REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Comparative
immunohistochemical
assessment
of
the
effect
of
repetitive
anesthesia
with
isoflurane
and
sevoflurane
on
rat
liver
夽
Flavia
Ruxanda
a,
Adrian
Florin
Gal
b,∗,
Cristian
Rat
¸iu
c,
Viorel
Micl˘
aus
¸
a,
Vasile
Rus
a,
Liviu
Ioan
Oana
daDepartmentofCellBiology,HistologyandEmbriology,UniversityofAgriculturalSciencesandVeterinaryMedicine,Cluj-Napoca, Romania
bDepartmentofPathologicalAnatomy,UniversityofAgriculturalSciencesandVeterinaryMedicine,Cluj-Napoca,Romania cDepartmentofImplantology,FacultyofMedicineandPharmacy,UniversityofOradea,Oradea,Romania
dDepartmentofAnesthesiologyandSurgicalPropedeutics,UniversityofAgriculturalSciencesandVeterinaryMedicine, Cluj-Napoca,Romania
Received14January2015;accepted11February2015 Availableonline26September2015
KEYWORDS
Anesthesia; Caspase-3; Isoflurane; Liver; Sevoflurane
Abstract
Backgroundandobjectives: Inhalationanesthetics areusedin human,aswell asveterinary medicalpractice.Inthepresentstudyweinvestigatedtheeffectofisofluraneandsevoflurane onrathepatocytes.
Methods:A total of40Wistarfemaleratswere usedinthisstudy.Animalswere dividedin groupsof5rats.GroupsIM,SMservedascontrolgroups.GroupsI1,I2,I3wereusedtostudy isofluraneandS1,S2,S3forsevofluranestudy.Theywereanesthetized3times,for2hlong,at 2daysintervalwithaconcentrationof:1.5%isoflurane(I1,I2,I3)and2%sevoflurane(S1,S2, S3).Theoxygensupplythroughouttheanesthesiawas1LO2/min.GroupsIM,IS,I1,S1were
sacrificedimmediatelyafterthelastanesthesia.GroupsI2,S2weresacrificed6hafterthelast anesthesia,andgroupsI3,S3,24hpost-anesthesia.Liversampleswereharvestedtohighlight caspase-3inapoptotichepatocytes.
Results:Followingisofluraneadministration,therewerelessthan1%cellsinapoptosis high-lighted in ratlivers from groups IM, I1 and I2. At24h post-anesthesia(group I3), a small number of apoptotic hepatocyteswas highlighted(around 3.23% cellsin apoptosis),with a strictlyperiacinardisposition,randomlydistributedinasmallnumberofhepaticlobules.After sevofluraneadministration,lessthan1%apoptotichepatocyteswereidentifiedatallcontrol momentsthroughoutthestudy.
夽 Thestudywas carriedoutat theDepartmentof Anesthesiologyand Surgical Propedeutics,Universityof AgriculturalSciences and
VeterinaryMedicine,Cluj-Napoca,Romania.
∗Correspondingauthor.
E-mail:adrianfloringal@yahoo.com(A.F.Gal).
http://dx.doi.org/10.1016/j.bjane.2015.02.003
Conclusions:Theresultssuggestthattheanestheticsdonotpresentaconsiderable hepatotox-icity.Thecomparativeassessmentofthetwoanestheticsshowsthatsevofluraneissuperiorto isoflurane.
©2015SociedadeBrasileiradeAnestesiologia.PublishedbyElsevierEditoraLtda.Thisisan openaccessarticleundertheCCBY-NC-NDlicense(
http://creativecommons.org/licenses/by-nc-nd/4.0/).
PALAVRAS-CHAVE
Anestesia; Caspase-3; Isoflurano; Fígado; Sevoflurano
Avaliac¸ãoimuno-histoquímicacomparativadoefeitodaanestesiarepetitiva
comisofluranoesevofluranosobreofígadoderato
Resumo
Justificativaeobjetivos: Anestésicosinalatóriossãousadosemhumanosetambémnaprática médicaveterinária.Nopresenteestudoinvestigamosoefeitodeisofluranoesevofluranoem hepatócitosderato.
Métodos: Nototal,40ratosWistarfêmeasforamusadosnesteestudo.Osanimaisforam dividi-dosemgruposdecincoratoscada.OsgruposIMeSMserviramcomogruposcontrole.Osgrupos I1,I2eI3foramusadosparaoestudodeisofluranoeosgruposS1,S2eS3paraoestudode sevoflurano.Osratosforamanestesiadostrêsvezes,duranteoperíodode2horasemintervalos dedoisdias,comumaconcentrac¸ãode1,5%deisoflurano(I1,I2,I3)e2%desevoflurano(S1,S2, S3).Ofornecimentodeoxigênioduranteaanestesiafoide1LO2/min.OsgruposIM,IS,I1eS1
foramsacrificadosimediatamenteapósaúltimaanestesia.OsgruposI2eS2foramsacrificados 6horasapósaúltimaanestesiaeosgruposI3eS3foramsacrificados24horasapósaanestesia. Amostrasdosfígadosforamcolhidaspararessaltaracaspase-3emhepatócitosapoptóticos.
Resultados: Apósaadministrac¸ãodeisoflurano,haviamenosde1%dascélulasemapoptose emdestaquenosfígadosdosratosdosgruposIM,I1eI2.Às24horasapósaanestesia(grupo I3),umpequenonúmerodehepatócitosapoptóticosfoidestacado(cercade3,23%decélulas emapoptose),comumadisposic¸ãoestritamenteperiacinar,distribuídosaleatoriamenteemum pequenonúmerodelóbuloshepáticos.Apósaadministrac¸ãodosevoflurano,menosde1%de hepatócitosapoptóticosfoiidentificadoemtodososmomentosdecontroleaolongodoestudo.
Conclusões:Osresultadossugeremqueosanestésicosnãoapresentamumahepatotoxicidade considerável.Aavaliac¸ãocomparativadosdoisanestésicosmostraquesevofluranoésuperior aoisoflurano.
©2015SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpen Accesssobumalicenc¸aCCBY-NC-ND(
http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Inhalational anesthetics are widely used in both human and veterinary medicine. They can be utilized in anes-theticmanagementinalargenumberofspecies,including reptiles, birds and wild animals.1---5 The primary site for metabolism of inhalational anesthetics is represented by theliver.6,7 Under thesecircumstances, it ispossible that somephysiologicalormorphologicalchangesappearinthe liver,duetoeitherthedirectactionoftheseanestheticsor someintermediarymetabolitsresultedduringtheir degra-dation.These aspects havemade the objectof numerous studieswhich broughtmuchusefulinformation, but there arestillcontroversiessurroundingtheinhalational anesthet-ics’actionontheliver.Thus,therearestudiesthatreport aminorliverenzymeselevation,whileothersreport fulmi-nantnecrotichepatitis,resultingeveninpatient’sdeath.8---10 Thegreatmajorityoftheinvestigationshavestudiedthe inhalationalanestheticeffectsonliverfunction.11Macroand especiallymicroscopicaspectsweretheobjectofstudyof
fatalsubacuteliverfailure,withmassivenecrosisof hepa-tocytes,atrepeatedsevofluraneanesthesia.
Consideringthesmallnumberofmorphological investiga-tionsandespeciallytheconflictingavailableinformationin thespecialty literature,we considered opportuneto con-duct histological and immunohistochemical investigations regardingtheisofluraneandsevofluraneeffectonratliver.
Methods
The experimentalprotocol wasapprovedby theScientific Research Ethics Committee of UASVM (University of Agri-culturalSciencesandVeterinaryMedicine)Cluj-Napocaand wasconducted in accordance with theMinistry of Health force and with the pertained European legislation. The experimentalstudywasconductedintheBiobaseofUASVM Cluj-Napoca andinthe DepartmentofAnesthesiology and SurgicalPropedeutics,UASVMCluj-Napoca.
Atotalof406week-oldWistarfemaleratswereusedin theexperimentalstudy.Theratswererandomlydividedin 8groups,5ratseach.Twoofthemservedascontrolgroups: IM(controlisoflurane)andSM(controlsevoflurane),ratsin thisgroupbeingexposedtooxygenalone(1L/min)3times, for2hlong,at2daysinterval.Threegroups(I1,I2andI3) weresubjectedtoisofluraneanesthesiaataconcentration of1.5%andtheotherthree(S1, S2andS3)tosevoflurane at a2%concentration.At theutilizedconcentrations, the minimumalveolar concentration(MAC)wasapproximately 1inthecase ofisoflurane,18 aswellassevoflurane.19 The concentrationofanestheticwasadjustedbyusinga vapor-izer.
Weusedaninductionboxforanesthesias.Theratswere introducedintheinductionbox15minbeforeinitiatingthe anesthesia,inordertoaccommodate.Experimentalgroups wereanesthetised3times,for2hlong,at2daysinterval. Theoxygensupplythroughouttheanesthesiawas1LO2/min
for both isofluraneand sevoflurane.We followedthe pro-tocolrecommendedbyHondaetal.,15 mentioningthatthe anesthesiaswererepeated3timesinordertoseetheeffects ofrepeatedanesthesiawithisofluraneandsevoflurane.
Aftercompletingtheanesthesias,theratsweresacrificed through cervical dislocation, at different post-anesthetic times:groupsIM,SM,I1andS2immediatelyafterthe anes-thesia,groupsI2,S2---6hpost-anesthesiaandI3,S3groups ---24hpost-anesthesia.
Immediately after the euthanasia, liver samples were harvested,introducedin10%bufferedformalinforfixation. After thefixation period,the samples wereembedded in paraffinandthensectionedata5mthicknessandmounted
onpoly-l-lysinecoatedmicroscopicslides(forthe
immuno-histochemicalreaction).
In ordertodetect theapoptotic hepatocytes,we used theimmunohistochemicalreactiontohighlightthe caspase-3expression,asmarkerofprogrammedcelldeath.Withthis purposeinview,LeicaBond-maxTM Immunohostochemistry
system(LeicaBiosystems Melbourne,BondMax model,M2 12154 series) wasused. The immunomarcation was made withRabbit Anti-Caspase-3 PolyclonalAntibodies (dilution 1/75,rabbitanti-caspase3polyclonal,Linaris,Biologische Produkte,Dossenheim,Germany---cat.n◦RB-1197-R7).The steps taken by the system in order to stain the sections
werethe following: (a) deparaffinizationand rinsing with anEDTA solutionat8.8 pH (EpitopeRetrievalSolution2), 98◦Cfor20min;(b)afterrinsing,theDC9800kitwasused for10min(LeicaMicrosystemGmbH)inordertoblockthe endogenousperoxidase;(c)rinsing,afterwhichthe‘‘rabbit anti-caspase-3’’primary antibody wasadded and incuba-tionfor 30min; (d) polymerase addingand incubation for 10min, obtainingthe color reactionwiththe help ofDAB (diaminobenzidine).
Evaluationofthemarkerwasmadethrough quantifica-tionofimmunomarkedcells.Cellswithadifferentintensity browncytoplasmwereconsideredpositive.Immunomarked cellsfrom5microscopicfields,at400×magnification,were counted.The followinggrading methodwasusedfor each case: (I)less than 1% cells in apoptosis, (II) 1---2% cells in apoptosis, and (III) more than 2% cells in apoptosis. The microscopicimageswereobtainedusingtheOlympusBX51 lightmicroscopeconnectedtoanOlympusDP-25digital pho-tocamera,imagesbeingstoredonadigitalmemorycardand shownonthemonitor.
Results
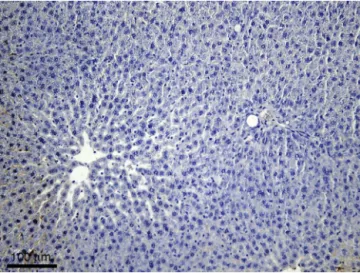
Immunohistochemicalreaction forcaspase-3 highlighted a smallnumberof caspase-3positivecells inratlivers from groupsIMandSM(i.e.,lessthan1%cellsinapoptosis), no morethatthenormalhepatocyteexchangerate.Apartfrom thefacttheywerescarce,thecaspase-3positivecellswere isolatedandscattered throughoutthe hepaticlobules.On mostofthesectionarea,thehepaticlobuleshadanormal structureanddidnotcontainapoptoticcells(Fig.1).
LiversfromratsinI1groupdidnotpresentmore caspase-3positive cells than the control group (i.e.,less than 1% cellsinapoptosis),sothatinthegreatmajorityofthe lob-ulestheywerenotpresent,notevenisolated.Thesituation maintainsinI2group,regardingboththegeneralaspectof theorganandthesmallnumberofapoptoticcells, compara-bletothecontrolgroup(i.e.,lessthan1%cellsinapoptosis). FirstnoticeablechangesappearonlyingroupI3andconsist ofthepresenceofcaspase-3positivecellsinsomelobules
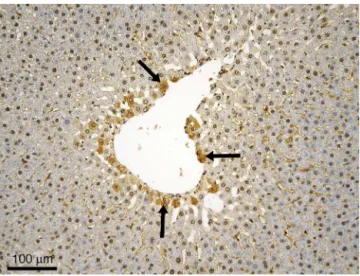
Figure2 Experimental groupI3 ---centrilobular distributed apoptotic cells (suggested by a darker cytoplasm) --- black arrows; immunohistochemical reaction anti-caspase-3 poly-clonalantibodies,Mayerhematoxylincounterstain.
(around3.23%cellsinapoptosis,whichrepresentmorethan 2% cells in apoptosis according to the suggested grading method).Someofthecellsintheclosevicinityofthe cen-trilobularveinappearaffected(Fig.2).Wementionthatthe phenomenonisnotpresentinallofthehepaticlobules,but onlyinsomeofthem.Therewerenoinflammatorychanges orcirculatorydisordersobservedafterexposureoftherats toisoflurane.
IntheliversharvestedfromratsingroupS1,thesituation iscomparabletotheoneinthecontrolgroup,thenumberof caspase-3positivecellsbeingverysmall.Amajorincrease ofthecaspase-3positivecellsisnoticedinneitherS2,nor S3groups.Nootherassociatedlesionswereobserved(e.g., dystrophic,inflammatorylesionsorcirculatorydisorders)in theliverafterexposureofthesubjectstosevoflurane.
Discussion
Liversfromrats inthe controlgroups presented anormal aspect regarding the lobulation and the lobular pattern. Caspase-3positivecells(i.e.,inapoptosis)wereidentified, buttheirnumberwassmall,actuallyrepresentingthe nor-mal hepatocyte exchange rate (aspect suggested by the isolateddisposition,scattered throughoutthehepatic lob-ules,withnumeroushepaticlobuleswithoutimmunomarked cells).
LiversfromratsingroupI1werecomparabletotheones from rats in the control group, from all points of view. Caspase-3positivecellswerepresentinsmallnumbersand onlyinsomelobules,sothatwecannotstatethatafterthe anesthetic administration there were immediate adverse reactions,notevendiscreteones. IngroupI2,nochanges wereobservedregardingtheapoptoses’distributioninliver in comparison to the two groups presented anteriorly. In groupI3aslightincreaseofthenumberofcaspase-3 posi-tivecellswasobserved,incomparisontothecontrol,I1and I2groups.ComparativetothethingsobservedingroupIM,I1 andI2,inthecaseofsubjectsfromgroupI3alargernumber ofimmunomarkedcells(inapoptosis)wasidentified,focally
disposed,prevailinglycentroacinar. Apossibleexplanation oftheaffectationofthecellsintheimmediatevicinityof thecentrilobularveincouldbethefactthattheanesthetic reachestheliverthroughthecirculatorysystem,butinthis situation,hepatocytesattheperipheryofthelobulesshould bethefirstcellstogivein,becausethatistheplacethat thesinusoidsenterthehepaticlobules.Buthepatocytesat theperipheryofthelobulesarefavoredintermsofnutrient andoxygensupplies,beingthefirsttobenefitduetotheir disposal,whichseemstoconferresistancetotheanesthetic action.Cellsinthevicinityofthecentrilobularveinarethe lasttosupply.Apparently,thismakesthemsomewhat vul-nerable,someofthementeringapoptosisunderanesthetic action.Otherreportssuggestthattheperiacinarzoneis par-ticularlyvulnerabletoapoptosis/necrosis(knownaszone3 or periacinar apoptosis/necrosis),partlybecausetheyare farthest from incoming arterial and portal venous blood, bearingoxygenandessentialnutrients.20,21
However,thenumberof apoptoticcells issosmall;we canstate that isofluraneisan anestheticwithagood tol-erance. Theadverse reactions appeared in theliver after itsadministrationin therapeutic dose is minimal,without other cellular lesions(e.g., dystrophies, necroses, vascu-lar disorders) that induce changes in the pattern of the organ.
Sevofluraneadministrationdidnotresultinthe increas-ingofcaspase-3positivehepatocytenumberinexperimental groups immediatelyaftertheanesthesiaortheother con-troltimes(6h and24hpost-anesthesia).At noneofthese moments,thecaspase-3positivecellswereatsuchalevel whichwouldsuggestthattheanestheticwouldtrigger hepa-tocyteapoptosis.Apoptoseswerepresentinasmallnumber ofcells,somehowcomparableinallgroups,whichmakesus statethattheirpresenceisabsolutelynormal,beinglinked to thenormal hepatocyte exchangerate. In other terms, sevoflurane does not seem to have adverse reactions on ratliver,detectablethroughhistologicalexamination.This demonstratesaverygoodtolerabilityofsevofluraneinthe liver.
knowingthatnecrosisappearswhenthehypoxiaissevere, whileapoptosistakesplacewhenthehypoxiaismild.24
The exact path of apoptosis induction by inhalational anesthetics is not entirely known, but there are studies which propose different hypotheses. Thus, Zhang et al.25 have shown that 2% isoflurane treatment for 6h long can cause apoptosis. Their study was conducted on cell cul-tures,primaryneuronsandmice.Theystatedthatisoflurane can increasethe pro-apoptoticBax factor level, decrease the anti-apoptotic Bcl-2 factor level, increase the accu-mulationofreactiveoxygenspecies,enablethereleaseof cytochromecfrommitochondriaincytosolandinducethe activation of caspase-9 andcaspase-3, thus inducing apo-ptosisthroughBcl-2proteinfamilyandmitochondrialpath. Wei et al.26 propose another hypothesis: activation of inositol1,4,5-triphosphate(IP3)receptorsonthe
endoplas-mic reticulum membranes by isoflurane. This fact causes the excessive release of calcium, triggering apoptosis. The authors specify that isoflurane induced apoptosis, dependent on the anestheticconcentration and duration, increasing the cytosolic and then the mitochondrial cal-cium. Turrilazzi et al.27 report a case of fatal fulminant hepaticnecrosisaftersevofluraneanesthesia,incriminating thecytoplasmiccalciumincreasebysevofluraneascause.
In conclusion, our results show that sevoflurane does nottriggerhepatocyteapoptosis,whereasisoflurane deter-mines a moderate inconstant periacinar apoptosis, 24h post-anesthesia. Our results suggest that the tested anesthetics do not present a significant hepatotoxicity, sevofluraneprovingtohaveasuperiortolerabilityin com-parisontoisoflurane.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgement
ThispaperwaspublishedundertheframeofEuropeanSocial Fund, Human Resources Development Operational Pro-gramme2007---2013,projectno.POSDRU/159/1.5/S/136893
References
1.LewisJCM.Fielduseofisofluraneandairanestheticequipment inwildlife.JZooWildlMed.2004;35:303---11.
2.WimsattJ,O’sheaTJ,EllisonLE,etal.Anesthesiaandblood samplingof wild bigbrown bats(Eptesicus fuscus) withand assessmentofimpactsonsurvival.JWildlDis.2005;41:87---95.
3.ParkerWT,MullerLI,GerhardtRR, etal.Field useof isoflu-raneforsafesquirrelandwoodratanesthesia.JWildlManage. 2008;72:1262---6.
4.EscobarA,ThiesenR,VitalianoSN,etal.Somecardiopulmonary effectsofsevofluraneincrestedcaracara(Caracaraplancus). VetAnaesthAnalg.2009;36:436---41.
5.GranoneTD,DeFranciscoON,KillosMB,etal.Comparisonof threedifferentinhalantanestheticagents(isoflurane, sevoflu-rane,desflurane)inred-tailedhawks(Buteojamaicensis).Vet AnaesthAnalg.2012;39:29---37.
6.WaskellL.Metabolismofthevolatileanesthetics.In:RiceSA, FishKJ,editors. Anesthetictoxicity. NewYork: RavenPress; 1994.p.49.
7.BadenJM,RiceSA.Metabolismandtoxicityofinhaled anesthet-ics.In:MillerRD,editor.Anesthesia.5thed.NewYork:Chruchill Livingstone;2000.p.147---73.
8.BruntEM,WhiteH,MarshJW,etal.Fulminanthepaticfailure afterexposuretoisofluraneanesthesia:acasereport. Hepatol-ogy.1991;13:1017---21.
9.ZizekD,RibnikarM,ZizekB,etal.Fatalsubacuteliverfailure afterrepeatedadministrationofsevofluraneanaesthesia.EurJ GastroeneterolHepatol.2010;22:112---5.
10.SoubhiaAF,LauzS,MonteroEF,etal.Effectsoftheinhalational anesthetics halothane and sevoflurane on an experimen-tal model of hepatic injury. Rev Bras Anestesiol. 2011;61: 591---603.
11.SahinSH,CinarSO,PaksoyI,etal.Comparisonbetweenlowflow seovflurane anesthesia and totalintravenous anesthesia dur-ingintermediate-durationsurgery:effectsonrenalandhepatic toxicity.Hippokratia.2011;15:69---74.
12.BylesPH,Dobkin AB, FergusonJH,et al. Forane(compound 469):2.Biochemicaleffectsofrepeatedadministrationto ani-mals,responsetobleeding,andcompatibilitywithepinephrine. CanAnaesthSocJ.1971;18:387---96.
13.Steffey EP, Zinkl J,Howland D Jr. Minimalchanges inblood cellcountsandbiochemicalvaluesassociatedwithprolonged isoflurane anesthesia of horses. Am J Vet Res. 1979;40: 1646---8.
14.Eger EI 2nd. Isoflurane: a review. Anesthesiology. 1981;55:559---76.
15.Honda M, Yamada T, Nomura T, et al. Differential, histo-chemicalandimmunohistochemicalchangesinrathepatocytes afterisofluraneorsevofluraneexposure.ActaMedOkayama. 2003;57:1---12.
16.Elena G, Amerio N, Ferrero P, et al. Effects of repetitive sevofluraneanaesthesiaonimmuneresponse,select biochem-ical parameters and organ histology in mice. Lab Anim. 2003;37:193---203.
17.Carrigan TW, Straughen WJ. A report of hepatic necrosis and death following isoflurane anesthesia. Anesthesiology. 1987;67:581---3.
18.MazzeRI,RiceSA,BadenJM.Halothane,isofluraneand enflu-raneMACinpregnantandnonpregnantfemaleandmalemice andrats.Anesthesiology.1985;62:339---41.
19.TamadaM,InoueT,WatanabeY,etal.MACvaluesof sevoflu-rane. Prog Med. 1986;6:3248---53 (in Japanese with English abstract).
20.JubbKVF,KennedyPC,PalmerN.Pathologyofdomestic ani-mals,vol.2,3rded.AcademicPress,Inc.;1985.p.240---307.
21.TreutingPM,DintzisSM.ComparativeAnatomyandHistology: AMouseandHumanAtlas.UnitedStatesofAmerica:Academic Press;2012.p.193---9[chapter13].
22.Nishiyama T, Yokoyama T, Hanaoka K. Liver and renal func-tionafterrepeatedsevofluraneorisofluraneanaesthesia.Can JAnaesth.1998;45:789---93.
23.NishiyamaT.Effectsofrepeatexposuretoinhalation anesthet-icsonliverandrenalfunction.JAnaesthesiolClinPharmacol. 2013;29:83---7.
24.KerrJF.Shrinkagenecrosis:adistinctmodeofcellulardeath.J Pathol.1971;105:13---20.
25.Zhang Y, Dong Y, Wu X, et al. The mitochondrial path-wayofanestheticisoflurane-inducedapoptosis.JBiol Chem. 2010;285:4025---37.
26.Wei H, Liang G, Yang H, et al. The common inhala-tional anesthtic isoflurane induces apoptosis via activa-tion ofinositol 1,4,5-triphosphate receptors. Anesthesiology. 2008;108:251---60.