UNCORRECTED
PROOF
Contents lists available at ScienceDirect
BBA - Biomembranes
journal homepage: www.elsevier.comStructural characterization of cardiolipin-driven activation of cytochrome C into a
peroxidase and membrane perturbation
Dariush Mohammadyani
a, b, 1, Naveena Yanamala
c, 1, Alejandro K. Samhan Arias
a, Alexander A. Kapralov
a,
German Stepanov
d, Nick Nuar
e, Joan Planas-Iglesias
f, Narinder Sanghera
f, Valerian E. Kagan
a,
Judith Klein-Seetharaman
f, ⁎aDepartment of Environmental and Occupational Health, University of Pittsburgh, PA 15219, USA bThomas C. Jenkins Department of Biophysics, Johns Hopkins University, Baltimore, MD 21218, USA
cNational Institute for Occupational Safety and Health/Centers for Disease Control and Prevention, Morgantown, WV 26505, USA dDepartment of Medical Biophysics, Russian State Medical University, Moscow 117997, Russia
eDepartment of Bioengineering, University of Pittsburgh, PA 15213, USA
fDivision of Metabolic and Vascular Health, Medical School, University of Warwick, Coventry CV4 7AL, UK
A R T I C L E I N F O
Keywords:
Protein-lipid interactions Conformational changes Coarse grained simulation
A B S T R A C T
The interaction between CL and cytochrome c (cyt-c), results in a gain of function of peroxidase activity by cyt-c. Despite intensive research, disagreements on nature and molecular details of this interaction remain. In particu-lar, it is still not known how the interaction triggers the onset of apoptosis. Enzymatic characterization of perox-idase activity has highlighted the need for a critical threshold concentration of CL, a finding of profound physio-logical relevance in vivo. Using solution NMR, fluorescence spectroscopy, and in silico modeling approaches we here confirm that full binding of cyt-c to the membrane requires a CL:cyt-c threshold ratio of 5:1. Among three binding sites, the simultaneous binding of two sites, at two opposing sides of the heme, provides a mechanism to open the heme crevice to substrates, resulting in “productive binding” in which cyt-c then sequesters CL, in-ducing curvature in the membrane. Membrane perturbation along with lipid peroxidation, due to interactions of heme/CL acyl chains, initiates the next step in the apoptotic pathway of making the membrane leaky. The third CL binding site while allowing interaction with the membrane, does not cluster CL or induce subsequent events, making this interaction “unproductive”.
1. Introduction
Cytochrome c (cyt-c) is an inter-membrane space mitochondrial pro-tein with a major role in electron transport from the inner mitochondr-ial membrane (IMM) respiratory complex III (cytochrome c reductase) to complex IV (cytochrome c oxidase) [1]. Cyt-c is a highly conserved, alpha helical globular protein, composed of 104 amino acids [2] with a net positive charge of +9.2–9.6 [3]. The prosthetic heme group of cyt-c is covalently linked to the peptide chain at positions Cys14 and Cys17. The heme iron is in hexa-coordinated form, with four covalent bonds from the porphyrin's nitrogen atoms and two axial ligands, His18 and Met80 [4]. Upon binding to Complex III, Met80 moves away from the covalently bonded iron to facilitate the reduction [5].
In addition to its well-established bioenergetic function, cyt-c has been identified as an essential pro-apoptotic factor acting as a switch in mitochondria-mediated cell death pathways upon its release into the cy-tosol [5–10]. The liberation of cyt-c is mediated by its binding to a mi-tochondria-specific phospholipid, cardiolipin (CL), an unusual anionic phospholipid with two negatively charged phosphate groups in its polar head and a bulky hydrophobic “body” containing four acyl chains [11, 12] (Fig. 1A). CL is the signature phospholipid of the inner mitochon-drial membrane (IMM), where its presence is important for the proper structural arrangements and functioning of respiratory complexes III, IV, and V [13–15]. For example, there are six CL binding sites on the res-piratory complex III alone [16], and CLs have been proposed to be in-volved in proton delivery to complex IV [17].
⁎ Corresponding author.
Email address: J.Klein-Seetharaman@warwick.ac.uk (J. Klein-Seetharaman)
1 These authors are joint co-authors.
https://doi.org/10.1016/j.bbamem.2018.01.009
Received 26 May 2017; Received in revised form 14 December 2017; Accepted 4 January 2018 Available online xxx
UNCORRECTED
PROOF
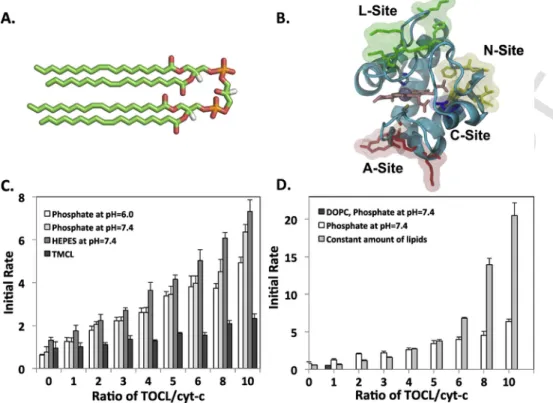
Fig. 1. The peroxidase activity of cyt-c increases significantly in the presence of CL. (A) The stick representation of TOCL structure; (B) The putative CL binding sites of cyt-c as proposed
previously in literature; (C) Peroxidase activity of cyt-c in presence of CL and variable amount of total lipids. Conditions: 10μM cyt-c, liposomes containing DOPC/TOCL (4:1), in presence of 100μM H2O2, 100μM Amplex Red. Fluorescence (λ exc 575nm, λ em 585nm) was measured during first 2min of the reactions and initial rates were calculated. For TOCL liposomes
measurements were preformed using 25mM HEPES buffer (pH7.4) and 12.5mM phoshate buffer (pH6.0 and 7.4), and for TMCL using 12.5mM phosphate buffer (pH7.4). All buffers con-tained 100μM DTPA. (D) Peroxidase activity of cyt-c in the presence of constant amounts of lipids. Conditions: similar to (C), except that for point marked by circles liposomes concon-tained a constant amount of lipids. So if TOCL/cyt-c ratio was increased, DOPC/TOCL ratio was decreased. At TOCL/cyt-c ratio equal to 10:1, DOPC/TOCL ratio is 2:1. For the sample contains DOPC without TOCL the initial rate is equal to 0.54.
Cyt-c is loosely bound to the IMM under normal conditions [18]. During apoptosis, trans-membrane migration of CL occurs from the IMM to the outer mitochondrial membrane (OMM), which increases the amount of CL in inter-membrane space-facing leaflets of IMM and OMM [19]. Hence, cyt-c can bind tightly to both CL-rich leaflets, and the in-teraction with CL results in a new peroxidase enzymatic activity [20, 21]. While soluble cyt-c displays only very weak peroxidase activity, it increases over 50-fold in the complex with CL, as measured in the presence of H2O2[22]. The gain of enzymatic activity is even up to 1000-fold with fatty acid hydroperoxides as sources of oxidizing equiva-lents [22, 23]. The newly emerged peroxidase activity is highly selective toward CL containing polyunsaturated fatty acid (PUFA) chains yielding a variety of oxygenated CL species (oxCL) [24, 25]. In the IMM, accumu-lation of oxCLs mobilizes the tightly bound cyt-c [18], because lipid oxi-dation causes release of CL from cyt-c resulting in decreased interaction with the membrane [26]. In OMM, simultaneous presence of cyt-c and generation of reactive oxygen species (ROS), particularly H2O2, leads to CL oxidation and OMM permeabilization (OMMP)—through yet to be established mechanisms—thus facilitating the release of cyt-c into the cytosol. These mitochondrial events designate the point of no return in apoptosis [27] initiated by the binding of cyt-c to the WD-40 portion of apaf-1 in apoptosomes [24].
Due to the lack of proper tools and accurate measurements of CL concentrations in each leaflet of IMM and OMM, controversially, vari-ous membrane compositions and disparate ratios of CL/cyt-c have been employed in previous in vitro studies. Most studies concluded that ex-tensive structural changes or even global unfolding of the protein upon interaction with the membrane accompany gain of peroxidase activ-ity [10, 28–30]. Furthermore, changes in cyt-c heme iron coordination state and redox potential upon binding to CL have been documented [31–33]. However, recent solid state NMR studies at a CL:cyt-c ratio of
8:1 suggest that CL can induce peroxidase activation in cyt-c even with little or no conformational changes in its structure [34], which was also recently confirmed by solution NMR experiments in reverse micelles [35]. Recent spectroscopic studies at varying ratios of CL to cyt-c (up to 200) support the notion that lipid oxidation is possible with a near na-tive structure containing an intact Met80-Fe3+linkage [36].
Four binding sites for CL on cyt-c, referred to as A, C, L and N (Fig. 1B) have been proposed. An unusual number of positively charged residues are found in these sites and are believed to be involved in elec-trostatic attraction of CL. In particular, Lys72 and Lys73 [30, 37–39] and Lys86 and Lys87 [40] in site A have been implicated in initiating complex formation, while high affinity binding may require additional interactions with site C, especially Asn52 [37, 41, 42]. At more acidic conditions (pH=6.2), Lys22, Lys25, His26, Lys27 and His33 in site L have been proposed to become available for interaction [43]. A fourth binding site, N, was recently identified by NMR [35]. Because sites A and L are not contiguous on the surface of cyt-c, it was proposed that binding of liposomes to these sites might mediate membrane fusion. Other models suggested that both hydrophobic and electrostatic forces facilitate partial penetration of cyt-c in the CL-containing membrane. On the other hand recent NMR studies conducted with bicelles cast into question if sites C and L exist [44]. Finally, there are also extended lipid anchorage models, in which one—or two—of the CL chains protrudes out of the membrane and inserts into a hydrophobic cavity of the pro-tein [38, 41, 45, 46], recently supported by crystallographic identifi-cation of detergents bound to a dimeric structure [47]. Despite recent progress, the current models still cannot be fully proven or refuted.
The diversity and often mutually exclusive character of existing models highlight the complexity of the interactions between cyt-c and CL. Furthermore, experimental observations such as the need for a
UNCORRECTED
PROOF
threshold concentration of CL required to induce peroxidase activity cannot be explained by any of these models. In order to better under-stand the relationship between functional properties of cyt-c and the multiple binding sites, we employed a combination of computational and experimental approaches to elucidate the molecular details of CL/ cyt-c interactions. Fluorescence spectroscopy was used for study of en-zymatic activity in vitro, and NMR spectroscopy was used to quan-tify dynamics of cyt-c and binding to CL. Molecular modeling meth-ods, including Gaussian network modeling (GNM), molecular docking, prediction of orientation of cyt-c with respect to the membrane and coarse-grained molecular dynamics simulations were used to comple-ment the NMR and fluorescence studies. The combined experimtal-computational approach enabled detection of the simultaneous en-gagement of two of the CL-binding sites, overlapping mostly with sites A and L, and the necessity of protein and membrane structural re-arrange-ments to boost peroxidase activity of cyt-c during apoptosis. The third binding site, overlapping with site N, also allows docking of cyt-c to the membrane but this binding is reversible and does not result in confor-mational perturbation in either cyt-c or the membrane.
2. Experimental procedures
2.1. Transformation, expression and purification of15N-labeled horse heart
cytochrome c
For details on purification of cyt-c, see Supplement. Briefly, compe-tent cells, strain C41 (DE3) SOLOs (Lucigen® corporation), were trans-formed with the plasmid, pJRhrsN [48] containing the recombinant pseudo-WT cyt-c (pWT). In pWT, His26 and His33 are replaced by Asn, to avoid mis-ligation with the heme during folding. Despite the mu-tations introduced, the pWT cyt-c is structurally similar to WT cyt-c [48, 49] and is a widely used model system [50–52]. Selection of pos-itive colonies was done by plating cells on Luria Broth (LB) agar plates containing the antibiotic ampicillin. Positive colonies were picked and grown for 24h at 37°C in 50ml of M9 minimal media supplemented with 4ml/L of glycerol and 100μg/ml of ampicillin, under vigorous shaking. The fully grown culture was added to 450ml of M9 minimum media, supplemented with15N-labeled NH
4Cl and grown at 37°C with shaking until the Abs600nm≈0.6 D.O. Cells were induced to express cyt-c by the addition of 2mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to the media and incubated for about 16h. They were then spun down by centrifugation for 25min at 5000g at 4°C. The supernatant was dis-carded and the cell pellet was frozen and stored at -80°C.
2.2. Preparation of liposomes for fluorescence and NMR studies
The optimization of conditions as well as the concentration/ratio of lipid used are described in detail in the Supplement. The optimized pro-tocol was as follows. Chloroform solutions of tetra-oleoyl CL (TOCL) or tetra-myristoyl CL (TMCL) and dioleoyl phosphatidylcholine (DOPC) were mixed in a glass tube and the solvent was evaporated under a flow of N2. After evaporation, 20mM HEPES pH7.4, or 50mM potas-sium phosphate pH6.0/7.4 as specified were added and the suspension was vortexed. Small unilamellar liposomes were prepared by sonication (Ultrasonic Homogenizer 4710, Cole-Parmer). The lipid mixtures were subjected to 10 pulses each, 5 times, and each step was followed by in-cubation on ice for 30s. A microtip with 20% power output was used.
2.3. Fluorescence spectroscopy
Assessment of peroxidase activity with Amplex Red reagent was per-formed as described previously [24]. Briefly, a Shimatzu RF-5301PC spectroflourophotometer was employed to measure the fluorescence of resorufin, which is an oxidation product of Amplex Red. Commercially available horse heart cyt-c (10μM, Sigma cat # C5572) was incubated with DOPC/TOCL liposomes of identical ratios as used in NMR exper-iments for 10min. Peroxidase reaction was started by addition of Am-plex Red (100μM) and H2O2(100μM) and was carried out for 30min (reaction rate was linear in the entire time interval). Fluorescence was detected by employing a “Fusion α” universal microplate analyzer and by using an excitation wavelength of 535nm and an emission wave-length of 585nm. The initial rates of cyt-c peroxidase activity at increas-ing TOCL/cyt-c ratios (1:1–10:1) were calculated by measurincreas-ing fluores-cence changes during the first 2min of the reaction with H2O2and Am-plex Red.
2.4. NMR spectroscopy
1H-15N HSQC NMR spectra of uniformly 15N isotope labeled cyt-c were obtained using a ~900MHz Bruker NMR spectrometer. Two di-mensional1H-15N HSQC spectra of cyt-c were acquired using a standard HSQC pulse sequence with 64 scans in first dimension and 160 scans in the second dimension and a D1 delay of 1s. Data acquisition was carried out using Topspin Version 3.0 Software (Bruker BioSpin Corp., Billerica, MA). Spectra were further processed and analyzed using NMRView [53] and Sparky [54] softwares. The backbone resonances observed in1H-15N HSQC spectra of pWT cyt-c [48] in phosphate buffer at pH6.0 were assigned using previously published NMR data, acquired under similar conditions [49]. As reported previously for this mutant, out of a total of 104 expected backbone amide, only 96 were observed. Signals corre-sponding to residues Gly1, Gln16, Thr28, Pro30, Pro44, Pro71, Pro76, and Gly84 were missing. The amide signals of cyt-c acquired at pH7.4 were unambiguously assigned based on their similarity to the chemical shifts observed at pH6.0, with the exception of a single residue, Asn26. At pH7.4, the signal corresponding to Asn26 disappeared and a new sig-nal appeared at 124.8ppm in the15N and 9.4ppm in the1H dimensions, respectively. We tentatively assign this signal to Asn26. As compared to previous studies [49] acquired using a Varian INOVA 750MHz NMR spectrometer, the data recorded using a Bruker 900MHz spectrometer here gave rise to five additional amide chemical signals (Fig. S1, labeled in ‘*’). The sign corresponding to these signals flipped from positive to negative in T2relaxation experiments (data not shown) suggesting that these signals are from residues containing N-atoms in their side-chains.
NMR T2relaxation measurements were carried out using a 600MHz Bruker NMR spectrometer. The pseudo-3D pulse sequence hsqcf3gpsi3d was used, where axes 1 and 3 correspond to1H and15N and the 2nd di-mension corresponds to different relaxation times with 1024×64 com-plex points. The CPMG mixing times used were 16, 32, 48, 64, 80, 96, 112, 128 and 160ms. Spectra with delays of 32, 48 and 96ms were recorded in duplicates for error estimation. Peak intensities were fit-ted using CURVEFIT (www.palmer.hs.columbia.edu/software/curvefit. html).
2.5. Molecular modeling – docking
Autodock Vina 1.1.2 was employed to computationally dock the structure of the CL to the crystal structure of native cyt-c (PDB ID:
UNCORRECTED
PROOF
1HRC) [4]. Three docking modeling sessions were run using three differ-ent random number generator seeds. The docking procedure used was similar to the studies performed earlier [55]. A cubic box was positioned at x, y, and z values of 46.839, 23.029, and 5.505, respectively. The cen-ter was built around the protein with 52×52×52 points and a spacing of 0.375Å between the grid points. The top 9 resulting orientations were clustered together based upon the RMSD value with respect to model 1. The best ligand bound receptor structure in each case was chosen based on lowest energy, visual inspection, as well as the total number of con-formations in a cluster.
2.6. Prediction of dynamics
Potential motions that define the equilibrium dynamics of the na-tive structure of cyt-c were predicted using the oGNM webserver, using Gaussian network model (GNM), using the default parameters [56, 57].
2.7. Prediction of membrane orientation
Protein orientation on the membrane was predicted using Orienta-tions of Proteins in Membranes (OPM) server [58].
2.8. Coarse-grained (CG) molecular dynamics (MD) simulations
CGMD was carried out based on the MARTINI force field [59]. In this approach, four heavy atoms are mapped to one CG bead. 400 lipids, including 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and TOCL, were used to construct the membrane. Five membranes with varying CL amount of 0 (control), 5, 7.5, 10, and 20mol% CL were chosen and subjected to simulation. All systems were subjected to neutralization. The composition of the lipids in each membrane listed in Table S1. The CG model of DOPC and TOCL molecules is illustrated in Fig. S2. The size of membrane patches used in this study was 12nm×12nm×vari-able thickness of the bilayer which is dependent on the CL concentra-tion. Each membrane system was subjected to 200ns simulations to be equilibrated. The effects of presence of oxidatively truncated- and hy-droxy-CLs in the bilayer on the interaction with cyt-c were evaluated using CG-MD simulations with the bilayer containing 21 oxCL and 86 DOPC molecules. We considered two levels of oxidation by adding the oxidized group in one chain or all four chains of CL, which led to four systems. Then, cyt-c was inserted into the box, the system was solvated and neutralized. Each system equilibrated with three different random seeds to produce different initial velocities and thus different trajec-tories. In total, 15 simulations were run for non-ox-CL systems, 1 mi-croseconds each, and 4 simulations for ox-CL containing system, 100ns each. All simulations were performed using the GROMACS v. 4.5.4 MD package [60] and visualized using VMD v. 1.9 software [61]. Initially, energy minimization was applied on each system for 20ps, before 1ns NVT and 1ns NPT ensemble equilibration. A 15fs time step was used to integrate the equations of motion. Non-bonded interactions have a cutoff distance of 1.2nm. Temperature and pressure were controlled using the velocity rescale (V-rescale) [62] and Berendsen [63] algo-rithms (time constant, tau-p=12.0ps, compressibility=3e−4 (bar−1), and reference pressure, ref_p=1.0bar), respectively. Simulations were run at 310K and at 1atm during NPT runs.
The extended experimental procedures are presented in supporting materials.
3. Results
3.1. Peroxidase activity
Previous solution NMR studies of cyt-c had been carried out in phos-phate buffer at pH6 [49], while previous quantification of cyt-c/CL per-oxidase activity was carried out in Hepes pH7 [22, 64]. We therefore first checked if there was any dependence of peroxidase activity on buffer used. The results are shown in Fig. 1C when TOCL was added in TOCL/DOPC mixed liposomes with DOPC:TOCL ratio of 4:1. The initial rate of substrate oxidation measured by fluorescence spectroscopy (see Methods) in Hepes pH7.4 buffer was consistently higher than in phos-phate buffer at pH6. To distinguish between pH and buffer chemistry ef-fects, we also tested phosphate buffer at pH7.4 and found it to be similar to pH6. As a negative control, TMCL did not significantly promote per-oxidase activity. Because the addition of TOCL in this experiment also increased the overall lipid concentration, we conducted a second experi-ment in which the overall lipid concentration was kept constant and the relative amounts of TOCL were increased (Fig. 1D). Thus, the relative amount of TOCL:DOPC was 1:2 at the highest concentration used. Un-der constant lipid conditions one can see that peroxidase activity rises dramatically after a threshold ratio of TOCL:cyt-c of 5:1. Therefore, the dilution of TOCL with DOPC suppresses the onset of peroxidase activity. The higher the local concentration of TOCL, the more active the per-oxidase becomes. The rationale to study two systems (constant versus variable total lipid) is to enable comparability between NMR, activity and simulation studies. While maintaining a constant CL to DOPC ra-tio of 4:1 ensures an identical negative charge distribura-tion on the mem-brane, it will increase the overall total lipid content in the experiment. However experiments using liposomes containing varying amounts of CL, while keeping the total lipid concentration in each experiment con-stant (1mM total lipid) are crucial to ensure that the observed changes in cyt-c activity are from its specific interaction with CL and not due to its unspecific interactions with increased liposomal content.
3.2. Dynamics of cyt-c in different buffers
Because the fluorescence experiments indicated a sensitivity of per-oxidase activity to buffer conditions, we first measured if buffers ex-erted an effect on the structure and dynamics of cyt-c alone. Solution NMR spectroscopy is the technique of choice to study dynamics of pro-teins, so we recorded1H,15N-HSQC spectra of fully15N-labeled cyt-c (see Fig. S1 and [65]). The assignment of cross-peaks was based on pub-lished spectra [49]. Previously, we pubpub-lished the HSQC spectrum of 50μM cyt-c in the presence and absence of CL in liposomes consisting of 250μM TOCL and 1mM DOPC, corresponding to a 20:80%-ratio of total lipid [65]. Here, we carried out analogous HSQC analysis for 12 additional samples in order to enable comparability between activity, NMR and simulation experiments, and to obtain quantitative informa-tion on the transiinforma-tion between the free and CL-bound states of cyt-c. A number of signals disappeared, while others shifts in position when comparing the spectra obtained in pH6 versus 7.4 in phosphate buffer (Fig. S3A) and further to Hepes buffer pH7.4 (Fig. S3B). These residues are mapped onto the cyt-c structure in Figs. S4A and S4B, respectively. As one can see the peaks primarily disappear due to the change in pH (Fig. S3A, red amino acids) as a result of exchange with the sol-vent. The chemical shift changes map to residues throughout the struc-ture. To quantify the extent to which the environment of these residues changes are due to changes in mobility, we recorded15N T
2relaxation rates in the three different buffer conditions. The inverse R2values are plotted along the amino acid sequence in Fig. 2. Careful inspection of
UNCORRECTED
PROOF
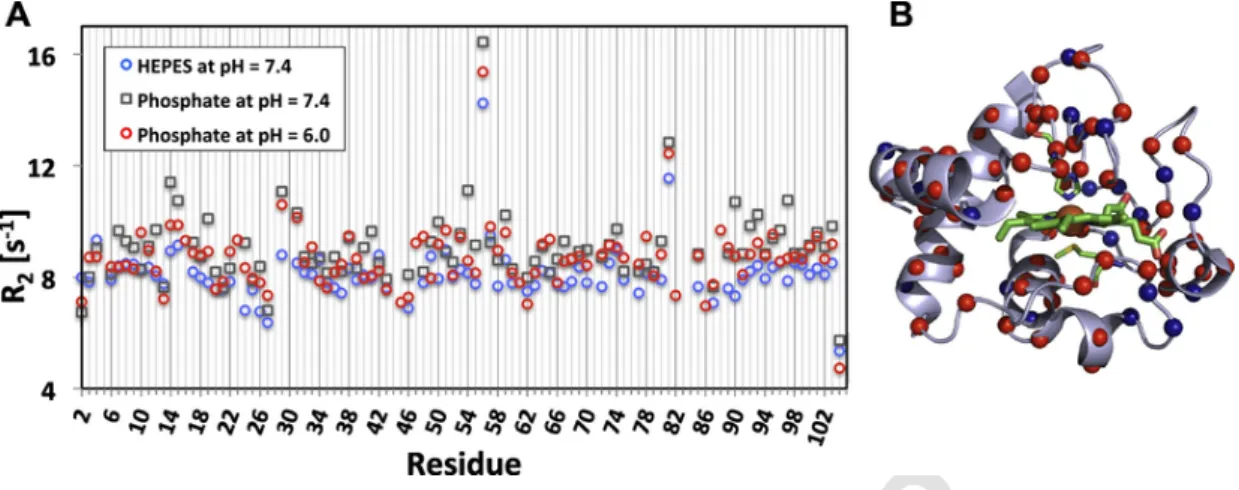
Fig. 2. Effect of pH and buffer composition on the structure and dynamics of horse heart cyt-c. (A) Overlay of R2relaxation rates of cyt-c acquired in phosphate buffer – at pH6 and 7.4 –
and HEPES buffer at pH7.4, and (B) Mapping of the changes in R2rates between phosphate and HEPES buffer on to the structure of cyt-c.
the data reveals that most residues have highest R2values in phosphate pH7.4 (gray squares), intermediate values in phosphate pH6 (red cir-cles) and lowest values in Hepes pH7.4. The majority of residues (49 residues) showed this pattern and are highlighted mapped on the cyt-c structure in red circles while those residues that showed other patterns (21 residues) or no change (19 residues) in different buffers are shown in blue. This indicates that the majority of residues in cyt-c are least mobile in phosphate buffer at pH7.4 and most mobile in Hepes buffer at pH7.4, with intermediate mobility in phosphate at pH6. Comparing this result with the peroxidase activity supports the notion that higher mobility may be a prerequisite for peroxidase activity. The mobility in-crease appears to affect the entire protein structure, rather than affect-ing individual regions preferentially (also refer to the color codaffect-ing of differences in R2values in Fig. S4D and S4F).
3.3. Predictions of protein dynamics with Gaussian Network Analysis
The NMR data indicated that cyt-c is a highly mobile protein. We therefore used Gaussian Network Analysis to model the dynamics by representing the protein as a network of beads (the amino acids) con-necting to each other by virtual bonds based on a chosen cut-off dis-tance. The three top modes of vibrations predicted by this model are shown in Fig. 3. As one can see all three modes affect opposing sides of the heme, namely H1 and H5 versus H2 in mode 1, the loop presenting His18 versus H2 in mode 2 and the loop presenting Met80 versus the end of H1 in mode 3. This suggests that the mobility in cyt-c would be expected to make the heme-binding pocket more accessible.
3.4. Solution NMR studies of CL binding to cyt-c
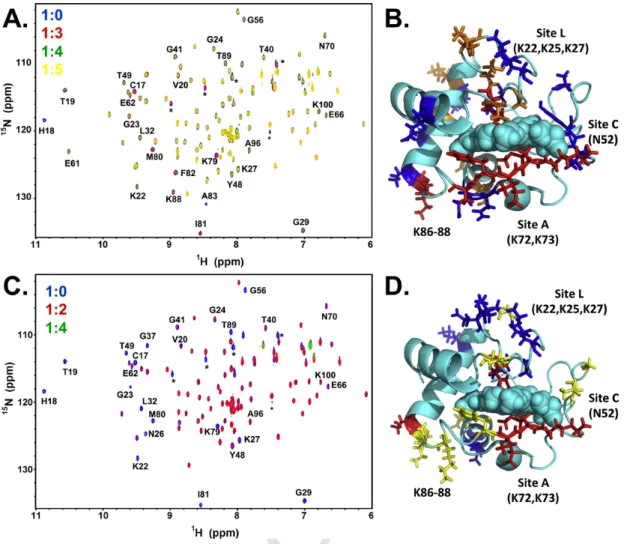
TOCL in DOPC was added in identical fashion to the activity exper-iments described above, either by maintaining the same molar ratio be-tween TOCL and DOPC in phosphate buffer pH6 (Fig. 4A, Table S2 and S3) or the same total lipid concentration in Hepes buffer pH7.4 (Fig. 4C, Table S2 and S3). Measurements at pH6 have the advantage of a larger number of signals being visible in the NMR spectra (see above, and residues highlighted in yellow in Fig. 4D). The gradual decrease in sig-nal intensity as a result of TOCL binding at pH6 predominantly affects the distal and proximal sides of the heme (red and orange residues in Fig. 4B) but then also extends to the left and right sides (blue in Fig. 4B). These regions are very similar to the regions of mobility predicted by GNM. In direct analogy to the activity measurements, the Hepes pH7.4 conditions result in an earlier disappearance of all peaks (complete dis-appearance of all peaks at ratio 1:5 in Hepes pH7.4 as opposed to 1:6 in phosphate pH6), which means that cyt-c is fully attached to the large liposomes which do not tumble sufficiently fast on the NMR time scale to allow peak detection. Thus, the strong interactions in pH7.4 demon-strated the involvement of both sites A and L and several residues ad-jacent to these sites in the interactions with CL-containing membranes. The comparison between fluorescence and NMR results shows that the TOCL/DOPC-liposome embedded state is the one most active as a per-oxidase. As a negative control, we studied the effects of TMCL —CL with saturated and non-oxidizable acyl chains— binding to cyt-c by NMR and found no changes in chemical shift or disappearance of peaks (Fig. S5).
Fig. 3. The intrinsic motions of cyt-c predicted using GNM model based approach. The three top ranking global/slow modes of cyt-c, (A) GNM mode 1, (B) GNM mode 2, and (C) GNM
mode 3. The cyt-c is rendered as cartoon and color coded in blue-white-red to indicate increase in flexibility. The ligand heme and residues His18 and Met80 are rendered as spheres and sticks, respectively. The different helices in cyt-c are labeled in (A) for ease of analysis.
UNCORRECTED
PROOF
Fig. 4. Interaction studies of cyt-c and CL. (A) An overlay of15N-1H-HSQC spectra of cyt-c in phosphate buffer at pH6.0 at 1:0 (blue), 1:3 (red), 1:4 (green) and 1:5 (yellow) cyt-c to CL
ratios. A molar concentration of 20% CL was used in each experiment. (B) Mapping of the chemical shifts that either disappeared at 1:4 (red)/1:5 (orange) cyt-c to CL ratio or showed decrease in signal intensities (blue) observed at 1:5 cyt-c to CL ratio are mapped onto the structure of cyt-c. (C) An overlay of15N-1H-HSQC spectra of cyt-c in Hepes buffer at pH7.4 at 1:0
(blue), 1:2 (red), and 1:4 (green) cyt-c to CL ratios. A constant lipid concentration of 1mM was maintained in each experiment. (D) Mapping of the signals that either disappeared (red) or exhibited significant line broadening effects (blue) at 1:2 cyt-c to CL ratio are mapped onto the structure of cyt-c. The signals that disappear in native cyt-c upon a change in pH from 6.0 to 7.4 are colored yellow.
3.5. Predicting CL binding sites on cyt-c by docking
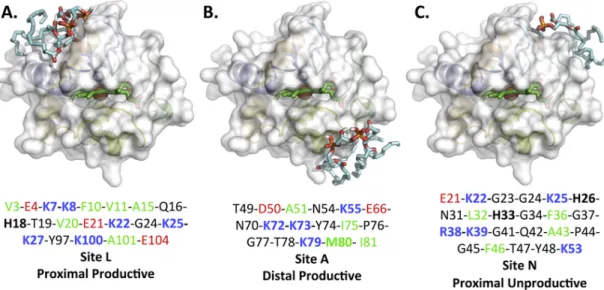
Autodock Vina was used to predict putative binding sites by molec-ular docking studies using the structure of native horse heart cyt-c (PDB ID: 1HRC) and TOCL. We grouped the nine top-ranked CL binding poses into three possible CL binding sites based on binding affinities, proba-bility of occurrence and visual inspection of the models. Representatives of each binding site are shown in Fig. 5A–C and the residues within 5Å of TOCL are listed under each model shown and for all nine models in Table S4. We found that these three binding sites largely overlap with three of the four previously identified binding sites, namely A, L and N (but not C). Because the previous naming of these sites is historical, we have chosen to rename the binding sites based on functional considera-tions.
The first binding site (Fig. 5A), formerly site L, which we will re-fer to henceforth as “proximal site” includes site L and several other residues in vicinity of proximal heme ligand His18. It consists of six positively charged Lys, six hydrophobic amino acids, three negatively charged Glu. His18 is also positively charged [66]. The acyl chains of TOCL are aligned toward the N- and C-terminal helices H1 and H5, respectively. The Glu residues may facilitate interactions of cyt-c with positively charged groups of zwitterionic lipids, such as the choline group of phosphatidylcholine, an abundant lipid in any biological
membrane. Thus, the nature of the amino acids and secondary struc-tures present in the proximal site supports involvement of electrosta-tic (specifically with CL, but potentially also other lipids in which CL is embedded) and hydrophobic forces in the cyt-c/CL interactions. A close proximity of Lys25 and Lys27 nitrogen atoms to oxygens in the CL phosphate groups may result in stabilization of the interaction through hydrogen bond formation. A recent survey of amino acids present in known CL binding pockets [67] identified the motif G as characteristic of CL binding. This motif is also present in the CL proximal site.
The second CL binding site (Fig. 5B), formerly site A, henceforth re-ferred to as the “distal site” includes site A and several residues in close proximity of the distal heme ligand, Met80. The composition of this site is overall similar to that of the proximal site and also comprises four positively charged Lys, four hydrophobic and two negatively charged amino acids (Asp50 and Glu66). In this site, the CL acyl chains are ex-tended around helices H2 and H4.
The third predicted binding site (Fig. 5C), formerly site N, is only composed of random coil. This site consists of five positively charged (R38 and 4 Lys), one negatively charged, and four hydrophobic residues. This site is also located on the proximal side of the heme but does not include the critical heme-coordinating residue His18Its loca-tion does not overlap but is in proximity to the previously proposed sites C (Asn52) and L. In this proximal site, the motif G{K,L} is present.
UNCORRECTED
PROOF
Fig. 5. Molecular docking predicts three CL-binding sites, which can be generalized in two main sites called “distal” and “proximal” sites. Molecular docking predicted three CL-binding
sites: (A) site one is located in vicinity of proximal His18, (B) site two is placed in neighboring of distal Met80, and (C) site three represents some overlaps with site one and remaining of this site is extended toward the opposite side of HEME crevice opening.
Furthermore, its high abundance of Gly and positively charged residues and low abundance of Leu, Val, Ile are all characteristic of CL binding [67]. Finally, this binding site contains Y48, which was recently identi-fied as a CL binding residue [68].
3.6. Predicted contacts of cyt-c with membrane surfaces
Clearly, the interaction of cyt-c with a single CL molecule as used in docking studies is an oversimplification as CL is embedded in a mem-brane in vivo. We therefore investigated the predicted orientation of cyt-c with respect to membranes using the OPM server [69, 70]. The orientation of cyt-c with respect to the membrane according to OPM is shown in Fig. S6. This orientation highly correlates with the proposed proximal and distal binding sites. The residues Lys22, Lys25, Lys27, His33, Arg38, Lys72 and Lys73 are all calculated to be part of the OPM membrane-binding interface on cyt-c. Moreover, several hydrophobic residues (Pro71, Ile75, Pro76, Gly77, Ile81, Phe82, and Ala83) around and including Met80 are predicted to be part of the interface. This in-dicates that the proximal and distal sites, formerly referred to as sites L and A, may be viewed as a joint albeit not contiguous binding site of hydrophobic and charged residues.
3.7. Coarse-grained molecular dynamics simulation of cyt-c interaction with membranes
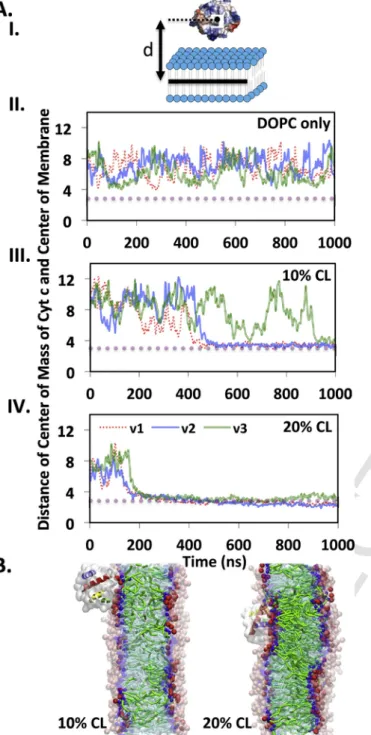
Ideally, we would like to study the interaction of cyt-c with CL fur-ther in the context of the lipid environment provided by the membrane. However, considering the large system size and long simulation time re-quired to study the interactions of proteins with membranes, we chose CG-MD simulations, rather than full atomistic MD simulations. CG-MD simulations offer advantages due to the greater length and time scales possible to capture possible interactions and to study the consequences of the interactions on both protein and lipid bilayer. Three indepen-dent CG-MD simulations of the interactions of cyt-c (based on PDB ID: 1HRC) with membranes of different compositions varying in the amount of CL (0, 5, 7.5, 10 and 20mol% embedded in DOPC) were run for 2μs each. The results are shown as distances of the center of mass of cyt-c with respect to the center of the membrane in Fig. 6A and visu-ally in Fig. 6B. In the absence of CL, cyt-c did not interact with the membrane (Fig. 6A, panel II), while in the presence of 10mol% TOCL a bound state was observed in 2 out of 3 simulations (Fig. 6A, panel III and 6B, panel I). The membrane containing DOPC and 20mol% TOCL
always resulted in a bound state (Fig. 6A, panel VI and 6B, panel II). Analysis of cyt-c residues located within 5Å distance of any CL mole-cules during the last 100ns of each simulation confirmed the simultane-ous presence of the distal (residues 72–88) and proximal (residues 5–18 and 22–27) CL binding sites on cyt-c in two of the simulations, strongly validating the indication from the OPM approach (Table S5). All the binding site analysis including data from chemical shift changes mea-surements, molecular docking, CGMD, OPM and relaxation calculations have been summarized in Fig. S7.
In one of the three CGMD simulations, the third binding site from docking was observed (Table S5). When relaxing the elastic network constraints on the protein and thus allowing greater flexibility in the protein structure, a 300ns further simulation resulted in a reorientation of cyt-c into the orientation found in the other two simulations, where proximal and distal binding sites are occupied together.
3.8. Effect of cyt-c interactions on CL-containing membranes
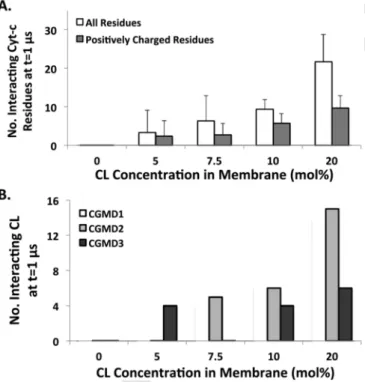
The CG-MD simulations not only shed light on the details of the amino acids on cyt-c involved in the interaction with the membrane, but also show how cyt-c affects the membrane. In particular, we ob-served very clearly that the strong interactions of cyt-c with CL-con-taining membranes lead to CL clustering (Figs. 6B and 7), in line with previous studies [71, 72]. We never observed such clustering in the absence of cyt c. Each bilayer system was simulated without protein for 500ns. This clustering in turn induces a negative curvature on the membrane surface, especially visible at the highest concentration of CL used (Fig. 7C, panel II). It is well known experimentally that not only in the eukaryotic mitochondrial membrane, but also in bacterial branes, CL localization can induce a negative curvature on the mem-brane [73]. Further analysis of the final configurations of the CG-MD trajectories revealed that the number of residues interacting with mem-brane proportionally increases with increasing CL concentration (Fig. 8A). Furthermore, the number of CL molecules interacting with cyt-c also increases with increasing CL concentration (Fig. 8B). The effect is even more pronounced when considering the two CGMD simulations that overlap with distal and proximal (or formerly A and L) binding sites and the CGMD simulation the overlaps with the third binding site separately (Fig. 8B). In the latter, the number of CL molecules inter-acting with cyt-c is significantly smaller (6 versus 14 and 15) and the membrane is not curved (Fig. S8). This finding strongly suggests that
UNCORRECTED
PROOF
Fig. 6. Cyt-c interacts with higher affinity to the membrane containing a larger amount of
CL. (A) I. Schematic representation of the distance between COM of cyt-c and center-line of the membrane; II. and III. The distance of cyt-c to the membranes containing pure DOPC and DOPC-20mol% CL over 1μs of three independent CG-MD simulations, respectively. Cyt-c never interacts with the membrane lacking CL, but recognizes the membrane con-taining 20mol% CL immediately. The high affinity interacts of cyt-c with the membrane leads to partial penetration of cyt-c into the membrane in all three simulations. (B) I. The comparison of the typical final configuration of the interactions of cyt-c with the mem-branes containing 10 and 20mol% CL. Representation guide: transparent cyan: DOPC acyl chains; green: CL acyl chains; transparent pink: choline and phosphate groups of DOPC; red: head groups of CL (two phosphate groups and one connected glycerol backbone); transparent blue: glycecrol backbone of DOPC; blue: glycerol backbone of CL; transparent white: protein; Helices: Blue: residues 3–13, Green: resides 50–54, Yellow: resides 61–67, Orange: resides 71–74 and Red: residues 88–103. II. The distance of cyt-c to the mem-branes containing DOPC-10mol% CL over 1μs of three independent CG-MD simulations.
the engagement of cyt-c's third binding site with the membrane is an unproductive one.
3.9. Effect of CL oxidation on cyt-c interaction with membranes
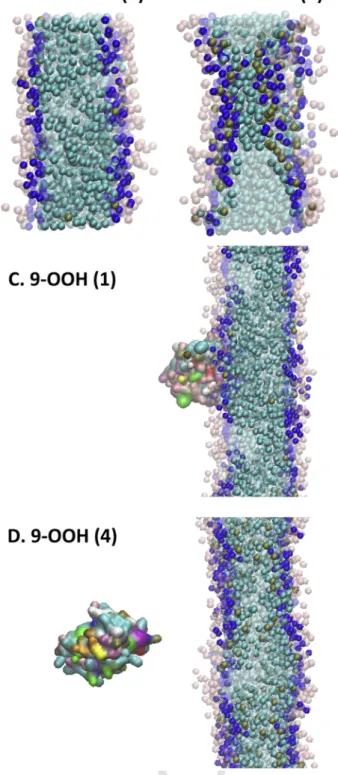
Once cyt-c gains peroxidase activity through its interaction with CL, it will oxidize the CL chains [24]. In order to study the functional con-sequences of this oxidation on its interaction with the membrane, we replaced CL with oxygenated species (see Methods). Because it was pre-viously shown that truncated lipid species can create holes in mem-branes [74], we first conducted control simulations with such truncated species, shown in Figure 9AB. Indeed, massive oxidation of CL also per-turbed our membranes, although we were not able to see actual holes. Next, we repeated CG-MD simulations with oxygenated CL species (Fig. 9C, D), which abolished cyt-c binding to the membrane when the per-oxy group was present in each of the four acyl chains of CL (Fig. 9D).
4. Discussion
The initiation and formation of the cyt-c/CL complex, which leads to cyt-c conformational changes of uncertain extent and peroxidase ac-tivity of cyt-c is pivotal for its role in the early stages of apoptosis. The nature of binding of CL to cyt-c, including affinity, stoichiometry and location of binding sites have remained unclear despite numerous stud-ies [22, 30, 31, 37, 38, 75–77]. Emphasis has been on the electrostatic interactions between negatively charged phosphate groups on CL and positively charged residues on cyt-c and it was proposed that these elec-trostatic interactions initiate the formation of the complex. Only subse-quent to electrostatic attraction, hydrophobic and hydrogen bonding in-teractions between cyt-c and the acyl chains are believed to develop and ensure the tight binding of cyt-c to anionic phospholipids, and fulfill-ment of the oxygenase function toward one of the PUFA-resides [22, 29, 30, 37, 76]. It has also been argued that this second stage tight binding is the major cause of partial unfolding of the protein [78, 79]. A number of experiments exhibit bi-phasic characteristics leading to the sugges-tion that binding is a 2-step process comprising electrostatic interacsugges-tion in step 1 followed by hydrophobic interaction in step 2 [21, 41, 75, 80]. To better understand the interplay of different binding sites and mul-tiple modes of binding proposed previously, we used NMR, fluorescence spectroscopy and molecular modeling to study the cyt-c/CL interaction and conformational heterogeneity of cyt-c structure. Molecular docking identified three possible locations for the interaction of CL with cyt-c, and NMR spectroscopy provided evidence that all of these sites are ac-tually engaged. How can this be possible given the locations on oppo-site ends of the protein? The answer lies in the fact that the interac-tion of cyt-c with CL has to be seen in the context of the membrane. Our CGMD and OPM results show that the two binding sites identified by docking are actually one when considering the extended size of the membrane and availability of multiple CL molecules within and that cyt-c embeds deeply into the membrane. The simulation further sup-ports the notion that both, electrostatics and hydrophobic forces com-bined lead to form a tight CL/cyt-c complex. The ability to bind cyt-c simultaneously at two opposing ends of the heme provides a natural path to formation of peroxidase by opening the heme crevice through “pulling” by the membrane. We thus consider the simultaneous occu-pation of the distal and proximal sites, which includes residues from the previously identified A and L sites, as “productive” binding. Pre-viously, simultaneous engagement of sites A and L has been proposed by binding two different vesicles to each site as a mechanism to initi-ate vesicle fusion, but only at low pH values [43]. In contrast to the use of the indirect binding readout of fusion, NMR spectroscopy al-lows measuring binding directly, and the NMR data presented here is
UNCORRECTED
PROOF
Fig. 7. Interactions of cyt-c induce CL clustering on the membrane, which leads in a negative curvature on the membrane surface. Final configurations of the head groups of the lipids in
the membrane containing (A) 7.5, (B) 10mol% and (C) 20mol% CL interacting with cyt-c, I. top and side views with cyt-c presentation, II. top and side views without cyt-c presentation – to represent CL-clustering underneath of the protein. Representation guide: transparent pink: choline and phosphate groups of DOPC; red: head groups of CL (two phosphate groups and one connected glycerol backbone); transparent blue: glycerol backbone of DOPC; blue: glycerol backbone of CL; colorful particle: cyt-c.
Fig. 8. Number of cyt-c residue interacting with membrane and number of CL
interact-ing with cyt-c enhance by increasinteract-ing CL concentration in the membrane, indicatinteract-ing higher affinity of cyt-c to interact with CL-rich membranes. (A) Average number of cyt-c resides, including all and positively charged residues, interacting with CL-containing membranes at t=1μs (n=3). (B) Average number of CL interacting with cyt-c at t=1μs (n=3).
consistent with the conclusion that simultaneous binding can occur at both low (pH6) and high (pH7.4) values. It is possible that differences in liposome composition contribute to the differences in conclusions on whether or not low pH is needed in the engagement of site L. The simul-taneous engagement of the two sites in our model in a single liposome as opposed to two liposomes is attractive because it provides a putative mechanism for the functional changes that follow the binding of cyt-c to the membrane, namely the CL clustering and bending of the membrane. We cannot distinguish if membrane curvature enhances binding of CL to cyt-c in this manner, or if the strain imposed on the membrane by bind-ing is the drivbind-ing factor for curvature formation. Either way, our results support a model in which cyt-c is partially and stably embedded in a lo-cally curved and CL-rich membrane patch. Recent independent CG-MD simulations strongly support the notion of CL enrichment in regions of high negative curvature [81].
Several recent studies have identified the presence of truncated oxi-dation products of lipids as a cause for structural deformation of mem-branes [82–84], including openings that make the membrane leaky [74]. Thus, it is possible that the oxidation of lipids alone may allow escape of cyt-c from the mitochondria into the cytoplasm. Other stud-ies have implicated the help of additional proteins [85]. Because the most frequently observed lipid oxidation species in apoptosis are those carrying oxygenation at one or more chains, we investigated the effect of oxygenation of CL on our membrane and on the binding with cyt-c. For comparison we also studied truncated CL species. Similar to the pre-vious studies with other truncated lipids, we observed severe pertur-bation of membrane structure at high level of oxidation (in our case when all 4 chains in CL were substituted with truncated species). In contrast, a model oxygenation product, where a peroxy-group was in-troduced at the 9-position in one or more acyl chains, did not cause
UNCORRECTED
PROOF
Fig. 9. The effect of ox-CL on the bilayer structure and on the interactions with cyt-c. The
color codes are (i) transparent (light blue, dark blue and pink): DOPC, (ii) light blue: CL chain, (iii) dark blue: CL head groups, (iv) brown: oxidized group of CL, (v) colorful par-ticle: cyt-c. (A) Truncation oxidation in one chain does not change the structure of the membrane. (B) Four chains representing oxidation via truncation changes the membrane structure and form ox-CL clusters. This may indicate that the highly oxidized system could be prone to developing a pore. (C) Monohydroxy-CL still allows cyt-c to bind the mem-brane. (D) Cyt-c shows lower affinity toward a membrane with a high level of oxidation using tetrahydroxy-CL (D) compared to the lower level of oxidation in (C).
perturbation of the membrane, even when all four chains in CL were oxygenated. In contrast, oxygenation strongly affected cyt-c binding, which was fully abolished in this case. When only one chain was oxy-genated, however, cyt-c was still able to bind to the membrane. These results indicate that massive peroxidation of CL will abolish cyt-c bind
ing to the membrane, which may contribute to the release into the cyto-plasm.
The difference in clustering of CL molecules between “productive” and “unproductive” binding sites is an interesting observation in view of the translocations of CL from the inner leaflet of the IMM to the OMM – the “arriving CLs” may change the catalytic landscape of cyt-c's per-oxidase activity, CL oxidation rates and the composition of the products formed. It is also possible that the “unproductive” site is still a very pro-ductive regulator – positive or negative – of peroxidase activity. These intriguing questions arising from our findings provide interesting av-enues for future research. For example, cyt-c carrying mutations at its different meaningful sites could be analyzed with respect to the CL ox-idation products by LC-MS. Furthermore, computational cross-correla-tion analysis could be performed using the new crystal structures re-solved carrying different lipids/detergents. This should allow deriving conclusions as to the changes taking place at the lipid-binding pockets as allosteric regulators of protein organization and peroxidase activity.
Although we have not studied the biphasic behavior of the inter-actions directly, the weak involvement of hydrophobic residues at the initial stages of the interactions supports a critical role of electrostatic interactions in the recognition stage (Table S5). We propose that one bound state is the productive binding at proximal and distal sites and that the other bound state is the unproductive binding at the third site. The NMR results indicate that all binding sites are engaged. The produc-tive binding may facilitate the extended lipid anchorage due to heme crevice opening [38, 41, 45], the nature of which still requires further investigation [44]. Therefore, cyt-c turns into a peroxidase due to pro-ductive binding in the presence of H2O2. This complex functions as a pro-apoptotic factor leading to lipid (mainly CL) peroxidation and reac-tive oxygen species generation.
The trans-membrane migration of CL from the IMM to the OMM is a dynamic process and increases the amount of available CL to interact with cyt-c [19]. Our results indicate that increasing the CL concentra-tion enhances the affinity of cyt-c for the membrane. The NMR and flu-orescence results are in good agreement with the CGMD results with re-spect to the ratio of CL needed to create a peroxidase. Remarkably, cyt-c recruits CL to form a cluster in the membrane, which demonstrates that multiple CL molecules (not one alone) stabilize the cyt-c/CL complex. Additionally, our data demonstrate that CLs with more PUFAs regulate the tight binding of cyt-c to the membrane. Thus, triggering transmigra-tion of CL at the onset of apoptosis acts as a regulatory factor controlling the catalytic competence of cyt-c.
Although our solution NMR studies demonstrate drastic effects of CL binding on cyt-c spectra, it is not possible to separate effects of liposome interaction from cyt-c structural changes. Thus, the large differences be-tween the previously observed global re-arrangements in cyt-c structure [78, 79, 86–89] vs very small or essentially lack of unfolding in solid state-NMR spectra [34] cannot be resolved here. The exquisite depen-dence of changes in the solution NMR spectra on CL concentration sup-port the hypothesis, that these differences will likely be related to the ratio of total lipid:CL:cyt-c used. Furthermore, peroxidase activity of the samples studied by solid state NMR increased only up to 2-fold, consis-tent with the notion that higher activity requires larger conformational changes. Finally, differences in oxygenation of samples may play a role as aerobic versus anaerobic conditions have been shown to modulate the extent of conformational rearrangements [36].
Taking together the conformational studies of cyt-c and interaction studies with CL, we propose that gain of peroxidase activity by the cyt-c/CL complex is not a small molecule induced effect, but rather is mediated by the interaction with the extensive surface area involved in binding the CL containing membrane bilayer. This has important bi-ological implications for modulating cyt-c peroxidase activity. The ex
UNCORRECTED
PROOF
tensive surface area formed between CL and cyt-c is reminiscent of pro-tein-protein interaction interfaces and the inherent conformational flex-ibility of cyt-c identified by the NMR relaxation measurements (Fig. 2) is crucial for enabling this interaction. Our recent discovery of imida-zole fatty acid inhibitors of peroxidase activity of cyt-c/CL complexes [90, 91] support the notion that a combination of multiple factors con-tributing to stable complex formation can effectively be used to exploit protein-lipid bilayer interfaces as a novel class of drug targets. A related approach is the CL targeting capability of peptide SS-31, a peptide that specifically binds CL and inhibits cyt-c peroxidase activity [92].
Transparency document
The Transparency document associated with this article can be found, in online version.
Acknowledgements
This work was in part supported by a Human Frontier Science Pro-ject Grant to VK and JKS.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https:// doi.org/10.1016/j.bbamem.2018.01.009.
References
[1]
V.P. Skulachev, Cytochrome c in the apoptotic and antioxidant cascades, FEBS Lett. 423 (3) (1998) 275–280.
[2]
R.E. Dickerson, The structure of cytochromec and the rates of molecular evolution, J. Mol. Evol. 1 (1) (1971) 26–45.
[3]
C. Putnam, Protein Calculator v3. 3, Available from: http://protcalc.sourceforge. net, 2006.
[4]
G.W. Bushnell, G.V. Louie, G.D. Brayer, High-resolution three-dimensional struc-ture of horse heart cytochrome c, J. Mol. Biol. 214 (2) (1990) 585–595. [5]
L.C. Godoy, C. Munoz-Pinedo, L. Castro, S. Cardaci, C.M. Schonhoff, M. King, V. Tortora, M. Marin, Q. Miao, J.F. Jiang, A. Kapralov, R. Jemmerson, G.G. Silkstone, J.N. Patel, J.E. Evans, M.T. Wilson, D.R. Green, V.E. Kagan, R. Radi, J.B. Mannick, Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells, Proc. Natl. Acad. Sci. U. S. A. 106 (8) (2009) 2653–2658.
[6]
M. Huttemann, P. Pecina, M. Rainbolt, T.H. Sanderson, V.E. Kagan, L. Samavati, J.W. Doan, I. Lee, The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis, Mi-tochondrion (2011).
[7]
B. Kadenbach, S. Arnold, I. Lee, M. Huttemann, The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases, Biochim. Biophys. Acta Biomembr. 1655 (2004) 400–408.
[8]
V. Kagan, Y. Tyurina, H. Bayir, C. Chu, A. Kapralov, I. Vlasova, N. Belikova, V. Tyurin, A. Amoscato, M. Epperly, The “pro-apoptotic genies” get out of mitochon-dria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin com-plexes, Chem. Biol. Interact. 163 (1–2) (2006) 15–28.
[9]
V.E. Kagan, H.A. Bayir, N.A. Belikova, O. Kapralov, Y.Y. Tyurina, V.A. Tyurin, J. Jiang, D.A. Stoyanovsky, P. Wipf, P.M. Kochanek, J.S. Greenberger, B. Pitt, A.A. Shvedova, G. Borisenko, Cytochrome c/cardiolipin relations in mitochondria: a kiss of death, Free Radic. Biol. Med. 46 (11) (2009) 1439–1453.
[10]
F. Sinibaldi, L. Fiorucci, A. Patriarca, R. Lauceri, T. Ferri, M. Coletta, R. Santucci, Insights into cytochrome c-cardiolipin interaction. Role played by ionic strength, Biochemistry 47 (26) (2008) 6928–6935.
[11]
G. Daum, Lipids of mitochondria, Biochim. Biophys. Acta 822 (1) (1985) 1–42.
[12]
M. Schlame, S. Brody, K.Y. Hostetler, Mitochondrial cardiolipin in diverse eukary-otes. Comparison of biosynthetic reactions and molecular acyl species, Eur. J. Biochem. 212 (3) (1993) 727–735.
[13]
B. Gomez Jr, N.C. Robinson, Phospholipase digestion of bound cardiolipin re-versibly inactivates bovine cytochrome bc 1, Biochemistry 38 (28) (1999) 9031–9038.
[14]
M. Zhang, E. Mileykovskaya, W. Dowhan, Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria, J. Biol. Chem. 280 (33) (2005) 29403.
[15]
E.J. Lesnefsky, T.J. Slabe, M.S.K. Stoll, P.E. Minkler, C.L. Hoppel, Myocardial is-chemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochon-dria, Am. J. Phys. Heart Circ. Phys. 280 (6) (2001) H2770.
[16]
C. Arnarez, J.P. Mazat, J. Elezgaray, S.J. Marrink, X. Periole, Evidence for cardi-olipin binding sites on the membrane-exposed surface of the cytochrome bc1, J. Am. Chem. Soc. 135 (8) (2013) 3112–3120.
[17]
C. Arnarez, S.J. Marrink, X. Periole, Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels, Sci. Rep. 3 (2013) 1263. [18]
M. Ott, J.D. Robertson, V. Gogvadze, B. Zhivotovsky, S. Orrenius, Cytochrome c re-lease from mitochondria proceeds by a two-step process, Proc. Natl. Acad. Sci. U. S. A. 99 (3) (2002) 1259–1263.
[19]
Y.Y. Tyurina, S.M. Poloyac, V.A. Tyurin, A.A. Kapralov, J. Jiang, T.S. Anthony-muthu, V.I. Kapralova, A.S. Vikulina, M.Y. Jung, M.W. Epperly, D. Moham-madyani, J. Klein-Seetharaman, T.C. Jackson, P.M. Kochanek, B.R. Pitt, J.S. Green-berger, Y.A. Vladimirov, H. Bayir, V.E. Kagan, A mitochondrial pathway for biosynthesis of lipid mediators, Nat. Chem. 6 (6) (2014) 542–552. [20]
V.E. Kagan, N.V. Konduru, W. Feng, B.L. Allen, J. Conroy, Y. Volkov, I.I. Vlasova, N.A. Belikova, N. Yanamala, A. Kapralov, Carbon nanotubes degraded by neu-trophil myeloperoxidase induce less pulmonary inflammation, Nat. Nanotechnol. 5 (5) (2010) 354–359.
[21]
Y.A. Vladimirov, E.V. Proskurnina, D.Y. Izmailov, A.A. Novikov, A.V. Brusnichkin, A.N. Osipov, V.E. Kagan, Mechanism of activation of cytochrome C peroxidase ac-tivity by cardiolipin, Biochemistry (Mosc) 71 (9) (2006) 989–997.
[22]
N.A. Belikova, Y.A. Vladimirov, A.N. Osipov, A.A. Kapralov, V.A. Tyurin, M.V. Potapovich, L.V. Basova, J. Peterson, I.V. Kurnikov, V.E. Kagan, Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing mem-branes, Biochemistry 45 (15) (2006) 4998–5009.
[23]
N.A. Belikova, Y.Y. Tyurina, G. Borisenko, V. Tyurin, A.K. Samhan Arias, N. Yana-mala, P.G. Furtmuller, J. Klein-Seetharaman, C. Obinger, V.E. Kagan, Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: an-tioxidant function in mitochondria, J. Am. Chem. Soc. 131 (32) (2009)
11288–11289. [24]
V.E. Kagan, V.A. Tyurin, J. Jiang, Y.Y. Tyurina, V.B. Ritov, A.A. Amoscato, A.N. Osipov, N.A. Belikova, A.A. Kapralov, V. Kini, I.I. Vlasova, Q. Zhao, M. Zou, P. Di, D.A. Svistunenko, I.V. Kurnikov, G.G. Borisenko, Cytochrome c acts as a cardi-olipin oxygenase required for release of proapoptotic factors, Nat. Chem. Biol. 1 (4) (2005) 223–232.
[25]
J.J. Maguire, Y.Y. Tyurina, D. Mohammadyani, A.A. Kapralov, T.S. Anthonymuthu, F. Qu, A.A. Amoscato, L.J. Sparvero, V.A. Tyurin, J. Planas-Iglesias, R.R. He, J. Klein-Seetharaman, H. Bayir, V.E. Kagan, Known unknowns of cardiolipin signal-ing: the best is yet to come, Biochim. Biophys. Acta 1862 (1) (2017) 8–24. [26]
D. Mohammadyani, V.E. Kagan, J. Klein-Seetharaman, Coarse grained molecular dynamics simulation of the interaction of cytochrome C with lipid bilayers, Bio-phys. J. 104 (2) (2013) 503a–504a.
[27]
N. Zamzami, S.A. Susin, P. Marchetti, T. Hirsch, I. Gómez-Monterrey, M. Castedo, G. Kroemer, Mitochondrial control of nuclear apoptosis, J. Exp. Med. 183 (4) (1996) 1533–1544.
[28]
L.V. Basova, I.V. Kurnikov, L. Wang, V.B. Ritov, N.A. Belikova, I.I. Vlasova, A.A. Pacheco, D.E. Winnica, J. Peterson, H. Bayir, D.H. Waldeck, V.E. Kagan, Cardi-olipin switch in mitochondria: shutting off the reduction of cytochrome c and turn-ing on the peroxidase activity, Biochemistry 46 (11) (2007) 3423–3434. [29]
A.A. Kapralov, I.V. Kurnikov, I.I. Vlasova, N.A. Belikova, V.A. Tyurin, L.V. Basova, Q. Zhao, Y.Y. Tyurina, J. Jiang, H. Bayir, Y.A. Vladimirov, V.E. Kagan, The hierar-chy of structural transitions induced in cytochrome c by anionic phospholipids de-termines its peroxidase activation and selective peroxidation during apoptosis in cells, Biochemistry 46 (49) (2007) 14232–14244.
UNCORRECTED
PROOF
[30]
M. Rytomaa, P.K. Kinnunen, Reversibility of the binding of cytochrome c to lipo-somes. Implications for lipid-protein interactions, J. Biol. Chem. 270 (7) (1995) 3197–3202.
[31]
M.R. Zucchi, O.R. Nascimento, A. Faljoni-Alario, T. Prieto, I.L. Nantes, Modulation of cytochrome c spin states by lipid acyl chains: a continuous-wave electron para-magnetic resonance (CW-EPR) study of haem iron, Biochem. J. 370 (Pt 2) (2003) 671–678.
[32]
Y.Y. Huang, T. Kimura, Thermodynamic parameters for the reduction reaction of membrane-bound cytochrome c in comparison with those of the membrane-free form: spectropotentiostatic determination with use of an optically transparent thin-layer electrode, Biochemistry 23 (10) (1984) 2231–2236.
[33]
Z. Salamon, G. Tollin, Interaction of horse heart cytochrome c with lipid bilayer membranes: effects on redox potentials, J. Bioenerg. Biomembr. 29 (3) (1997) 211–221.
[34]
A. Mandal, C.L. Hoop, M. DeLucia, R. Kodali, V.E. Kagan, J. Ahn, P.C. van der Wel, Structural changes and proapoptotic peroxidase activity of cardiolipin-bound mito-chondrial cytochrome c, Biophys. J. 109 (9) (2015) 1873–1884.
[35]
E.S. O'Brien, N.V. Nucci, B. Fuglestad, C. Tommos, A.J. Wand, Defining the apop-totic trigger: the interaction of cytochrome C and cardiolipin, J. Biol. Chem. 290 (52) (2015) 30879–30887.
[36]
L. Serpas, B. Milorey, L.A. Pandiscia, A.W. Addison, R. Schweitzer-Stenner, Autoxi-dation of reduced horse heart cytochrome C catalyzed by cardiolipin-containing membranes, J. Phys. Chem. B 120 (48) (2016) 12219–12231.
[37]
M. Rytomaa, P.K. Kinnunen, Evidence for two distinct acidic phospholipid-binding sites in cytochrome c, J. Biol. Chem. 269 (3) (1994) 1770–1774.
[38]
E.K. Tuominen, C.J. Wallace, P.K. Kinnunen, Phospholipid-cytochrome c interac-tion: evidence for the extended lipid anchorage, J. Biol. Chem. 277 (11) (2002) 8822–8826.
[39]
F. Sinibaldi, L. Milazzo, B.D. Howes, M.C. Piro, L. Fiorucci, F. Polticelli, P. Ascenzi, M. Coletta, G. Smulevich, R. Santucci, The key role played by charge in the inter-action of cytochrome c with cardiolipin, J. Biol. Inorg. Chem. (2016). [40]
A. Kostrzewa, T. Pali, W. Froncisz, D. Marsh, Membrane location of spin-labeled cytochrome c determined by paramagnetic relaxation agents, Biochemistry 39 (20) (2000) 6066–6074.
[41]
F. Sinibaldi, B.D. Howes, M.C. Piro, F. Polticelli, C. Bombelli, T. Ferri, M. Coletta, G. Smulevich, R. Santucci, Extended cardiolipin anchorage to cytochrome c: a model for protein-mitochondrial membrane binding, J. Biol. Inorg. Chem. 15 (5) (2010) 689–700.
[42]
G.P. Gorbenko, J.G. Molotkovsky, P.K. Kinnunen, Cytochrome C interaction with cardiolipin/phosphatidylcholine model membranes: effect of cardiolipin protona-tion, Biophys. J. 90 (11) (2006) 4093–4103.
[43]
C. Kawai, F.M. Prado, G.L. Nunes, P. Di Mascio, A.M. Carmona-Ribeiro, I.L. Nantes, pH-Dependent interaction of cytochrome c with mitochondrial mimetic mem-branes: the role of an array of positively charged amino acids, J. Biol. Chem. 280 (41) (2005) 34709–34717.
[44]
H. Kobayashi, S. Nagao, S. Hirota, Characterization of the cytochrome c mem-brane-binding site using cardiolipin-containing bicelles with NMR, Angew. Chem. Int. Ed. Eng. 55 (45) (2016) 14019–14022.
[45]
E. Kalanxhi, C.J. Wallace, Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models, Biochem. J. 407 (2) (2007) 179–187.
[46]
V.M. Trusova, G.P. Gorbenko, J.G. Molotkovsky, P.K. Kinnunen, Cytochrome c-lipid interactions: new insights from resonance energy transfer, Biophys. J. 99 (6) (2010) 1754–1763.
[47]
L.J. McClelland, H.B. Steele, F.G. Whitby, T.C. Mou, D. Holley, J.B. Ross, S.R. Sprang, B.E. Bowler, Cytochrome c can form a well-defined binding pocket for hydrocarbons, J. Am. Chem. Soc. (2016).
[48]
J.N. Rumbley, L. Hoang, S.W. Englander, Recombinant equine cytochrome c in Es-cherichia coli: high-level expression, characterization, and folding and assembly mutants, Biochemistry 41 (47) (2002) 13894–13901.
[49]
W. Liu, J. Rumbley, S.W. Englander, A.J. Wand, Backbone and side-chain het-eronuclear resonance assignments and hyperfine NMR shifts in horse cytochrome c, Protein Sci. 12 (9) (2003) 2104–2108.
[50]
M.M. Krishna, H. Maity, J.N. Rumbley, Y. Lin, S.W. Englander, Order of steps in the cytochrome C folding pathway: evidence for a sequential stabilization mecha-nism, J. Mol. Biol. 359 (5) (2006) 1410–1419.
[51]
H. Maity, J.N. Rumbley, S.W. Englander, Functional role of a protein foldon—an Omega-loop foldon controls the alkaline transition in ferricytochrome c, Proteins 63 (2) (2006) 349–355.
[52]
M.M. Krishna, H. Maity, J.N. Rumbley, S.W. Englander, Branching in the sequen-tial folding pathway of cytochrome c, Protein Sci. 16 (9) (2007) 1946–1956. [53]
B.A. Johnson, Using NMRView to visualize and analyze the NMR spectra of macro-molecules, Methods Mol. Biol. 278 (2004) 313–352.
[54]
Goddard, T.D.A.K., D. G., SPARKY 3. University of California: San Francisco. [55]
C.T. Chu, J. Ji, R.K. Dagda, J.F. Jiang, Y.Y. Tyurina, A.A. Kapralov, V.A. Tyurin, N. Yanamala, I.H. Shrivastava, D. Mohammadyani, K.Z. Qiang Wang, J. Zhu, J. Klein-Seetharaman, K. Balasubramanian, A.A. Amoscato, G. Borisenko, Z. Huang, A.M. Gusdon, A. Cheikhi, E.K. Steer, R. Wang, C. Baty, S. Watkins, I. Bahar, H. Bayir, V.E. Kagan, Cardiolipin externalization to the outer mitochondrial mem-brane acts as an elimination signal for mitophagy in neuronal cells, Nat. Cell Biol. 15 (10) (2013) 1197–1205.
[56]
L.W. Yang, X. Liu, C.J. Jursa, M. Holliman, A.J. Rader, H.A. Karimi, I. Bahar, iGNM: a database of protein functional motions based on Gaussian Network Model, Bioinformatics 21 (13) (2005) 2978–2987.
[57]
L.W. Yang, A.J. Rader, X. Liu, C.J. Jursa, S.C. Chen, H.A. Karimi, I. Bahar, oGNM: online computation of structural dynamics using the Gaussian Network Model, Nu-cleic Acids Res. 34 (Web Server issue) (2006) W24–31.
[58]
M.A. Lomize, I.D. Pogozheva, H. Joo, H.I. Mosberg, A.L. Lomize, OPM database and PPM web server: resources for positioning of proteins in membranes, Nucleic Acids Res. 40 (Database issue) (2012) D370–6.
[59]
S.J. Marrink, H.J. Risselada, S. Yefimov, D.P. Tieleman, A.H. de Vries, The MAR-TINI force field: coarse grained model for biomolecular simulations, J. Phys. Chem. B 111 (27) (2007) 7812–7824.
[60]
D. Van der Spoel, E. Lindahl, B. Hess, G. Groenhof, A.E. Mark, H.J.C. Berendsen, GROMACS: fast, flexible, and free, J. Comput. Chem. 26 (16) (2005) 1701–1718. [61]
W. Humphrey, A. Dalke, K. Schulten, VMD: visual molecular dynamics, J. Mol. Graph. Model. 14 (1) (1996) 33–38.
[62]
G. Bussi, D. Donadio, M. Parrinello, Canonical sampling through velocity rescaling, J. Chem. Phys. 126 (1) (2007).
[63]
H.J.C. Berendsen, J.P.M. Postma, W.F. Vangunsteren, A. Dinola, J.R. Haak, Molec-ular-dynamics with coupling to an external bath, J. Chem. Phys. 81 (8) (1984) 3684–3690.
[64]
N. Yanamala, A.A. Kapralov, M. Djukic, J. Peterson, G. Mao, J. Klein-Seetharaman, D.A. Stoyanovsky, J. Stursa, J. Neuzil, V.E. Kagan, Structural re-arrangement and peroxidase activation of cytochrome c by anionic analogues of vitamin E, toco-pherol succinate and tocotoco-pherol phosphate, J. Biol. Chem. 289 (47) (2014) 32488–32498.
[65]
A.A. Kapralov, N. Yanamala, Y.Y. Tyurina, L. Castro, A.S. Arias, Y.A. Vladimirov, A. Maeda, A.A. Weitz, J. Peterson, D. Mylnikov, V. Demicheli, V. Tortora, J. Klein-Seetharaman, R. Radi, V.E. Kagan, Topography of tyrosine residues and their involvement in peroxidation of polyunsaturated cardiolipin in cytochrome c/cardi-olipin peroxidase complexes, Biochim. Biophys. Acta (2011).
[66]
G.R. Grimsley, J.M. Scholtz, C.N. Pace, A summary of the measured pK values of the ionizable groups in folded proteins, Protein Sci. 18 (1) (2009) 247–251. [67]
J. Planas-Iglesias, H. Dwarakanath, D. Mohammadyani, N. Yanamala, V.E. Kagan, J. Klein-Seetharaman, Cardiolipin interactions with proteins, Biophys. J. 109 (6) (2015) 1282–1294.
[68]
P. Pecina, G.G. Borisenko, N.A. Belikova, Y.Y. Tyurina, A. Pecinova, I. Lee, A.K. Samhan-Arias, K. Przyklenk, V.E. Kagan, M. Huttemann, Phosphomimetic substitu-tion of cytochrome C tyrosine 48 decreases respirasubstitu-tion and binding to cardiolipin and abolishes ability to trigger downstream caspase activation, Biochemistry 49 (31) (2010) 6705–6714.
[69]
A.L. Lomize, I.D. Pogozheva, M.A. Lomize, H.I. Mosberg, Positioning of proteins in membranes: a computational approach, Protein Sci. 15 (6) (2006) 1318–1333. [70]
M.A. Lomize, A.L. Lomize, I.D. Pogozheva, H.I. Mosberg, OPM: orientations of pro-teins in membranes database, Bioinformatics 22 (5) (2006) 623–625.
UNCORRECTED
PROOF
[71]
L.R. Brown, K. Wuthrich, NMR and ESR studies of the interactions of cytochrome c with mixed cardiolipin-phosphatidylcholine vesicles, Biochim. Biophys. Acta 468 (3) (1977) 389–410.
[72]
P.J. Spooner, A. Watts, Cytochrome c interactions with cardiolipin in bilayers: a multinuclear magic-angle spinning NMR study, Biochemistry 31 (41) (1992) 10129–10138.
[73]
L.D. Renner, D.B. Weibel, Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes, Proc. Natl. Acad. Sci. U. S. A. 108 (15) (2011) 6264–6269.
[74]
L. Cwiklik, P. Jungwirth, Massive oxidation of phospholipid membranes leads to pore creation and bilayer disintegration, Chem. Phys. Lett. 486 (4–6) (2010) 99–103.
[75]
Z. Salamon, G. Tollin, Surface plasmon resonance studies of complex formation be-tween cytochrome c and bovine cytochrome c oxidase incorporated into a sup-ported planar lipid bilayer. II. Binding of cytochrome c to oxidase-containing cardi-olipin/phosphatidylcholine membranes, Biophys. J. 71 (2) (1996) 858–867. [76]
M. Rytomaa, P. Mustonen, P.K. Kinnunen, Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes, J. Biol. Chem. 267 (31) (1992) 22243–22248.
[77]
G. Stepanov, O. Gnedenko, A. Mol'nar, A. Ivanov, Y. Vladimirov, A. Osipov, Evalu-ation of cytochrome c affinity to anionic phospholipids by means of surface plas-mon resonance, FEBS Lett. 583 (1) (2009) 97–100.
[78]
C.L. Bergstrom, P.A. Beales, Y. Lv, T.K. Vanderlick, J.T. Groves, Cytochrome c causes pore formation in cardiolipin-containing membranes, Proc. Natl. Acad. Sci. U. S. A. 110 (16) (2013) 6269–6274.
[79]
J. Hanske, J.R. Toffey, A.M. Morenz, A.J. Bonilla, K.H. Schiavoni, E.V. Pletneva, Conformational properties of cardiolipin-bound cytochrome c, Proc. Natl. Acad. Sci. U. S. A. 109 (1) (2012) 125–130.
[80]
N.C. Robinson, Specificity and binding affinity of phospholipids to the high-affinity cardiolipin sites of beef heart cytochrome c oxidase, Biochemistry 21 (1) (1982) 184–188.
[81]
K.J. Boyd, N.N. Alder, E.R. May, Buckling under pressure: curvature-based lipid segregation and stability modulation in cardiolipin-containing bilayers, Langmuir 33 (27) (2017) 6937–6946.
[82]
M. Amirkavei, P.K. Kinnunen, Interactions and dynamics of two extended confor-mation adapting phosphatidylcholines in model biomembranes, Biochim. Biophys. Acta 1858 (2) (2016) 264–273.
[83]
K.A. Runas, N. Malmstadt, Low levels of lipid oxidation radically increase the pas-sive permeability of lipid bilayers, Soft Matter 11 (3) (2015) 499–505. [84]
R. Volinsky, L. Cwiklik, P. Jurkiewicz, M. Hof, P. Jungwirth, P.K. Kinnunen, Oxi-dized phosphatidylcholines facilitate phospholipid flip-flop in liposomes, Biophys. J. 101 (6) (2011) 1376–1384.
[85]
U. Schlattner, M. Tokarska-Schlattner, S. Ramirez, Y.Y. Tyurina, A.A. Amoscato, D. Mohammadyani, Z. Huang, J. Jiang, N. Yanamala, A. Seffouh, M. Boissan, R.F. Epand, R.M. Epand, J. Klein-Seetharaman, M.L. Lacombe, V.E. Kagan, Dual func-tion of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch, J. Biol. Chem. 288 (1) (2013) 111–121.
[86]
P.J. Spooner, A. Watts, Reversible unfolding of cytochrome c upon interaction with cardiolipin bilayers. 2. Evidence from phosphorus-31 NMR measurements, Bio-chemistry 30 (16) (1991) 3880–3885.
[87]
P.J. Spooner, A. Watts, Reversible unfolding of cytochrome c upon interaction with cardiolipin bilayers. 1. Evidence from deuterium NMR measurements, Biochem-istry 30 (16) (1991) 3871–3879.
[88]
Y. Hong, J. Muenzner, S.K. Grimm, E.V. Pletneva, Origin of the conformational heterogeneity of cardiolipin-bound cytochrome C, J. Am. Chem. Soc. 134 (45) (2012) 18713–18723.
[89]
A. Bernabeu, L.M. Contreras, J. Villalain, Two-dimensional infrared correlation spectroscopy study of the interaction of oxidized and reduced cytochrome c with phospholipid model membranes, Biochim. Biophys. Acta 1768 (10) (2007) 2409–2420.
[90]
A. Bakan, A.A. Kapralov, H. Bayir, F. Hu, V.E. Kagan, I. Bahar, Inhibition of peroxi-dase activity of cytochrome c: de novo compound discovery and validation, Mol. Pharmacol. 88 (3) (2015) 421–427.
[91]
J. Jiang, A. Bakan, A.A. Kapralov, K.I. Silva, Z. Huang, A.A. Amoscato, J. Peterson, V.K. Garapati, S. Saxena, H. Bayir, J. Atkinson, I. Bahar, V.E. Kagan, Designing in-hibitors of cytochrome c/cardiolipin peroxidase complexes: mitochondria-targeted imidazole-substituted fatty acids, Free Radic. Biol. Med. 71 (2014) 221–230. [92]
A.V. Birk, S. Liu, Y. Soong, W. Mills, P. Singh, J.D. Warren, S.V. Seshan, J.D. Pardee, H.H. Szeto, The mitochondrial-targeted compound SS-31 re-energizes is-chemic mitochondria by interacting with cardiolipin, J. Am. Soc. Nephrol. 24 (8) (2013) 1250–1261.