w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Development
of
standardized
extractive
solution
from
Lippia
sidoides
by
factorial
design
and
their
redox
active
profile
Bruno
S.
Lima
a,
Cledison
S.
Ramos
a,
João
P.A.
Santos
b,
Thallita
K.
Rabelo
b,
Mairim
R.
Serafini
a,
Carlos
A.S.
Souza
a,
Luiz
A.L.
Soares
c,
Lucindo
J.
Quintans
Júnior
d,
José
C.F.
Moreira
b,
Daniel
P.
Gelain
b,
Adriano
A.S.
Araújo
a,
Francilene
A.
Silva
a,∗aDepartamentodeFarmácia,UniversidadeFederaldeSergipe,SãoCristóvão,SE,Brazil bDepartamentodeBioquímica,UniversidadeFederaldeRioGrandedoSul,PortoAlegre,RS,Brazil cDepartamentodeFarmácia,UniversidadeFederaldePernambuco,Recife,PE,Brazil
dDepartamentodeFisiologia,UniversidadeFederaldeSergipe,SãoCristóvão,SE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16December2014 Accepted17December2014 Availableonline24April2015
Keywords: Lippiasidoides Factorialdesign Antioxidant Flavonoids
a
b
s
t
r
a
c
t
Theaimofthisstudywastoevaluatetheinfluencesofvariablesofpreparationontotalflavonoidscontent fromextractivesolutionofLippiasidoidesCham.,Verbenaceae.Thusa23factorialdesignwasusedtostudy
theimportanceofplantproportion,theextractionmethodandsolventontheextractionofflavonoid.The methodologyofdeterminationofchemicalsinfactorialdesignwasvalidatedaccordingtotheparameters requiredbyBrazilianHealthAgency.Theextractionsolutionwasselectedthroughafullfactorialdesign wherethebestconditionstoachievethehighestcontentofflavonoidswere:7.5%(w/v)ofplantwith ethanol50%(v/v)assolvent.ThepolyphenolscontentwasdeterminedbyLCmethodanditsrelationship withtheantioxidantandfreeradicalscavengingactivitieswasevaluated.Thefreeradicalscavenging activitiesandantioxidantpotentialsweredeterminedfordifferentconcentrationsusingvariousinvitro
models.Ourresultsindicatethatextractsexhibitedasignificantdose-dependentantioxidanteffectas evaluatedbyTRAP/TARassays.Besides,weobservedanantioxidantactivityagainsthydroxylradicalsand nitricoxide,andprotectionagainstlipidperoxidationinvitro.Ourresultssuggestthattheextractpresents significantinvitroantioxidantpotentialindicatingpromisingperspectivesforitsuseaspharmaceutical/or foodadditive.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Lippia sidoides Cham., Verbenaceae, popularly known as
“alecrim-pimenta”, isa typicalshrubcommonlygrowinginthe
NortheastofBrazil.Thisspeciesproducesanessentialoilrichin thy-molandcarvacrol,whichhasapotentantimicrobialactivityagainst fungiandbacteria(Lemosetal.,1990;Lacosteetal.,1996).The hydro-alcoholicextractsofthisplantarelargelyusedfortreatment ofskinwounds,asamouthantisepticandinliquidsoap prepa-rationstotreatandpreventgeneralfungalinfectionsofthebody (Matos,2000).
Inthisway,thosegroupsofsubstancescanbesuccessfullyused aschemicalsmarkerswhichcanassistinthestudiesof standard-izationofextractivesolutionsfrommedicinalplants.
∗ Correspondingauthor.
E-mail:francilene.silva@pq.cnpq.br(F.A.Silva).
However,validatedqualitycontrolmethodsneedtobe
devel-oped inorder tocomplywithregulatoryrequirements, ifsome
plantistobeusedasrawmaterialbythepharmaceutical indus-try;theabsenceofthosestudieshampersthereproducibilityofthe extractivesqualities,whichcouldaffecttheefficiencyandsafety. Thus,thestandardizationofplantextractivesolutionsshouldbe thefirststepduringthetechnologicaldevelopmentof phytophar-maceuticals.Theinfluenceofseveralparameterssuchasextractive methodandtechnology,typeandconcentrationofsolvent,aswell asplantconcentration,andtheirinfluenceinthephysical-chemical properties of theextractive solutions should be evaluated and quantified(Audietal.,2001;Cunhaetal.,2009).
Thefactorialdesignisastatistictoolusedtoscreeningand/or
optimization process,with rapid and economic way,and
max-imization the quality of final products. Besides, the statistical analysisawardresultsarereliable(MyersandMontgomery,1995; Montgomery,1997).Thus,itispossibletorankeachindependent variable according toits significance on thestudied responses. Therefore,withreducedtimeandexperimentaleffort,itmaybe
http://dx.doi.org/10.1016/j.bjp.2014.12.004
possibletochoicetheextractiveconditionsthatareabletoproduce
themaximumexperimentalresponse (MyersandMontgomery,
1995; Montgomery,1997; Canteri-Scheminet al., 2005; Soares etal.,2005).
Theaimofthisworkwastousethefactorialdesigntoevaluate theeffectoftheplantproportion,solventtype,concentrationand theextractionmethodonthetotalflavonoidscontentinextractives solutionsfromL.sidoides.Additionally,theaimwasalsotoevaluate theinvitroantioxidantactivityoftheoptimizedextractivesolution.
Materialsandmethods
Plantmaterial
Aerialpartsof LippiasidoidesCham., Verbenaceae,were col-lectedinthegardenofmedicinalplantsoftheFederalUniversity ofSergipe(SãoCristóvão,Brazil)inMay2009andwasidentified byProf.AnaPaulaN.Pratawhoisa planttaxonomistfromthe
DepartmentofBiology.Avoucherspecimen(ASE2626)hasbeen
depositedintheHerbariumofDepartmentofBiology.Theplant materialafterharvestingwassubjectedtodryingprocessina circu-latingairatatemperatureof40±2◦Cuntilstabilizationofresidual moisture.Afterdryingtheplantmaterialwasmanuallyselected, subjectedtogrindinginagrinderofknives,weighed,sampledand wascharacterizedthroughanalysisof moisturecontent, granu-lometricanalysisusingsieveof150,250,355,500,600,710and 1000m,lossofdryingbygravimetricmethodanddetermination
ofextractivecontent.
Evaluationofanalyticmethodologybyspectrophotometry
Throughthetechniqueofdeterminationwithoutacid hydroly-sis(Petryetal.,1998;Silvaetal.,2009;Marquesetal.,2012),the sampleofrawmaterialwassubjectedtoextractionunderreflux (7.5%,w/v)andwasheldinadilutionof3:50mlofstocksolutionin waterdistilled;then,inflasksof20mlaliquotswereaddedto3.2ml ofthedilutedsolutionand1.6mlofAlCl3(2.5%and7.5%)inorderto assesswhatwouldbetheidealconcentrationofAlCl3necessaryfor chelatingflavonoidslikequercetinfoundintheextractivesolution ofL.sidoides.Then,theballoonswerefilledwithethanol(40%)and
30minafterthereadingswereperformedina
spectrophotome-terat423nm(maximumofAlCl3-quercetincomplexed).Giventhe optimalconcentrationofAlCl3 tocomplexationwasperformeda readingofscanningtheextractivesolutionbeforeandafter
com-plexationwhich comprised a range of200–800nm inwhich it
wasintended to strengthen the displacement of bathochromic
flavonoidsolution.
Validationmethodology
Amethodologytodetermineandquantifytheflavonoidsinraw materialmustbevalidatedconsideringthefollowingparameters: specificity,linearity,precision,accuracy androbustness(Anvisa, 2003).
Thestatisticanalysisandexperimentaldesign
Theexperimentalmatrixwasa23factorialdesignanditwas usedtoevaluatetheinfluenceofplantproportions(5.0/7.0%;w/v), solventtype(water/ethanol50%)andtheextractionmethod (infu-sion/decoction)ontheefficiencyoftheextractionofflavonoids.The experimentswereperformedintriplicate,andthetotalflavonoids content(TFC)wasusedasresponses.Thestatisticalanalysisofthe factorialdesignwasperformedusingthesoftwareStatistica®
6.0
(StatSoft,USA).TheexperimentaldatawereanalyzedbyANOVA
andt-testforstandardizedeffects(MyersandMontgomery,1995).
HPLCanalysisofextractivesolution
PreparationofL.sidoidesextracts
Dryextract(100ml)obtainedbydecoction(7.5%,w/vin15min) wasdilutedwithmethanol/milli-Qwater(50:50,v/v)toachievea concentrationof10g/ml.ForinjectionintheHPLC,thesolutions
werefilteredina0.44m(regeneratedcellulose)membrane.
AnalyticalandpreparativeHPLC
TheHPLCanalysiswasperformedonaShimadzusystem
con-sisting of a degasser DGU-20A3, a SIL-20A autosampler, two
LC-20AD pumps and a SPDM20Avp photodiode array detector
(DAD),coupledwithaCBM20Ainterface.Beforeinjectionintothe HPLC,thesolutionswerefilteredina0.44m(regenerated
cellu-lose)membrane.Theanalyticalmethodemployedalineargradient systemwhich consisted of (A) acetic acid:water 1.0%(v/v); (B)
methanol.Thechromatographic separation hasbeenperformed
usingaPhenomenexLunaC18analyticalcolumn250mm×4.6mm
(5mmparticlesize).Theflowratewas0.6ml/minandtheinjection volumewas20l.Themobilephaseconsistedofgradientofwater
andmethanolstartingwith10%Bduring20min;40–45%Bduring 5min;45–60%Bduring10min;60–75%Bduring25mintotaltime of60min.Photodiodearraydetectorwassetat254nmfor acquir-ingchromatograms.Theanalysisofpeakswasbasedinaccordance withtheretentiontimeofthestandardsubstanceandmass
spec-trum(MS/MS).Massspectrometricanalysiswasperformedona
Bruckermassspectrometerfittedwithiontrapionizationsource. Thenegativeionmode[m/zM−H]wasusedforallcompounds.Ion trapionizationwasoperatedinMRMandconditionswere: nebu-lizerpressure:40psi;drygasflow:9l/min;dryinggastemperature: 300◦C;flowrate:200l/min.
Redoxactiveprofile
Totalantioxidantpotential(TRAP)andtotalantioxidantreactivity (TAR)
TRAP/TAR was determined by measuring the
chemi-luminescence (CL) intensity of luminol induced by 2,2′-azobis (2-amidinopropan) dihydrochloride (AAPH) (Lissi et al., 1995).
ThebackgroundCLwasmeasuredbyaddingAAPHandluminol.
Then,thesamples(OELSfrom1ngml−1to1mgml−1)wereadded,
and the CL was measured in a liquid scintillator counter. The
lastcountbeforetheadditionsampleswasconsideredas100%. Graphswereobtainedbyplottingpercentageofcountspermin(% cpm)versustime(s).TheAUC(TRAPassay)wascalculatedusing GraphPadPrismsoftware.TheTARwascalculatedastheratioof lightintensityinabsenceofsamples(Io)/lightintensityrightafter OELSaddition(I).
TBARS(ThiobarbituricAcidReactiveSpecies)
TBARSassaywasemployedtoquantifylipidperoxidationand
anadaptedTBARSmethodwasusedtomeasuretheantioxidant
at1200gfor10min.Analiquotof0.5mlfromthesupernatantwas mixedwith0.5mlTBAandheatedat95◦Cfor30min.After cool-ing,samplesabsorbancewasmeasuredusingaspectrophotometer at532nm.TheresultswereexpressedasapercentageofTBARS formedbyAAPHalone(inducedcontrol).
Hydroxylradical(•OH−)scavengingassay
HydroxylradicalsweregeneratedbyaFentonsystem(FeSO2 -H2O2).Whenexposedtohydroxylradicals,thesugardeoxyribose isdegradedtomalonaldehyde(MDA),whichgeneratesapink chro-mogenonheatingwithTBAatlowpH.Themethodfordetermining thescavengingonhydroxylradicalswasperformedaccordingtoa previouslydescribedprocedure.
Nitricoxide(NO)scavengingassay
Nitricoxidewasgenerated fromspontaneousdecomposition
ofsodiumnitroprusside(SNP)inthephosphatebuffer(pH7.4). OncegeneratedNOinteractswithoxygentoproducenitriteions, whichweremeasuredbytheGriessreaction.Thereactionmixture containingSNPinphosphatebufferandOELSatdifferent concen-trationswereincubatedat37◦Cfor1h.Analiquotwastakenand homogenizedwithGriessreagent.Theabsorbanceofchromophore wasmeasuredat540nm.Percentinhibitionofnitricoxide gener-atedwasmeasuredbycomparingtheabsorbancevaluesofnegative controls(SNPandvehicle)andassaypreparations.
Resultsanddiscussion
Characterizationoftheplantmaterial
TheplantmaterialofL.sidoidesusedinthisresearchpresented particlemeandiameterof325m,lossondryingof11.25%and
extractivecontentof19.83%.Theseproprietiesareimportantfor standardizationofextractiveprocess,sinceparticlesmean diam-eterinfluencestheextractionefficiencyandthelossondryingis importanttoconservationofrawmaterial(ListandSchimdt,1989).
Developmentofanalyticmethodologybyspectrophotometry
Thealuminumcationformsstablecomplexeswithflavonoids, whichleadstoadeviationofthepeakabsorptiontolonger wave-length.Thusitispossibletodeterminetheamountofflavonoids, avoidingtheinterferenceofotherphenolicsubstances,mainly phe-nolicacids,whichinvariably accompanytheflavonoidsinplant tissues.Thisreadingisdoneinspectrophotometerat425nm,using aluminumchlorideto2%.Therewasnostatisticallysignificant dif-ferenceinamplitudeofthepeakabsorptionoratthetimethatthis pointhasbeenreachedregardingtheconcentrationofAlCl3was used.
Validationofthemethodology
AccordingtoBrazilianHealthAgency(Anvisa,2003),the pur-poseofvalidationistodemonstratethatthemethodissuitable for their intended purpose, and to identify qualitative, semi-quantitativeand/orquantitiesof drugsand othersubstances in
pharmaceuticals. Thus, the method is validated when has the
specificity,linearity,precision,accuracyandrobustnesswithinthe parametersrequired.
Thespecificityofmethodwasdemonstratedacrossthe spec-trumscanningoftheextractivesolutionfromL.sidoides(7.5%,w/v) andthestandardquercetin(50.0g/ml),bothcomplexedwith
alu-minumchloride(2.5%),onlyonepeakbeingshownofmaximum
4.500
4.000
2.000
–2.000
–4.000
–4.500
200.00 400.00 600.00
L.sidoides
Quercetin
nm.
Abs.
800.00 000
Fig.1. Spectrumscanningof theextractivesolution fromL. sidoidesandthe quercetinstandard.
absorptionat423nmfor quercetinandotherat405nmfor the extractivesolution(Fig.1).
Thelinearitywasobtainedthroughofthecalibrationcurveof quercetindilutedinethanol(40%).Thiscurvewasmadewithfive differentconcentrations:10,20,30,40and50g/ml.The
param-eterlinearityisveryimportantbecauseitdemonstratesthatthe resultsobtainedaredirectlyproportionaltotheconcentrationof analyteinthesample,withinaspecifiedrange.
Thetestwasmadeintriplicateandtheresultsofthestatistical analysisareshowedinTable1.Theequationofthelineobtained wasy=0.01467x+0.3062,theaccuracyofthedeterminationswas between102.10and101.11%(Table1)andthedetermination coef-ficientobtainedwasgreaterthan0.99(r2=0.9992)whichiswithin theparametersofexistinglegislation(Anvisa,2003).
Theprecisionwasobtainedattwolevels:repeatability,which
evaluated the concordance betweenthe results within a short
periodoftimewiththesameanalystandsameinstrumentation. Thislevelwascarriedintriplicatewithsixdeterminationsof100% oftheconcentrationofthetest;andthesecondlevelwasthe
inter-mediate precision,which analyzes the correlationbetween the
resultsfromthesamelaboratory,obtainedindifferentdayswith differentanalystsperformedinduplicatewithsixdeterminations. Theresultsofthestatisticalanalysisoftheprecisiontestareshown inTable2.
Theaccuracyobtainedasindicatedbytheresolutionforthis testwasthusappliedintheanalyticalmethodologyproposedin theanalysisofasubstanceofknownpurity;inlow(22.5g/ml),
medium(45g/ml)andhigh(65g/ml)concentrationaccording
totheminimumandmaximumvaluesonthecurvealltheresults areshowninTable3.
The robustness was evaluated by variation of temperature
between25and35◦Candthesolutionconcentrationofaluminum
Table1
Resultsofthestatisticalanalysisofthelinearitytest.
TC C SD RSD A
10 10.2152 1.723 1.68 102.10 20 20.4642 2.351 1.15 102.32 30 30.6136 2.093 0.68 101.65 40 40.6821 1.254 0.28 101.90 50 50.5461 2.372 0.46 101.11 TC(g/ml),theoreticalconcentration;C(g/ml),meanconcentrationofthree
Table2
Resultsofthestatisticalanalysisoftheprecisiontest.
Test TC C SD RSD A
Repeatability 50.0 50.47 2.45 1.09 99.59 50.0 50.91 1.75 0.38 100.32 50.0 49.97 2.12 0.32 100.21 Intermediate
precision
Analyst1 44.0 44.05 2.11 0.47 100.76 Analyst2 44.0 44.16 2.09 0.47 100.32 TC(g/ml),theoreticalconcentration;C(g/ml),meanconcentrationofthree
deter-minations;SD,standarddeviation;RSD(%),relativestandarddeviation;A(%), accuracy.
Table3
Resultofthestatisticalanalysisoftheaccuracytest.
Test CL TC C SD RSD A
Accuracy Low 22.5 22.475 2.45 1.09 99.89 Medium 45.0 44.912 1.75 0.38 99.80 High 65.0 64.978 2.12 0.32 99.95 CL,concentrationlevel;TC(g/ml),theoreticalconcentration;C(g/ml),mean
concentrationofthreedeterminations;SD,standarddeviation;RSD(%),relative standarddeviation;A(%),accuracy.
chlorideinmethodbetween2.5and7.5%.Theanalysesweredonein triplicate.Themethodwasrobustforthevariationoftemperature andsolutionconcentrationofaluminumchloride.
Experimentaldesign
Theresultsforthetotalflavonoidscontentofeachextractive solutionarepresentedinTable4.Thestandardizedeffectsofeach main factor as wellas respectiveinteractions are presented in Table5.Regarding thestatisticalanalysisof experimentaldata, themostimportanteffectcouldbeattributedtothefactorSolvent, whichprovidesmoreefficientextractiveprocedurewhenethanol 50%(v/v)wasusedassolvent.Accordingtot-test,thesecondmain factorwasthedrugproportion.Nostatisticallysignificanteffect couldbeimputedtoextractionmethods(infusion/decoction).On theotherhand,theinteractionsofextractionmethodwereableto provideimprovementsontheextractiveefficiencyeitherwith Sol-ventorPlantproportion.Theexpectedimprovementsintheyield ofTFCduetoincreasingdrugamountaswellasthepositiveeffect ofethanol:watermixtureundergoextraenhancedeffectderived frommethodofextraction.Althoughnosignificanteffectswere observedfortheMethodofExtraction,theinfluenceof maintain-ingthetemperaturebydecoctionseemstobeessentialtopromote betterwettabilityoftherawmaterialandhigherdiffusivityofthe solvent.
SeparationpolyphenolsbyHPLC
Photodiodearraydetectorwassetat254nmforacquiring
chro-matograms.ThechromatogramL.sidoidesextract showedthree
Table5
Statisticalanalysisofexperimentaldata:standardizedeffects(mainfactorsand interactions).
Standardizedeffect t-Test ANOVA(F) Mean/interc. 14.64 53.11* –
(1)Method 1.014 1.84 3.38
(2)Plant(%) 2.69 4.88* 23.85*
(3)Solvent 4.37 7.92* 62.67*
1by2 1.33 2.42* 5.83*
1by3 1.41 2.57* 6.58*
2by3 1.00 1.82 6.30
*˛=0.05.
majorpeakswellseparated:P1(R.T.21min),P2(R.T.34min)and P3(R.T.41min).P1wasidentifiedaschlorogenicacid(Fig.2).P2 andP3 werenot identifiedbut exhibitedUV spectrapattern of caffeoylquinicacidderivatives,indicatingthatthisclassof polyphe-nolsare themajorconstituents inthe extract.P1 had itsmass
spectrum analysis and Showed MS 353 m/z and MS2 191 m/z
(chlorogenicacidmolecule).
Redoxactiveprofile
TheTRAPandTARmethodsarewidelyemployedtoestimatethe generalantioxidantcapacityofsamplesinvitro.Thus,thegeneral antioxidantpotentialofextractfromL.sidoideswasfirstevaluated bytheTRAP/TARassays.TheTRAP/TARassaysindicatethat opti-mizedextractivesolutionofL.sidoides(OELS)presentsasignificant antioxidantactivityattheallconcentrationsstudied(Fig.3).
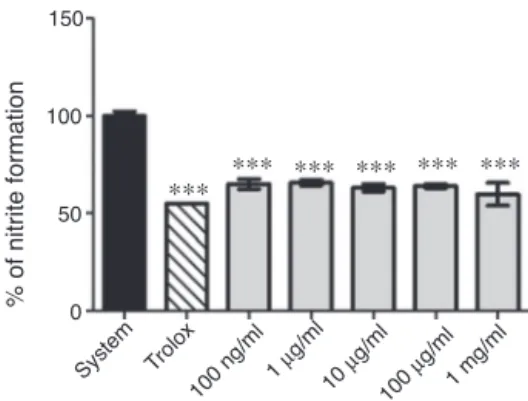
Lipidperoxidation(LPO)hasbeendefinedasthebiological dam-agecausedbyfreeradicalsthatareformedunderoxidativestress (Zinetal.,2002).Severalplantextractshavebeenshowntoinhibit LPOasmeasuredbythelevelsofTBARS.Thelipidsinmembrane arecontinuouslysubjectedtooxidantchallenges.Oxidantinduced abstractionofahydrogenatomfromanunsaturatedfattyacylchain ofmembranelipidsinitiatestheprocessofLPO,whichpropagates asachainreaction.Intheprocess,cyclicperoxides,lipid peroxi-desandcyclicendperoxidesaregenerated,whichultimatelyare fragmentedintoaldehydeslikeMDA.Similarly,theOELSwereable topreventlipoperoxidationinducedbyAAPHinvitroina lipid-enriched system(Fig.4).TheOELSat 100ngml−1 to1mgml−1 showedantioxidantactivityagainsthydroxylradicals(Fig.5).To determinetheabilityofextracttoactasareactivenitrogenspecies (RNS)scavenger,weevaluatedtheNO-scavengingactivitybythe Griessmethodand OELSat100ngml−1 to1mgml−1 showeda significant(p<0.05)NO-scavengingactivity(Fig.6).
The resultsfoundin this studyare in agreementwith both observationsandsuggestadirectcorrelationbetweenantioxidant activityandpolyphenolcontent.Itisprobablethattheactive prin-cipleresponsiblebytheredoxactivityinthisworkisthepolyphenol content.Severalstudieshaveshownthattheredoxactivity asso-ciatedwithnaturalantioxidantsisattributedtothetotalcontent
Table4
Matrixofthefactorialdesign23.
Exp Codedvariable Naturalvariable TFT(g/ml)
Method Plant Solvent Method Plant(w/v) Solvent
1 +1 +1 −1 Decoction 7.5 Water 13.043
2 +1 +1 +1 Decoction 7.5 EtOH50% 21.280
3 +1 −1 +1 Decoction 5.0 EtOH50% 14.798
4 +1 −1 −1 Decoction 5.0 Water 11.476
5 −1 +1 −1 Infusion 7.5 Water 13.568
6 −1 +1 +1 Infusion 7.5 EtOH50% 16.064
7 −1 −1 +1 Infusion 5.0 EtOH50% 15.157
8 −1 −1 −1 Infusion 5.0 Water 11.752
65.0
254nm, 4nm (1.00)
A
62.0 60.0 57.5 55.0 52.5 50.0 47.5 45.0 42.5 40.0 37.5 35.0 32.5 30.0 27.5 25.0 22.5 20.5 17.5 15.0 12.5 10.0 7.5 5.0 2.5 0.0
0.0 5.0 10.0 15.0 20.0 25.0 30.0 P1
P3 P2
35.0 40.0 45.0 50.0 55.0 min mAU
950 900 850 800 750
650
550
450
350
250
150 100 50 0 200 300 400 500 600 700
254nm, 4nm (1.00)
B
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 50.0 55.0 min mAU
Fig.2.(A)ChromatogramofextractivesolutionofL.sidoides.(B)Chromatogramofthereferencesubstance(chlorogenicacid).
30 0000
A
20 0000
10 0000
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗∗∗
TRAP
(AUC arbitary units)
0
System Trolox
100 ng/ml 1 1 mg/ml µg/ml
10 µ g/ml
100 µg/ml
100
80
60
40
20
B
∗∗∗
∗∗∗
∗∗∗
∗∗∗
T
AR (Io/I)
0
System Trolox
100 ng/ml 1 1 mg/ml µg/ml
10 µ g/ml
100 µg/ml
Fig.3.(A)TotalRadical-TrappingAntioxidantParameter(TRAP)atdifferent con-centrations.Valuesrepresentmean±S.E.D.,experimentsintriplicate,***p<0.001 differentfromsystemandtrolox(ANOVAfollowedbyTukey).(B)Thetotal antiox-idantreactivity(TAR)wascalculatedastheratiooflightintensityinabsenceof samplesexpressedaspercentofinhibition(I0/I).Valuesrepresentmean±S.E.D.,
experimentsintriplicate,***p<0.001differentfromsystemandtrolox(ANOVA followedbyTukey).
100
50
0 150
System Trolox
100 ng/ml 1 1 mg/ml µg/ml
10 µ g/ml
100 µg/ml
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗
TBARS
(% AAPH-induced
damage)
Fig.4. ThiobarbituricAcidReactiveSpecies(TBARS)wasevaluatedfromL.sidoides
(100ngml−1to1mgml−1).Valuesrepresentmean
±S.E.D.,experimentsin tripli-cate,ANOVAfollowedbyTukey,*p<0.05;***p<0.001differentfromsystem.
100
50
0 150
System Trolox
100 ng/ml 1 1 mg/ml µg/ml
10 µg/ml
100 µg/ml
∗∗∗
∗∗
∗∗
∗∗∗
∗∗∗
∗∗
% of hydroxyl
Fig. 5.Hydroxyl radical-scavenging activity from L. sidoides (100ngml−1 to
1mgml−1).Valuesrepresentmean
100
50
0 150
System Trolox
100 ng/ml1 1 mg/ml µg/ml
10 µg/ml
100 µg/ml
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗∗∗
∗∗∗
% of nitrite formation
Fig.6. Nitricoxide (NO) scavenging activity from L.sidoides (100ngml−1 to
1mgml−1).Valuesrepresentmean±S.E.D.,experimentsintriplicate,ANOVA
fol-lowedbyTukey*p<0.05;***p<0.001differentfromsystem(SNP).
ofphenoliccompounds(Halliwell,2008;Rice-Evansetal.,1997; Scalbertetal.,2005;Serafinietal.,2011).
Conclusions
Theanalysisof datafromthis studyconfirmedtheability of
thedeveloped methodforevaluatingthepolyphenolcontentin
extractsofL.sidoidesandtheanalysisoffactorialdesignshowed thattheoptimumconditiontopreparetheextractivesolutionswith maximumtotalflavonoidcontent,foundextractionbydecoction, using7.5%(w/v)ofplantandethanol50%(v/v)astheextractive solvent.Concluding,thedatepresentedhereinindicatesthattheL. sidoidesextracthaveinvitroantioxidantactivityandshouldbe con-sideredasnewsourcesofnaturalantioxidantsjointlywithother phenolicrichplants.Furtherstudiesareneededtoexaminethe potentialuseoftheseextractsinthepreventionortreatmentof
pathologies where oxidativestress seemstoplay an important
role.
Authors’contributions
BSLandCSRcontributedwithchromatographicanalysis, fac-torialdesignandwritingofthemanuscript.JPAS,TKRandCASS contributedwiththeredoxactiveprofile.MRS,LALS,LJQJ,JCFM, DPG,AASAandFASdesignedthestudy,supervisedthelaboratory work,contributedtocriticalreadingofthemanuscript,writingof themanuscriptandfinaleditingofthemanuscript.Alltheauthors havereadthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors are grateful to CAPES, CNPq, FACEPE/PE and
FAPITEC/SEforfinancialsupportandfellowships.
References
Audi,E.A.,Campos,E.J.V.,Rufino,M.,Cortez,D.G.,Bersani-Amado,C.A.,Soares, L.A.L.,Petrovick,P.R.,Mello,J.C.P.,2001.PetiveriaalliaceaL.:plantdrug qual-itycontrol,hydroalcoholicextractstandardizationandpharmacologicalassay oflyophilizedextract.ActaFarm.Bonaer.20,225–232.
Anvisa,2003.Resolutionn◦899,2003,AgênciaNacionaldeVigilânciaSanitária.
MinistériodaSaúde,Brasília,DF.
Canteri-Schemin,M.H.,Fertonani, H.C.R.,Waszczynskyj,N.,Wosiacki,G.,2005. Extractionofpectinfromapplepomace.Braz.Arch.Biol.Technol.48,259–266. Cunha,F.P.,Costa,L.J.L.,Fernandes,A.J.D.,Souza,T.P.,Soares,L.A.L.,2009. Develop-mentandoptimizationofextractivesfromAstroniumurundeuva(allemão)Engl. byfactorialdesign.Braz.Arch.Biol.Technol.52,23–29.
Halliwell,B.,2008.Arepolyphenolsantioxidantsorpro-oxidants?Whatdowelearn fromcellcultureandinvivostudies?Arch.Biochem.Biophys.476,107–112. Lacoste,E.,Chaumont,J.P.,Mandin,D.,Plumel,M.M.,Matos,F.J.,1996.Antiseptic
propertiesofessentialoilofLippiasidoides(Cham).applicationtothecutaneous microflora.Ann.Pharm.Fr.54,228–230.
Lemos,T.L.,Craveiro,A.A.,Alencar,J.W.,Matos,F.J.,Clarck,A.M.,MacChesney,J.D., 1990.AntimicrobialactivityofessentialoilofBrazilianplants.Phytother.Res. 4,82–84.
Lissi,E.,Salim-Hanna,M.,Pascual,C.,DelCastillo,M.D.,1995.Evaluationoftotal antioxidantpotential(TRAP)andtotalantioxidantreactivityfrom luminol-enhancedchemiluminescencemeasurements.FreeRadic.Biol.Med.2,153–158. List,P.H.,Schimdt,P.C.,1989.PhytopharmaceuticalTechnology.CRCPress,Florida. Marques,G.S.,Monteiro,R.P.M.,Leão,W.F.,Lyra,M.A.M.,Peixoto,M.S.,Rolim-Neto, P.J.,Xavier,H.S.,Soares,L.A.L.,2012. Evaluationofproceduresfor spectro-photometricquantificationoftotalflavonoidsinleavesofBauhiniaforficata. Quím.Nova35,517–522.
Matos,F.J.A.,2000.PlantasMedicinais.ImprensaUniversitária,Fortaleza. Montgomery,D.C.,1997.DesignandAnalysisofExperiments.Willey,NewYork. Myers,R.H.,Montgomery,D.C.,1995.ResponseSurfaceMethodology:Processand
ProductOptimizationUsingDesignedExperiments.Willey,NewYork. Petry,R.D.,DeSouza,K.C.B.,Bassani,V.L.,Petrovick,P.R.,González,O.G.,1998.
Dosea-mentodoteordeflavonóidestotaisemextratoshidroalcoólicosdePassiflora alataDryander(maracujá).Rev.Bras.Farm.79,7–10.
Rice-Evans,C.A.,Miller,N.J.,Paganga,G.,1997.Antioxidantpropertiesofphenolic compounds.TrendsPlantSci.2,152–159.
Scalbert,A.,Manach,C.,Morand,C.,Rémésy,C.,2005.Dietarypolyphenolsandthe preventionofdiseases.Crit.Rev.FoodSci.Nutr.45,287–306.
Serafini,M.R.,Santos,R.C.,Guimarães,A.G.,DosSantos,J.P.,DaConceicão,A.D.S., Alves,I.A.,Gelain,D.P.,DeLima,P.C.N.,Quintans-Júnior,L.J.,Bonjardim,L.R., Araújo,A.A.S.,2011.MorindacitrifoliaLinnleafextractpossessesantioxidant activitiesandreducesnociceptivebehaviorandleukocytemigration.J.Med. Food14,1–8.
Silva,I.V.,Ferreira,M.S.,Wanderley,A.G.,Fernandes,M.J.B.C.,Soares,L.A.L.,DeSouza, T.P.,2007.Theinfluenceofextractiveparametersonthepreparationofasolution fromPsidiumguajavaL.ActaFarm.Bon.28,116–120.
Silva,K.G.H.,Xavier-Júnior,F.H.,Farias,I.E.G.,Silva,A.K.A.,Caldas-Neto,J.A.,Souza, L.C.A.,Santiago,R.R.,Alexandrino-Júnior,F.,Nagshima-Júnior,T.,Soares,L.A.L., Santos-Magalhães,N.S.,Egito,E.S.T.,2009.Stationarycuvette:anewapproach toobtaininganalyticalcurvesbyUV–VISspectrophotometry.Phytochem.Anal. 20,265–271.
Soares,L.A.L.,González,O.G.,Petrovick,P.R.,Schmidt,P.C.,2005.Optimizationof tabletscontainingahighdoseofspray-driedplantextract:atechnicalnote. AAPSPharmSciTech6,368–371.