. Nature408,239–247.
Fitzpatrick,T.B.,1975.Soleiletpeau.J.Med.Esthétique2,33–34.
Freitas,Z.M.F.,Gonc¸alves,J.C.S.,Santos,E.P.,Vergnanini,A.L.,2001.Glyceridestersof p-methoxycinnamicacid:anewsunscreenofthecinnamateclass.Int.J.Cosmet. Sci.23,147–152.
Gálvez,M.V.,2010.Antioxidantsinphotoprotection:dotheyreallywork?Actas Dermosifiliogr.101,197–200.
Gilaberte,Y.,González,S.,2010.Updateonphotoprotection.ActasDermosifiliogr. 101,659–672.
Gilbert,E.,Pirot,F.,Bertholle,V.,Roussel,L.,Falson,F.,Padois,K.,2013.Commonly usedUVfiltertoxicityonbiologicalfunctions:reviewoflastdecadestudies.Int. J.Cosmet.Sci.35,208–219.
Greul,A.K.,Grundmann,J.U.,Heinrich,F.,Pfitzner,I.,Bernhardt,J.,Ambach,A., Biesal-ski,H.K.,Gollnick,H.,2002.PhotoprotectionofUV-irradiatedhumanskin:an antioxidativecombinationofvitaminsEandC,carotenoids,seleniumand proan-thocyanidins.SkinPharmacol.Physiol.15,307–315.
Gutteridge, J.M.C., Halliwell, B., 2000. Free radicals and antioxidants in the year 2000: a historical look to the future. Annals N. Y. Acad. Sci. 899, 136–147.
Haag,S.F.,Tscherch,K.,Arndt,S.,Kleemann,A.,Gersonde,I.,Lademann,J.,Rohn,S., Meinke,M.C.,2014.Enhancementofskinradicalscavengingactivityandstratum corneumlipidsaftertheapplicationofahyperforin-richcream.Eur.J.Pharm. Biopharm.86,227–233.
Hayes,J.E.,Allen,P.,Brunton,N.,O’Grady,M.N.,Kerry,J.P.,2011.Phenolic com-positionandinvitroantioxidantcapacityoffourcommercialphytochemical products:oliveleafextract(OleaeuropaeaL.),lutein,sesamolandellagicacid. FoodChem.126,948–955.
Invitox,1996.ProtocolNumber108,CAM-TBStest,EuropeanCenterforthe Vali-dationofAlternativeMethodsECVAM.
Jain,S.K.,Jain,N.K.,2010.Multiparticulatecarriersforsun-screeningagents.Int.J. Cosmet.Sci.32,89–98.
Jansen,R.,Wang,S.Q.,Burnett,M.,Osterwalder,U.,Lim,H.W.,2013.Photoprotection: partI.Photoprotectionbynaturallyoccurring,physical,andsystemicagents.J. Am.Acad.Dermatol.69,1–12.
Jarzycka,A.,Lewinska,A.,Gancarz,R.,Wilka,K.A.,2013.Assessmentofextractsof
Helichrysumarenarium,Crataegusmonogyna,Sambucusnigrainphotoprotective UVAandUVB;photostabilityincosmeticemulsions.J.Photochem.Photobiol. 128,50–57.
Labsphere.UsermanualUV-2000SUltravioletTransmittanceAnalyzer. AQ-02755-000,Rev.3.,Labsphere,Inc.,NorthSutton,2008.
Lagarto, A.,Veja,R.,Guerra,I.,González,R.,2006. Invitroquantitative deter-minationofophthalmicirritancybythechorioallantoicmembranetestwith trypanbluestainingasalternativetoeyeirritationtest.Toxicol.InVitro20, 699–702.
Laguerre,M.,Lecomte,J.,Villeneuve,P.,2007.Evaluationofabilityofantioxidantsto counteractlipidoxidation:existingmethods,newtrendsandchallenges.Prog. LipidRes.46,244–282.
Liebsch,M.,Spielman,H.,2002.Currentlyavailableinvitromethodsusedinthe regulatorytoxicology.Toxicol.Lett.127,127–134.
Luepke,N.P.,Kemper,F.H.,1986.TheHET-CAMtest:analternativetothedraizeeye test.FoodChem.Toxicol.24,495–496.
Mansur, J.S.,Breder, M.N.R.,Mansur, M.C.A.,Azulay, R.D.,1986. Determinac¸ão dofatordeprotec¸ãosolarporespectrofotometria.An.Bras.Dermatol. 61, 121–124.
Mansur,M.C.P.P.R.,Leitão,S.G.,Lima,L.M.T.R.,Ricci-Júnior,E.,Souza,G.R.,Barbi, N.S.,Martins,T.S.,Dellamora-Ortiz,G.M.,Leo,R.R.T.,Vieira,R.C.,Leitão,G.G., Santos,E.P.,2012.Evaluationoftheantioxidantandphototoxicpotentialsof
Bauhiniamicrostachyavar.massambabensisVazleafextracts.Lat.Am.J.Pharm. 31,200–206.
Meinke,M.C.,Friedrich,A.,Tscherch,K.,Haag,S.F.,Darvin,M.E.,Vollert,H.,Groth, N.,Lademann,J.,Rohn,S.,2013.Influenceofdietarycarotenoidsonradical scavengingcapacityoftheskinandskinlipids.Eur.J.Pharm.Biopharm.84, 365–373.
Meinke, M.C., Schanzer, S., Haag, S.F., Casetti, F., Müller, M.L., Wölfle, U., Kleemann, A., Lademann, J., Schempp, C.M., 2012. In vivo photoprotec-tive and anti-inflammatory effect of hyperforin is associated with high antioxidant activity in vitro and ex vivo. Eur. J. Pharm. Biopharm. 81, 346–350.
Menezes,P.R.,Schwarz,E.A.,Santos,C.A.M.,2004.Invitroantioxidantactivityof speciescollectedinParaná.Fitoterapia75,398–400.
Mensor,L.L.,Menezes,F.S.,Geitão,G.G.,Reis,A.S.,Santos,T.C.,Coube,C.S.,Leitão, S.G.,2008.ScreeningofBrazilianplantextractsforantioxidantactivitybythe useofDPPHfreeradicalmethod.Phytother.Res.15,127–130.
Monteiro, M.S.S.B., Ozzetti, R.A., Vergnanini, A.L., Brito-Gitirana, L., Volpato, N.M.,Freitas, Z.M., Ricci-Júnior, E., Santos,E.P., 2012. Evaluationof octyl
p-methoxycinnamateincludedinliposomesandcyclodextrinsinanti-solar preparations:preparations,characterizationsandinvitropenetrationstudies. Int.J.Nanomed.17,3045–3058.
Morabito,K.,Shapley,N.C.,Steeley,K.G.,Tripathi,A.,2011.Reviewofsunscreen andtheemergenceofnon-conventionalabsorbersandtheirapplicationsin ultravioletprotection.Int.J.Cosmet.Sci.33,385–390.
Mota,A.C.V.,Freitas,Z.M.F.,Ricci-Jr,E.,Dellamora-Ortiz,G.M.,Santos-Oliveira,R., Ozzetti,R.A.,Vergnanini,A.L.,Ribeiro,V.L.,Silva,R.S.,Santos,E.P.,2013.Invivo andinvitroevaluationofoctylmethoxycinnamateliposomes.Int.J.Nanomed. 8,4689.
Nascimento,D.F., Silva, N.A.,Mansur, C.R.E., Presgrave,R.F., Alves, E.N.,Silva, R.S.,Ricci-Júnior,E.,Freitas,Z.M.F.,Santos,E.P.,2012.Characterizationand evaluation of poly(caprolactone) nanoparticles containing 2-ethylhexyl-p -methoxycinnamate,octocrylene,andbenzophenone-3inanti-solar prepara-tions.J.Nanosci.Nanotechnol.12,1–12.
Oktay,M.,Gulcin,I.,Kufrevioglu,O.I.,2003.Determinationofinvitroantioxidant activityoffennel(Foeniculumvulgare)seedextracts.Lebensmittel-Wissenschaft Technol36,263–271.
Piccaglia,R.,Marotti,M.,2001.CharacterizationofsomeItaliantypesofwildfennel (FoeniculumvulgareMill).J.Agric.FoodChem.49,239–244.
Pietta,P.,2000.Flavonoidsasantioxidants.J.Nat.Prod.63,1035–1042.
Podda,M.,Grundmann-Kollmann,M.,2001.Lowmolecularweightantioxidantsand theirroleinskinageing.Clin.Exp.Dermatol.26,578–582.
Rouanet,J.M.,Decordé,K.,Del-Rio,D.,Auger,C.,Borges,G.,Cristol,J.-P.,Lean, M.E.J.,Crozier,A.,2010.Berryjuices,teas,antioxidantsandthepreventionof atherosclerosisinhamsters.FoodChem.118,266–271.
Ruberto,G.,Baratta,M.T.,Deans,S.G.,Dorman,H.J.D.,2000.Antioxidantand antimi-crobialactivityofFoeniculumvulgareandCrithmummaritimumessentialoils. PlantaMed.66,687–693.
Salama,Z.A.,ElBaz,F.K.,Gaafar,A.A.,Zaki,M.F.,2013.Antioxidantactivitiesof phen-olics,flavonoidsandvitaminCintwocultivarsoffennel(Foeniculumvulgare
Mill.)inresponsestoorganicandbio-organicfertilizers.J.SaudiSoc.Agric.Sci. 14,91–99.
Sambandan,D.R.,Ratner,D.,2011.Sunscreens:anoverviewandupdate.J.Am.Acad. Dermatol.64,748–758.
Santos,E.P.,Freitas,Z.M.,Souza,K.R.,Garcia,S.,Vergnanini,A.,1999.Invitroand
invivodeterminationsofsunprotectionfactorsofsunscreenlotionswith octyl-methoxycinnamate.Int.J.Cosmet.Sci.21,1–5.
Silva,E.G.,Behr,G.A.,Zanotto-Filho,A.,Lorenzi,R.,Pasquali,M.A.,Ravazolo,L.G., 2007.AntioxidantactivitiesandfreeradicalscavengingpotentialofBauhinia microstachya(RADDI)MACBR.(Caesalpinaceae)extractslinkedtotheir polyphe-nolcontent.Biol.Pharm.Bull.30,1488–1496.
Vergnanini,A.,Freitas,Z.M.F.,Souza,K.R.,Garcia,S.,Santos,E.P.,1999.Invitroand
invivodeterminationsofsunprotectionfactorsofsunscreenlotionswith octyl-methoxycinnamate.Int.J.CosmeticSci.21,15.
Worth,A.P.,Balls,M.,2001.TheroleofECVAMinpromotingtheregulatory accep-tanceofalternativemethodsintheEuropeanUnion.ATLA–Altern.Lab.Anim. 29,525–535.