w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Brief
communication
Rituximab
use
in
young
adults
diagnosed
with
juvenile
idiopathic
arthritis
unresponsive
to
conventional
treatment:
report
of
6
cases
Ana
Paula
Sakamoto
a,
Marcelo
M.
Pinheiro
a,
Cássia
Maria
Passarelli
Lupoli
Barbosa
b,
Melissa
Mariti
Fraga
a,
Claudio
Arnaldo
Len
a,
Maria
Teresa
Terreri
a,∗aUniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
bHospitalInfantilDarcyVargas,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10July2014 Accepted24December2014 Availableonline20July2015
Keywords:
Juvenileidiopathicarthritis Children
Rituximab Refractoriness
a
b
s
t
r
a
c
t
Juvenileidiopathicarthritis(JIA)isthemostcommonrheumaticdiseaseinchildhood. With-outaneffectivetherapy,patientsmayprogressquicklytofunctionaldisability.Recently, depletionofBcellsemergedasanewapproachforthetreatmentofautoimmunediseases, includingJIA.
WedescribesixcasesofJIApatientsfollowedatareferralcenterforRheumatologyand PediatricRheumatology,submittedtotreatmentwithrituximab(RTX)afterrefractoriness tothreeanti-TNFagents.
PatientsreceivedRTXcycleswithtwoinfusionseverysixmonths.Responsetotreatment wasassessedbyDAS28,HAQ/CHAQ,andanoverallassessmentbythedoctorandthepatient. Ofoursixpatients,fourweregirls(meanageatonsetofdisease:6.1years;meandisease evolutiontime:15.1years;meanageuponreceivingRTX:21.6years).Fourpatientsbelonged topolyarticularsubtype(1rheumatoidfactor[RF]-negative,3FR-positive),apatientwith systemicJIAsubtypewithapolyarticularcourseandarthritisrelatedtoenthesitis.Ofour sixpatients,fiverespondedtotreatment;andduringthecourseof12months,theclinical responsewasmaintained,althoughnotsustained.However,discontinuationbyinfusion reactionscausedthewithdrawalofRTXintwopatients.
TheuseofRTXinJIAisrestrictedtocasesrefractorytootherbiologicalagentsand,even consideringthatthisstudywasheldinasmallnumberofadvancedpatients,RTXproved tobeaneffectivetherapeuticoption.
©2015ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor.
E-mail:teterreri@terra.com.br(M.T.Terreri). http://dx.doi.org/10.1016/j.rbre.2015.05.002
Uso
de
rituximabe
em
adultos
jovens
com
diagnóstico
de
artrite
idiopática
juvenil
refratária
ao
tratamento
convencional:
relato
de
6
casos
Palavras-chave:
Artriteidiopáticajuvenil Crianc¸as
Rituximabe Refratariedade
r
e
s
u
m
o
Aartrite idiopáticajuvenil(AIJ)éadoenc¸areumáticamaisfrequentenainfância.Sem terapia efetiva, os pacientes podem evoluir rapidamentepara incapacidadefuncional. Recentemente,adeplec¸ãodoslinfócitosBsurgiucomonovaabordagemparaotratamento dedoenc¸asautoimunes,incluindoaAIJ.
DescrevemosseiscasosdepacientescomAIJ,acompanhadosemumcentrodereferência emReumatologiaeReumatologiaPediátrica,submetidosaotratamentocomrituximabe (RTX)apósrefratariedadeatrêsanti-TNF.
OspacientesreceberamciclosdeRTXcomduasinfusõesacadaseismeses.Arespostaao tratamentofoiavaliadapeloDAS28,HAQ/CHAQ,avaliac¸ãoglobaldomédicoedopaciente. Dosseispacientes,quatroerammeninas(médiadeidadedeiníciodadoenc¸a:6,1anos; médiadetempodeevoluc¸ãodedoenc¸a:15,1anos;médiadeidadeaoreceberRTX:21,6anos). Quatropacientespertenciamaosubtipopoliarticular(1fatorreumatoide(FR)negativo,3FR positivo),umpacientecomAIJsubtiposistêmicocomevoluc¸ãopoliarticulareumcomartrite relacionadaàentesite.Dosseispacientes,cincoresponderamaotratamentoedurantea evoluc¸ãode12meses,arespostaclínicafoimantida,emboranãosustentada.Noentanto,a descontinuac¸ãoporreac¸õesinfusionaismotivaramasuspensãodoRTXemdoispacientes. OusodoRTXemAIJérestritoaoscasosrefratáriosaoutrosbiológicose,mesmotendo sidorealizadaemumnúmeropequenodepacientesedeformatardia,mostrouseruma opc¸ãoterapêuticaeficaz.
©2015ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Juvenileidiopathicarthritis(JIA)isthemostcommonchronic rheumaticdiseaseinchildhoodandwithoutappropriate treat-mentitcanquicklyresultinfunctionaldisability.1
Recently,Blymphocytedepletionemergedasanew ther-apeutic approach for JIA and SLE.2–7 Rituximab (RTX) is a
chimericmonoclonalantibodydirectedagainsttheCD20 pre-Bcellsand matureBcellswithefficacyand safetyinadult patientswithrheumatoid arthritis(RA)withaninadequate responsetodisease-modifyingdrugs(DMARDs)and anti-TNF-alphainhibitors.8–12
Theobjectiveofthisstudywastodescribesixpatientswith JIAfollowedinaPediatricRheumatologyUnitbetween1993 and2014whounderwenttreatmentwithRTX.
Methods
SixpatientsdiagnosedwithJIAaccordingtotheILAR (Inter-nationalLeagueofAssociationsofRheumatology)criteria13
receivedRTXcycleswithtwointravenousinfusionsof1geach ondays1and15,everysixmonths.
Theclinical response was measured byDAS28 (Disease ActivityScorein28joints),14erythrocytesedimentationrate
(ESR),HealthAssessmentQuestionnaire(HAQ)15ortheChild
HealthAssessmentQuestionnaire(CHAQ)16andevaluationof
visualanalogscale(VAS)ofthepatientandthephysician. The assessments were performed before and every six monthsoftreatment.AccordingtoEULAR(EuropeanLeague AgainstRheumatism)criteria,thepatientswereclassifiedas
goodrespondersifimprovementinDAS28wasgreaterthan 1.2, moderate responders ifbetween 0.6and 1.2,and non-respondersiflessthan0.6,intwoconsecutivemeasurements. ClinicalremissionwasdefinedasaDAS28lessthan2.6.17
Primary failure was defined as a reduction lower than 0.6ofDAS28after12 weeksand secondaryfailure,suchas lossofefficacyover24weeksinpatientswhohadresponded inthefirst12weeks.17Adverseeventswererecorded.
Case
report
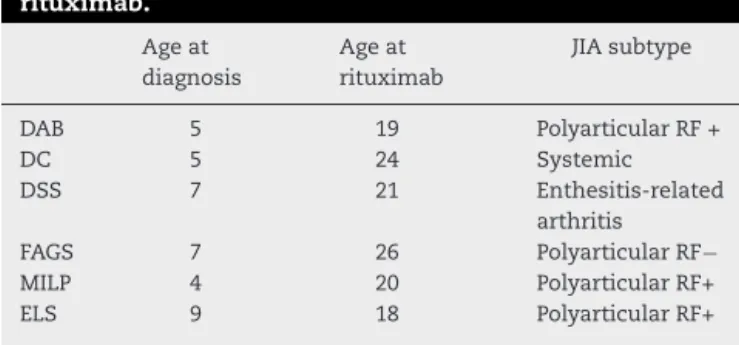
Themeanonsetageofthediseasewas6.1yearsandmean diseasedurationwas15.1years.ThemeanageatstartofRTX was21.6years(18–26years)(Table1).Uveitiswasnotobserved inanypatient.
Table1–Clinicaldataof6patientstreatedwith rituximab.
Ageat diagnosis
Ageat rituximab
JIAsubtype
DAB 5 19 PolyarticularRF+
DC 5 24 Systemic
DSS 7 21 Enthesitis-related
arthritis
FAGS 7 26 PolyarticularRF−
MILP 4 20 PolyarticularRF+
Case1
DAB,19yearsold,female,erosivepolyarticularJIAdiagnosed sincethe ageoffive,positiverheumatoid factor(RF),being followedsincetheageof11.
Thepatienthastakenglucocorticoids(GCs),methotrexate (upto1mg/kg/week)forfiveyears,cyclosporine(5mg/kg/day) for five months and cyclophosphamide (up to 1g/m2) for
three months with partial response to all treatments. She initiated treatment with infliximab (5mg/kg every six weeks)fortwoyears,followedbyetanercept(0.8mg/kg/week) for four months and adalimumab (24mg/m2) for four
years.
Duetorefractorinessandintensediseaseactivity,RTXwas introduced at19 years ofage, combined with leflunomide (20mg/day)andprednisoneataninitialdoseof20mg,with progressivetaperinguptowithdrawal.
After6monthsofthefirstintravenousinfusion,thepatient presentedclinicalimprovement.Currently,thepatientis clin-icallystable,withnoarthritisandnomorningstiffness.She remainsonleflunomide,andiscurrentlyreceivingthefourth cycleofRTX.
Case2
DC, 25 years, male, systemic onset JIA and polyarticular course,negativeRF,diagnosedsincethe ageoffive,started follow-upatthisclinictwoyearslater.
Initially, the patientwas onindomethacin (2mg/kg/day) and methotrexate (1mg/kg/week) associated with GCs (up to1mg/kg/day).Whenhewas14,hepresentedmacrophage activation syndrome, with pulse therapy being performed with intravenous methylprednisolone, and cyclosporine being started (5mg/kg/day). After two years thalidomide (2.5mg/kg/day)wasinitiated,withpartialresponse.Whenhe was19,hestartedinfliximab(5mg/kgevery6weeks), develop-inganaphylaxisinthe9thdose.Heswitchedtoadalimumab (24mg/m2)andetanercept(0.8mg/kg/week),withsecondary
failureafter9and10months,respectively.
RTX was started at 24 years old, presenting sustained response,althoughwithinfusionreactionscontrolledwiththe useofintravenousGC,antihistaminesandadecreasein infu-sionrate.Hewasconcomitantlyoncyclosporine(150mg/day) andprednisone(20mg/day).However,afterthefourthcycleof RTX,thepatientdevelopedserumsickness,anddiscontinued thetreatment.
After stopping RTX, the disease became more active, requiringother medications and he hadprimary failure to tocilizumab(8mg/kg/dose)andabatacept(10mg/kg/dose)and was referred to autologous bone marrow transplantation (ABMT).
Case3
DSS, 26, male, diagnosed with enthesitis-related arthritis (ERA)JIAsincetheageofseven.He beganfollow-upwhen hewas13yearsold.Theevolutionwaspolyarticularerosive, associatedwithsevereintestinalinvolvement,withCrohn’s diseasebeingdiagnosed.
Hetookmethotrexate(upto1mg/kg/week)for10years, sulfasalazine(40mg/kg/day)forfiveyears,plus methylpred-nisolone and cyclophosphamide intravenous pulsetherapy (upto1g/m2)foroneyear,andmultiplejointinjectionswith
GCs.Hestartedinfliximab(5mg/kg/doseevery6weeks)with goodinitialresponseandfailureaftertwoyearsoftreatment. Heusedcyclosporine(5mg/kg/day)andmethylprednisolone pulsetherapy, withno responseforoneyear.Adalimumab (24mg/m2)wasintroducedbuttherewasnoresponseafter
fourmonthsoftreatment(primaryfailure).
When he was 21,he received the 1st cycle of RTX. He showedsignificantjointimprovementafter12months. How-ever,ithadtobediscontinuedduetorecurrenceoffistulizing intestinaldisease.Althoughhepreviouslyhadsecondary fail-uretoIFX,thiswasreintroducedcombinedwithmethotrexate (25mg/week)andprednisone(20mg/day).However,dueto dis-easeactivityandrefractoriness,ABMTwasindicated.
Case4
FAGS, 32, female, erosive polyarticular JIA diagnosedsince sevenyearsofage,negativeRF,followedsinceshewas15years old.
She presented polyarthritis of small and large joints, withgood responsetotreatment withmethotrexate(upto 1mg/kg/week)andtapereddosesofGCsinthefirstyear.She remained asymptomatic until shewas 20 years old, when arthritisreappeared.
Inthe firstyearofrecurrence,the patientwasrestarted on methotrexate (25mg/week) and prednisone (20mg/day). Duetoelevatedtransaminaselevels,thedoseofmethotrexate was reduced (15mg/week) and sulfasalazine(40mg/kg/day) and chloroquine(5mg/kg/day)were associated.At24 years old,leflunomide (20mg/day)wasstarted,but therewas no response aftertwelvemonths. Shepresented primary fail-ure tothethreeanti-TNFblockers,and RTXwasstartedat theageof26.Concomitanttotreatment,thepatientreceived methotrexate(25mg/week) and prednisone(10mg/day).Six monthsafterthefirstintravenousinfusiontherewasa sig-nificantimprovementinpainwhilesynovitispersisted.She maintaineddiseaseactivityalthoughwithsatisfactoryresults, accordingtotheEULARresponse.After12monthsof treat-ment with RTX, cyclosporine (150mg/day) was introduced withnosuccess.
AfterthefourthcycleofRTX,thepatientpresented clin-ical worsening (secondary failure) and it was replaced by tocilizumab(8mg/kg/dose)withpartialresponse.Atpresent, sheisonabatacept(10mg/kg/dose),butwithpoorresponse andtheneedformultipleintra-articularinjectionsandhigh dosesofprednisone(20mg/day).Giventherefractorinessof thecase,thepatientwasreferredtoABMT.
Case5
MILP,20,female,polyarticularJIAsincetheageoffour,positive RF,followedsince12yearsold.
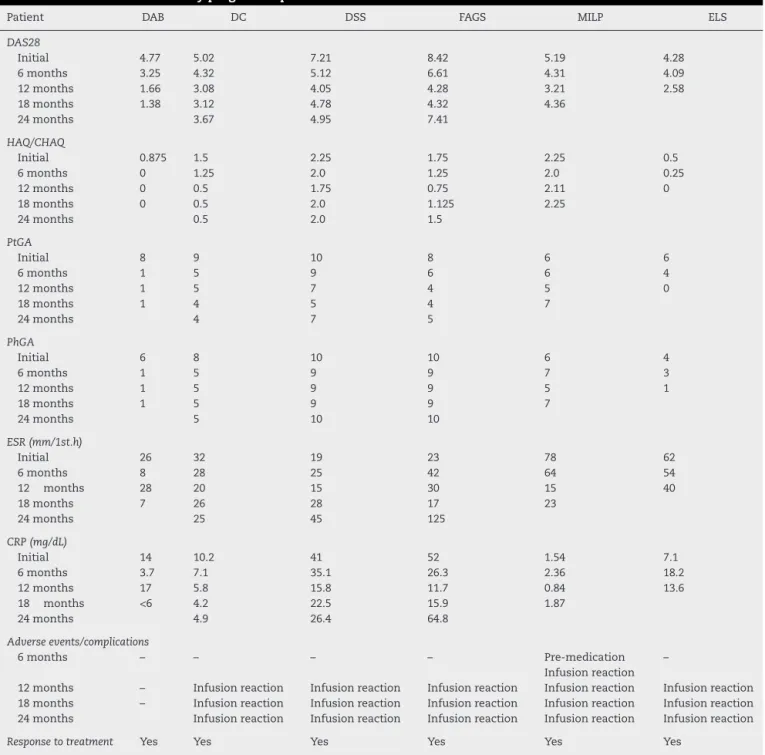
Table2–Clinicalandlaboratoryprogressofpatientsaftertreatmentwithrituximab.
Patient DAB DC DSS FAGS MILP ELS
DAS28
Initial 4.77 5.02 7.21 8.42 5.19 4.28
6months 3.25 4.32 5.12 6.61 4.31 4.09
12months 1.66 3.08 4.05 4.28 3.21 2.58
18months 1.38 3.12 4.78 4.32 4.36
24months 3.67 4.95 7.41
HAQ/CHAQ
Initial 0.875 1.5 2.25 1.75 2.25 0.5
6months 0 1.25 2.0 1.25 2.0 0.25
12months 0 0.5 1.75 0.75 2.11 0
18months 0 0.5 2.0 1.125 2.25
24months 0.5 2.0 1.5
PtGA
Initial 8 9 10 8 6 6
6months 1 5 9 6 6 4
12months 1 5 7 4 5 0
18months 1 4 5 4 7
24months 4 7 5
PhGA
Initial 6 8 10 10 6 4
6months 1 5 9 9 7 3
12months 1 5 9 9 5 1
18months 1 5 9 9 7
24months 5 10 10
ESR(mm/1st.h)
Initial 26 32 19 23 78 62
6months 8 28 25 42 64 54
12 months 28 20 15 30 15 40
18months 7 26 28 17 23
24months 25 45 125
CRP(mg/dL)
Initial 14 10.2 41 52 1.54 7.1
6months 3.7 7.1 35.1 26.3 2.36 18.2
12months 17 5.8 15.8 11.7 0.84 13.6
18 months <6 4.2 22.5 15.9 1.87
24months 4.9 26.4 64.8
Adverseevents/complications
6months – – – – Pre-medication
Infusionreaction –
12months – Infusionreaction Infusionreaction Infusionreaction Infusionreaction Infusionreaction 18months – Infusionreaction Infusionreaction Infusionreaction Infusionreaction Infusionreaction 24months Infusionreaction Infusionreaction Infusionreaction Infusionreaction Infusionreaction
Responsetotreatment Yes Yes Yes Yes Yes Yes
DAS28,DiseaseActivityScorein28joints;HAQ,HealthAssessmentQuestionnaire;CHAQ,ChildHealthAssessmentQuestionnaire;PtGA, patient’sglobalassessmentofvisual-analogicalscale;PhGA,physician’sglobalassessmentofvisual-analogscale;CRP,Creactiveprotein;ESR, erythrocytesedimentationrate.
1mg/kg/week)and intravenouspulsetherapy with methyl-prednisolonewerestarted.
Attheageof12,infliximab(5mg/kgevery6weeks)was startedincombinationwithcyclosporine(5mg/kg/day),with partial response after one year. After two years, a com-bination of methotrexate and leflunomide was introduced (20mg/day)withgoodresponseforninemonths.Attheage of15etanercept(0.8mg/kg/dose)wasstartedandbilateralhip arthroplasty was performed, and as she presentedclinical
andlaboratorydiseaseactivity,adalimumab(24mg/m2)was
dosesofGCs.Duetorefractorinessofthedisease,treatment withtocilizumabwasindicated.
Case6
ELS,20,female,erosivepolyarticularJIApositiveRF,sinceshe was9yearsoldandontreatmentsince10yearsofage.
ShehadalreadyreceivedGCs(upto1mg/kg/day)orallyfor fivemonthswithsignificantadverseevents.Atthefirstvisit, methotrexate(0.4mg/kg/week)andnaproxenwereinitiated, andGCstapered.Duringfollow-up,intravenouspulsetherapy withmethylprednisolone wascarriedoutdueto inflamma-tion,anemiaandpersistenceofthejointdisease.
When she was 10, cyclosporin (up to 5mg/kg/day) was associated, and disease activity was maintained. After ayear,therapywithinfliximabwasinitiated(5mg/kgevery6 weeks).At13yearsold,therapywithadalimumab(24mg/m2)
was initiated, with good response for the first two years. Afterthisperiod,atreatmentwasconductedwithetanercept (0.8mg/kg/dose)for11monthswithpoorresponse,andRTX wasindicated.
Shereceivedthe1stcycleofRTXwhenshewas18,with clinicalimprovementforthreemonths.Afterthreemonths ofmedication,therewasclinical and laboratoryworsening andinfection.However,inthefollowingmonths,thepatient hadsignificantimprovementinpain.Thepatientisawaiting afourthdoseofRTXincombinedusewithsubcutaneousMTX (40mg/week).
Discussion
RTXisanalternativetherapyforrefractoryJIApatients.2,3,5–7
TherearenocontrolledclinicaltrialswithRTXinJIAand evi-denceislimitedtocaseseriesreports.6,7,18,19 Thedoseand
dosing intervals were determined taking into account the experienceandcliniclogistics.
The clinical improvement observed by patients treated with RTX suggests an important role for B cells in JIA (Table2).2,6,19,20Itwasobservedthatinthechildrenjointswith
JIAthereisoligoclonalexpansionofBcellsandincreased IL-12production,andsubsequentactivationofTcells.Inadults, thereisBcelldepletioninbloodandsynovialtissue.10
All six patients responded to treatment at six and 12months,buttheclinicalresponsewasnotmaintainedin threeofthem,experiencingdisease activityand refractori-ness,sincehalfofthepatientshadindicationofABMT.Itis alsoimportanttonotethatRTXwasintroducedlateandafter failuretothreeanti-TNFblockers.Thus,Blymphocyte deple-tiontherapy,eventhoughitwasemployedinasmallnumber ofpatientsandbelatedly,provedtobeaneffectiveandsafe therapeuticoptioninhalfthecases.
RandomizedcontrolledandpivotaltrialsofRTXshowed that efficacy was greater in adult patients with positive RF or anti-citrullinated proteinantibodies,highlightingthe humoralresponseinthesepatients.21–23Inourstudy,halfof
thesampledidnothaveRFandnoneofthepatientshad anti-citrullinatedproteinantibodies.Thisaspectdidnotaffectthe therapeuticresponseintheshortandmediumterm,although,
interestingly,itwasthesepatientswhodidnotclaimbenefits andhavebeenreferredtotheABMT.
Adverse eventsobservedinourpatientswere similarto those described in the literature. The main one was the infusionreactionthatoccurredintwopatientsandledto per-manentdiscontinuationofthemedicationdespiteadequate responseofthedisease.
Somelimitationsmaybelisted,suchasthesmall num-ber of patients, lack of data on the CD19 cell count and radiographicinformationofstructuraldamageintheinitial evaluationand follow-up. However,thedataare consistent withthepopulationofreallifeinthisagegroup.
Thus,RTXisatreatmentoptioninactive,severeJIAand unresponsivetoDMARDsandanti-TNFblockers.Thesafety and efficacy demonstrated bythis study should encourage studieswithmorepatients,inordertodeterminethe possi-bilityofawindowofopportunityinpatientswithJIA.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.CassidyJT,PettyRE.Textbookofpediatricrheumatology. 6thed.Philadelphia:Elsevier;2011.
2.AlexeevaEI,ValievaSI,BzarovaTM,SemikinaEL,IsaevaKB, LisitsynAO,etal.Efficacyandsafetyofrepeatedcoursesof rituximabtreatmentinpatientswithsevererefractory juvenileidiopathicarthritis.ClinRheumatol.2011;30:1163–72. 3.JanssonAF,SenglerC,Kuemmerle-DeschnerJ,GruhnB,
KranzAB,LehmannH,etal.Bcelldepletionforautoimmune diseasesinpaediatricpatients.ClinRheumatol.
2005;30:87–97.
4.MarksSD,PateyS,BroganPA,HassonN,PilkingtonC,WooP, etal.BLymphocytedepletiontherapyinchildrenwith refractorysystemiclupuserythematosus.ArthritisRheum. 2006;52:3168–74.
5.El-HallakM,BinstadtBA,LeichtnerAM,BennetCM, NeufeldEJ,FuhlbriggeRC,etal.Clinicaleffectsandsafetyof rituximabfortreatmentofrefractorypediatricautoimmune diseases.JPediatr.2007;150:376–82.
6.KuekA,HazlemanBL,GastonJH,ÖstörAJK.Successful treatmentofrefractorypolyarticularjuvenileidiopathic arthritiswithrituximab.Rheumatology.2006;45:1448–9. 7.FeitoJG,PeredaCA.Rituximabtherapyproducedrapidand
sustainedclinicalimprovementinapatientwithsystemic onsetjuvenileidiopathicarthritisrefractorytoTNFalpha antagonists.JClinRheumatol.2009;15:363–5.
8.WeinerGJ.Rituximab:mechanismofaction.SeminHematol. 2010;47:115–23.
9.LeandroMJ,EdwardsJCW,CambridgeG.Clinicaloutcomein 22patientswithrheumatoidarthritistreatedwithB lymphocytedepletion.AnnRheumDis.2002;61:883–8. 10.KeystoneE,BurmesterGR,FurieR,LovelessJE,EmeryP,
KremerJ,etal.Improvementinpatient-reportedoutcomesin arituximabtrialinpatientswithsevererheumatoidarthritis refractorytoanti-tumornecrosisfactortherapy.Arthritis Rheum.2008;59:785–93.
responsetotumornecrosisfactorinhibitortherapies.Ann RheumDis.2009;68:216–21.
12.NasonovEL.Newtrendsintreatmentofrheumatoidarthritis: perspectivesofuseofmonoclononalanti-B-lymphocyte antibodies(rituximab).RussMedJ.2006;25:1778–82. 13.PettyRE,SouthwoodTR,MannersE,BaumJ,GlassDN,HeX,
etal.InternationalLeagueofAssociationforRheumatology classificationofjuvenileidiopathicarthritis:secondrevision. JRheumatol.2004;31:390–2.
14.PrevooML,van´tHofMA,KuperHH,vanLeeuwenMA,vande PutteLB,vanRielPL.Modifieddiseaseactivityscoresthat includetwenty-eight-jointcounts.Developmentand validationinaprospectivelongitudinalstudyofpatientswith rheumatoidarthritis.ArthritisRheum.1995;38:44–8.
15.FerrazMB,OliveiraLM,AraujoPM,AtraE,TugwellP. Cross-culturalreliabilityofthephysicalabilitydimensionof thehealthassessmentquestionnaire.JRheumatol. 1990;17:813–7.
16.MachadoCSM,RupertoN,SilvaCHM,FerrianiVP,RoscoeI, CamposLM,etal.TheBrazilianversionoftheChildhood HealthAssessmentQuestionnaire(CHAQ)andtheChild HealthQuestionnaire(CHQ).ClinExpRheumatol.2001;19 Suppl.23:S25–9.
17.vanGestelAM,PrevooML,vanHofMA,vanRijswijkMH,van dePutteLBA,vanRielPLCM.Developmentandvalidationof theEuropeanLeagueAgainstRheumatismresponsecriteria forrheumatoidarthritis.Comparisonwiththepreliminary AmericanCollegeofRheumatologyandtheWorldHealth
Organization/InternationalLeagueAgainstRheumatism criteria.ArthritisRheum.1996;39:34–40.
18.Kasher-MeronM,UzielY,AmitalH.Successfultreatment withB-celldepletingtherapyforrefractorysystemiconset juvenileidiopathicarthritis:acasereport.Rheumatology. 2009;48:445–6.
19.NarváezJ,Diaz-TornéC,JuanolaX,GeliC,LlobetJM,NollaJM, etal.Rituximabtherapyforrefractorysystemic-onset juvenileidiopathicarthritis.AnnRheumDis.2009;68:607–8. 20.MorbachH,WiegeringV,RichlP,SchwarzT,SuffaN,Eichhorn
EM,etal.ActivatedmemoryBcellsmayfunctionas
antigen-presentingcellsinthejointsofchildrenwithjuvenile idiopathicarthritis.ArthritisRheum.2011;63:3458–66. 21.MorbachH,GirschickHJ.B-celltargetedtherapyforchildren
andadolescentswithrheumaticdiseases.ZRheumatol. 2013;72:347–53.
22.EmeryP,FleischmannR,Filipowicz-SosnowskaA,
SchechtmanJ,SzczepanskiL,KavanaughA,etal.Theefficacy andsafetyofrituximabinpatientswithactiverheumatoid arthritisdespitemethotrexatetreatment:resultsofaphase IIBrandomized,double-blind,placebo-controlled,
dose-rangingtrial.ArthritisRheum.2006;54:1390–400. 23.CohenSB,EmeryP,GreenwaldMW,DougadosM,FurieRA,
GenoveseMC.Rituximabforrheumatoidarthritisrefractory toanti-tumornecrosisfactortherapy:resultsofa