www.jped.com.br
ORIGINAL
ARTICLE
Bone
mineral
density
in
children
with
idiopathic
nephrotic
syndrome
夽
Ghada
Mohamed
El-Mashad
a,
Mahmoud
Ahmed
El-Hawy
a,∗,
Sally
Mohamed
El-Hefnawy
b,
Sanaa
Mansour
Mohamed
aaMenoufiaUniversity,FacultyofMedicine,PediatricsDepartment,Menoufia,Egypt bMenoufiaUniversity,FacultyofMedicine,BiochemistryDepartment,Menoufia,Egypt
Received26November2015;accepted24May2016 Availableonline5November2016
KEYWORDS
Nephroticsyndrome; Bonemineraldensity; DXAscan
Abstract
Objectives: Toassessbonemineraldensity(BMD)inchildrenwithidiopathicnephroticsyndrome (NS)andnormalglomerularfiltrationrate(GFR).
Methods: Cross-sectional case---controlstudy carried outon 50children: 25 casesofNS (16 steroid-sensitive [SSNS] and nine steroid-resistant [SRNS] under follow up in the pediatric nephrologyunitofMenoufiaUniversityHospital,whichistertiarycarecenter,werecompared to25healthycontrolswithmatched ageandsex. Alloftheparticipants weresubjectedto completehistorytaking,thoroughclinicalexamination,laboratoryinvestigations(serum creat-inine,bloodureanitrogen[BUN],phosphorus[P],totalandionizedcalcium[Ca],parathyroid hormone[PTH],andalkalinephosphatase[ALP]).Bonemineraldensitywasmeasuredatthe lumbarspinalregion(L2---L4)inpatientsgroupusingdual-energyX-rayabsorptiometry(DXA). Results: TotalandionizedCaweresignificantlylowerwhile,serumP,ALP,andPTHwerehigher inSSNSandSRNScasesthanthecontrols.OsteopeniawasdocumentedbyDXAscanin11patients (44%)andosteoporosisintwopatients(8%).Fractureriskwasmildinsix(24%),moderatein two(8%),andmarkedinthree(12%)ofpatients.
Conclusion: Bonemineralizationwasnegativelyaffectedbysteroidtreatmentinchildrenwith NS.
©2016SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
夽 Pleasecitethisarticleas:El-MashadGM,El-HawyMA,El-HefnawySM,MohamedSM.Bonemineraldensityinchildrenwithidiopathic nephroticsyndrome.JPediatr(RioJ).2017;93:142---7.
∗Correspondingauthor.
E-mails:mahmodelhawy18@yahoo.com,mahmoud.elhawi@med.menofia.edu.eg(M.A.El-Hawy).
http://dx.doi.org/10.1016/j.jped.2016.05.010
PALAVRAS-CHAVE
Síndromenefrótica; Densidademineral óssea;
ExameDXA
Densidademineralósseaemcrianc¸ascomsíndromenefróticaidiopática
Resumo
Objetivos: Avaliar a densidade mineral óssea (DMO) em crianc¸as com síndrome nefrótica idiopática(SNI)ecomtaxadefiltrac¸ãoglomerular(TFG)normal.
Métodos: Oestudotransversaldecaso-controlefoirealizadocom50crianc¸as:25casosdeSNI [16sensíveisaesteroides(SNSE)enoveresistentesaesteroides(SNRE)comacompanhamento naunidade de nefrologiapediátricadohospital daMenoufiaUniversity, centrode cuidados terciário]foramcomparadoscom25controlessaudáveisdogrupodecontrolecomidadeesexo equivalentes.Todososparticipantesforamsubmetidosaanamnesecompleta,exameclínico completo,exameslaboratoriais[creatininasérica,nitrogênioureiconosangue(BUN),fósforo (P),cálcio(Ca)totaleionizado,paratormônio(PTH)efosfatasealcalina(ALP)].Adensidade mineralósseafoimensuradanaregiãodacolunalombar(L2-L4)nogrupodepacientesusando aabsorciometriaporraio-Xdeduplaenergia(DXA).
Resultados: Osníveisdecálciototaleionizadoeramsignificativamentemenores,aopassoque ofósforosérico,aFAeoPTHerammaioresemcasosdeSNSE eSNRE quenoscontroles.A osteopeniafoidocumentadapeloexameDXAem11pacientes(44%)eaosteoporose,emdois pacientes(8%).Oriscodefraturaeraleveemseis(24%),moderadoemdois(8%)eacentuado emtrês(12%)dospacientes.
Conclusão: A mineralizac¸ão dos ossos foi afetada negativamente pelo tratamento com esteroidesemcrianc¸ascomSN.
©2016SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
Introduction
Childhoodnephroticsyndrome(NS)isdefinedby nephrotic-range proteinuria, generalized edema, hypoalbuminuria, andhyperlipidemiawithnormalrenalfunction.1Idiopathic
nephroticsyndrome(INS)isthemostfrequentrenaldisease in children.2 Childhood NS typically follows a
relapsing-remitting course, often requiring recurrent courses of glucocorticoids (GC), but with low systemic inflammation duringremission.3
Bone massdeposition begins during fetallife and con-tinues during infancy and adolescence, stabilizing at the beginningofadulthood.4Duringchildhoodandadolescence,
skeletal modeling results in sex- and maturation-specific increasesinbonedensity.Metabolicbonedisease(MBD)is characterizedbychangesinskeletalmineralizationdueto poorbone mineralcontent(BMC).5Childrenmaybe
espe-ciallyvulnerabletotheeffectsofGConboneformationand peakbonemass.6
Prednisone is the first-linetreatment for INS toinduce remission, to prevent relapses and to avoid side effects of the disease.7 Prolonged administration of prednisone
interferes with growth and bone mineralization, and has deleterious effect on basic cellular mechanisms that are important in the development and maintenance of bone strength.7,8 Steroids are knownto causeosteoporosis and
affect BMC and bone mineral density (BMD) in children.9
Glucocorticoids have a suppressiveeffect on osteoblasto-genesis in the bone marrow and promote the apoptosis of osteoblasts and osteocytes, thus leading to decreased bone formation.10 Thereis someevidence tosuggest that
GC may increase bone resorption by extending the life-spanofpre-existingosteoclasts.11Glucocorticoidsmayalso
promote calcium loss through the kidneys and gut, and thisnegativecalciumbalancecan itselfleadtoincreased boneremodelingandosteoclasticactivityduetosecondary hyperparathyroidism.12
ChildrenwithINSare at riskfor MBD, accompanied by importantalterationsofmineralandbonemetabolism.13
Therefore, it was hypothesized that patients with NS wouldhaveBMDdeficitswhencomparedtotheirpeers.This studywasdesignedtodetermineBMD inchildrenwithINS andnormalglomerularfiltrationrate(GFR).
Methods
This study was carried out on 50 children after approval oftheEthicalCommitteeofFacultyofMedicine, Menoufia University,and a written consent was obtained from the guardiansof patients andcontrols. Children weredivided intotwogroups:
GroupI
Included25childrenaged1---15yearswhofulfilltheclinical criteriaforINS(heavyproteinuria>40mg/m2/h, hypoalbu-minemia<2.5g/L, hypercholesterolemia>250mg/dL, and edema)withnormalrenalfunction(normalglomerular fil-trationbySchwartzformula).14PatientswithsecondaryNS,
with other conditions unrelated to NS that could affect bone health, and patients who received prior medication forosteoporosisorvitaminDpreparationsbeforeorduring thestudywereexcluded.
of daily prednisone 60mg/m/day (80mg daily max) for fourweeks, followed by 40mg/m2/day given every other day as a single daily dose for at least four weeks. The alternate-day dose wasthen slowly tapered and dis-continued over the next one to two months. Relapses (proteinuria>40mg/h/m2 forthreeconsecutivedaysafter havingbeeninremission)weretreatedwith60mg/m2/day in a single morning dose until the child entered remis-sion(proteinuria<4mg/m2/h for threeconsecutive days). Dietaryadvicewasgivenfamiliestoprovidetheirchildren adiet rich in calcium,adequate caloric intake,adequate
protein(1g/kg/d),withnoaddedsalttolimitfluidoverload. The prednisone dose was then changed to alternate-day dosingasnotedwithinitialtherapy,andgraduallytapered over four to eight weeks. Patients with INS were clas-sified into three groups depending on their response to GC therapy: [i] steroid-dependent NS: two consecutive relapses during corticosteroid therapy or within 14 days aftercessationoftherapy(SDNS:14patients);[ii] steroid-resistant NS: failure to achieve remission following four weeks of prednisone60mg/m2 followed by three methyl-prednisolone pulses (SRNS: nine patients); [iii] infrequent
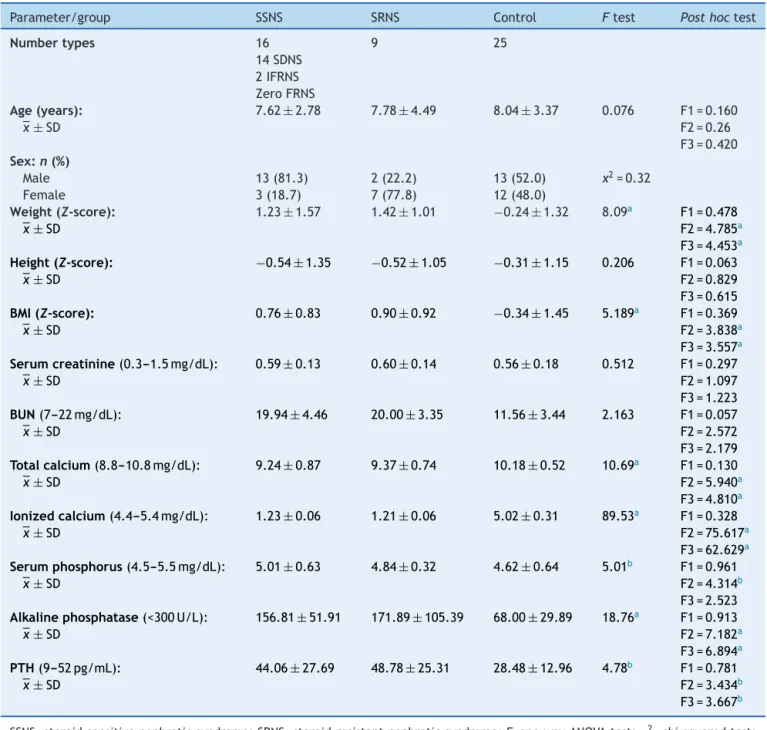
Table1 Demographicandclinicaldataofthestudiedgroups.
Parameter/group SSNS SRNS Control Ftest Posthoctest
Numbertypes 16 9 25
14SDNS 2IFRNS ZeroFRNS
Age(years): 7.62±2.78 7.78±4.49 8.04±3.37 0.076 F1=0.160
¯
x±SD F2=0.26
F3=0.420
Sex:n(%)
Male 13(81.3) 2(22.2) 13(52.0) x2=0.32
Female 3(18.7) 7(77.8) 12(48.0)
Weight(Z-score): 1.23±1.57 1.42±1.01 −0.24±1.32 8.09a F1=0.478
¯
x±SD F2=4.785a
F3=4.453a
Height(Z-score): −0.54±1.35 −0.52±1.05 −0.31±1.15 0.206 F1=0.063 ¯
x±SD F2=0.829
F3=0.615
BMI(Z-score): 0.76±0.83 0.90±0.92 −0.34±1.45 5.189a F1=0.369
¯
x± SD F2=3.838a
F3=3.557a
Serumcreatinine(0.3---1.5mg/dL): 0.59± 0.13 0.60± 0.14 0.56± 0.18 0.512 F1=0.297 ¯
x±SD F2=1.097
F3=1.223
BUN(7---22mg/dL): 19.94± 4.46 20.00± 3.35 11.56± 3.44 2.163 F1=0.057
¯
x±SD F2=2.572
F3=2.179
Totalcalcium(8.8---10.8mg/dL): 9.24±0.87 9.37±0.74 10.18±0.52 10.69a F1=0.130
¯
x± SD F2=5.940a
F3=4.810a
Ionizedcalcium(4.4---5.4mg/dL): 1.23± 0.06 1.21± 0.06 5.02± 0.31 89.53a F1=0.328
¯
x±SD F2=75.617a
F3=62.629a
Serumphosphorus(4.5---5.5mg/dL): 5.01± 0.63 4.84± 0.32 4.62± 0.64 5.01b F1=0.961
¯
x±SD F2=4.314b
F3=2.523
Alkalinephosphatase(<300U/L): 156.81±51.91 171.89±105.39 68.00±29.89 18.76a F1=0.913
¯
x± SD F2=7.182a
F3=6.894a
PTH(9---52pg/mL): 44.06± 27.69 48.78± 25.31 28.48± 12.96 4.78b F1=0.781
¯
x± SD F2=3.434b
F3=3.667b
SSNS,steroid-sensitivenephroticsyndrome;SRNS,steroid-resistantnephroticsyndrome;F,onewayANOVAtest;2,chi-squaredtest; ¯
x±SD,mean±standarddeviation;PTH,parathyroidhormone;BMI,bodymassindex;BUN,bloodureanitrogen;F1,differencebetween SSNSandSRNS;F2,differencebetweenSSNSandcontrol;F3,differencebetweenSRNSandcontrol.
Table2 DrugreceivedandDXAscanamongpatientgroups.
Parameter/group SSNS SRNS Student’st-test p-Value
Drugsreceived
Steroidduration(years) 2.55±2.49 2.56±1.62 0.02 0.49
Cumulativesteroiddose(mg/m2) 17,300.94±17,221.76 11,570.00±6,776.68 0.95 0.35 Immunosuppressivedrugs No(%) No(%)
Yes 9(56%) 9(100%)
No 7(44%) 0(0%)
DXAscan
BMD 0.61±0.10 0.56±0.24 0.81 0.42
Z-score −1.11±1.08 −1.00±0.86 0.27 0.79
SSNS,steroid-sensitivenephroticsyndrome;SRNS,steroid-resistantnephroticsyndrome;BMD,bonemineraldensity;DXA,dual-energy X-rayabsorptiometry.
relapsers:lessthanfourtimesina12-monthperiod(IFR:two patients).14
GroupII
Twenty-fiveapparentlyhealthychildrenofmatchedageand sex were enrolled as a control group. They were chosen fromtheoutpatientpediatricclinic,complainingfromacute transientillnesses.
All patients and controls were subjected to complete history taking, including type of treatment, its duration, and datesand number of relapses. The cumulative doses of prednisone that each patient received during therapy werecalculatedfromtheirmedicalcharts.Thoroughclinical examinationincludingheight,weight,andbodymassindex (BMI)were recordedand plotted onWorldHealth Organi-zationstandarddeviationcurves.Laboratoryinvestigations includedserumcreatinine,bloodureanitrogen(BUN, phos-phorous (P), calcium (total and ionized; Ca), parathyroid hormone(PTH),andalkaline phosphatase(ALP)were esti-mated.
BMDwasmeasuredatthelumbarspinalregion(L2---L4)in patientsgroupusingdual-energyX-rayabsorptiometry(DXA) (ChallengerEnvisionosteodensitometer,DMS,England).BMD wasclassified according toBakr15 on thebasis of BMD
Z-score.Scoreswerecalculatedfromthefollowingequation: Z-score=(BMD[g/cm3]ofthepatient----BMDpredictedfor age andsex/SD forBMD [age, sex, andheightmatched]). A patient was considered osteopenic if the Z-score was <---1.0.IftheZ-scorewas≤---2.5,thepatientwasclassified ashavingsevereosteopenia(osteoporosis).Chanceof osteo-poroticfracture <10%,10---19%,and >20%wereconsidered low,medium,andhighriskoffracture,respectively.16
Statisticalanalysis
TheresultswereanalyzedstatisticallyusingSPSSsoftware (version17;SPSSInc.,Chicago,IL,USA).Statistical analy-siswasperformedusingone-wayANOVA(Ftest)withpost hoc test, Student’s t-test, and chi-squared test. Correla-tions were determined by Pearson correlation and linear regressionanalysis.Continuousvariableswerepresentedas mean±standarddeviation,whileforcategoricalvariables,
numbers(%) wereused.Significancewasconsidered at p -value<0.05.
Results
Demographicandanthropometriccharacteristicsofthe par-ticipantswithsteroid-sensitivenephroticsyndrome(SSNS), steroid-resistantnephroticsyndrome(SRNS), andthe con-trol groups are summarized in Table 1. Weight and BMI Z-scores were significantly higher in the SSNS and SRNS patients than the controls, with no significant difference between them regarding the height Z-scores. In terms of serummarkers ofbone turnover,serumCa (totaland ion-ized)weresignificantlylower,whileserumphosphorusand alkalinephosphataseweresignificantlyhigherinbothSSNS andSRNSpatientsvs.thecontrols.Boneacheswerefound ineightpatients(32%).
Nosignificantstatisticaldifferenceswerefoundbetween SSNSand SRNS patients regardingthe drugs receivedand DXAmeasurements(Table2).Seventy-twopercentofSSNS patientsreceivedimmunosuppressivedrugs,asfollows:48% werecyclosporinetherapy,4%onMycophenolateMofetil,8% oncyclophosphamide,and12%onmixedimmunosuppressive therapy.
Bonemineral density (BMD) and fracture risk between nephrotic syndrome participants are given in Table 3. OsteopeniawasdocumentedbyDXAscanin11patients(44%) (sevenSDNS,fourSRNS),andosteoporosis intwopatients (8%)(twoSDNS).Fractureriskwasmildinsixpatients(24%)
Table 3 Bone mineral density and fracture risk among patientgroups.
Parameter n(%)
Bonemineraldensity
Average 12(48.0)
Osteopenicrange 11(44.0)
Osteoporoticrange 2(8.0)
Fracturerisk
No 14(56.0)
Mild 6(24.0)
Moderate 2(8.0)
Table4 Un-standardizedandstandardizedlinearregressioncoefficientsforcorrelationsbetweenZ-scoreandsomestudied parameters.
Parameter Un-standardizedcoefficientsB StandardizedcoefficientsB p-Value
Age(years) 0.06 0.19 >0.05
BMI 0.103 0.268 >0.05
Steroidduration(years) 0.05 0.11 >0.05
Cumulativesteroiddose(mg/m2) 0.12 0.23 <0.05a
BMI,bodymassindex. aSignificant.
(oneIFRNS,twoSDNS,threeSRNS), moderateintwo(8%) (oneSDNS,oneSRNS),andmarkedinthree(12%)(twoSDNS, oneSRNS).
Asignificantstatisticalcorrelationwasobservedbetween BMD Z-scores and age of patients (r=0.43; p<0.05), weightZ-score(r=0.56;p<0.001),heightZ-score(r=0.57; p<0.05), BMI (r=0.34; p<0.05), duration (r=−0.46; p<0.05), and cumulative dose of GC therapy(r=−0.88; p<0.001).Linearregressionanalyses inTable 4showthat thesteroidcumulativedose wastheonly significant inde-pendentriskfactor.
Discussion
Although GCs are the treatment of choice for children with idiopathic NS, obesity and bone mineralization side effects should beconsidered.7 In this study, the analyses
clearlyshowedan impactof GCsonbody weightandBMI in nephrotic syndrome participants. Not surprisingly, the SSNSandSDNSpatientshadsignificantlyhigherweightand BMIZ-scores thanthe controls, but withinsignificant sta-tisticaldifferencebetweenSSNSand SDNS.Similarresults were reported by Lestari et al.17 and Ribeiro et al.7 in
theiranalysesofobesityinSSNSandSDNS.Theuseof high-doseandlong-termsteroidsleadstoincreasedfoodintake and inhibited energy expenditure through stimulation of neuropeptide-Yandinhibitedreleaseofcorticotrophin hor-mone.Theprocesstriggersananabolicprocessandleadsto obesity.14HypocalcemiainpatientswithNSreportedinthis
studywasinlinewithKos¸anetal.18GCscausehypocalcemia
bydecreasedCaadsorptionfromgutandkidneys.7
However,somestudieshavereportednormalserum cal-ciumlevelsinchildrenwithNSduetoincreasedPTH.19
Therewere significant elevated levelsofserumP, ALP, and PTH in patients with NS vs. controls. These results arein accordancewithPa´nczyk-Tomaszewskaet al.19 and
Esmaeeili et al.20 Kos¸an et al.18 suggested that GCs
indi-rectlyaffectbonebyreducedintestinalcalciumabsorption andincreasedurinarycalciumlosses.Hyperparathyroidism reported in this study was likely due to hypocalcemia induced by GCs;high levels of PTH are knownto induce reabsorption of Ca from bone, as mentioned by Aceto etal.21 Bone-specificALP,whichisoneoftheisoenzymes,
is produced by osteoblasts and is a good marker of bone formation22; it washigherin the patients thanin healthy
children.Its increase duringGCs therapy in children with NSwasalsofoundby Kos¸anetal.18 Thiselevation maybe
relatedtoincreasedboneturnoverandimprovementof mas-siveproteinuria.
ThisstudyhasreportedadverseeffectofGCsontheBMD; GC therapy was associated with decreased BMD Z-score, osteoporosis,andanincreasedriskoffractureinnephrotic syndromechildren,withinsignificantstatisticaldifference betweenSSNSandSRNS.Indeed,linearcorrelationbetween thecumulativedose ofGCsandBMD wasrecorded.These resultswereinagreementwiththosereportedbyPa´ nczyk-Tomaszewskaetal.,19whoconcludedthatchildrenwithNS
treatedwithcorticosteroids areatrisk ofbonemass loss. Also,Acetoetal.21showedthatGCsreducedBMDZ-scorein
SSNS,andthatBMDZ-scoresignificantlycorrelateswiththe totaldosageofprednisone.
Canalis23 elucidated that corticosteroids suppress the
differentiation of osteoblastic cells andenhance the apo-ptosisofmature osteoblasts,whichresultinadecreaseof boneformation andlossofBMD. Ithasalsobeenreported thatsteroidtherapy cancauseosteoporosis orexacerbate apre-existingosteoporoticcondition,leadingtopathologic fractures.24Basiratniaetal.25concludedthatbonelosscan
occurinsomesteroid-dependentnephroticpatients, espe-ciallythosewithahighercumulativedoseofsteroid;higher cumulative doseswere associated withmore steroid con-sumptionandconsequentlymoreboneloss.
Itwasconcluded thatosteopenia assessedby DXAwas frequentinchildrenwithNS,especiallythoseadministered higher dosesof steroids (SDNSor SRNS). Bone mineraliza-tionwasnegativelyaffectedbysteroidtreatmentinchildren withNS.Thepresentstudyhadsomelimitations,suchasthe smallnumber of patientsand shortdurationavailable for thestudy.Therefore,theauthorsrecommendfurther stud-ieswithalargersamplesize andlongerduration.Regular BMDevaluationshouldbeperformedonNSchildren,andan appropriatetherapeuticapproachshouldbeplanned.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorswouldliketothankProf.MohamedHamed Bah-bah, Head and Creator of the pediatric nephrology unit, MenoufiaUniversity,Egypt.
References
initiationofglucocorticoidtherapyforpediatricnephrotic syn-drome.OsteoporosInt.2014;25:627---37.
2.Zhang Sy, Audard V, Fan Q, Pawlak A, Lang P, Sahali D. Immunopathogenesisofidiopathicnephroticsyndrome.Contrib Nephrol.2011;169:94---106.
3.MoonRJ,GilbertRD, PageA, MurphyL, Taylor P,CooperC, etal.Childrenwithnephroticsyndromehavegreaterbonearea butsimilarvolumetricbonemineraldensitytohealthycontrols. Bone.2014;58:108---13.
4.FortesCM,GoldbergTB,KurokawaCS,SilvaCC,MorettoMR, BiasonTP,etal.Relationshipbetweenchronologicalandbone ages and pubertal stage of breasts with bone biomarkers and bone mineral density in adolescents. J Pediatr (Rio J). 2014;90:624---31.
5.QuintalVS,DinizEM,CaparboVdeF,PereiraRM.Bone densit-ometrybydual-energyX-rayabsorptiometry(DXA)inpreterm newbornscomparedwithfull-termpeersinthefirstsixmonths oflife.JPediatr(RioJ).2014;90:556---62.
6.LeonardMB,FeldmanHI,ShultsJ,ZemelBS,FosterBJ,Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syn-drome.NEnglJMed.2004;351:868---75.
7.Ribeiro D, Zawadynski S, Pittet LF, ChevalleyT, Girardin E, ParvexP. Effectof glucocorticoidsongrowth and bone min-eraldensityinchildrenwithnephroticsyndrome.EurJPediatr. 2015;174:911---7.
8.HodsonEM,KnightJF,WillisNS,CraigJC.Corticosteroidtherapy fornephroticsyndromeinchildren.CochraneDatabaseSystRev. 2005:CD001533.
9.Yildirim ZK, Büyükavci M, Eren S, Orbak Z, Sahin A, Karakelleo˘gluC.Latesideeffectsofhigh-dosesteroidtherapy onskeletalsysteminchildrenwithidiopathicthrombocytopenic purpura.JPediatrHematolOncol.2008;30:749---53.
10.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibi-tion of osteoblastogenesis and promotion of apoptosis of osteoblastsandosteocytesbyglucocorticoids.Potential mech-anisms of their deleterious effects on bone. J Clin Invest. 1998;102:274---82.
11.HofbauerLC,GoriF,RiggsBL, LaceyDL,DunstanCR, Spels-berg TC, et al. Stimulation of osteoprotegerin ligand and inhibitionofosteoprotegerinproductionbyglucocorticoidsin humanosteoblastic lineage cells:potential paracrine mech-anismsofglucocorticoid-inducedosteoporosis.Endocrinology. 1999;140:4382---9.
12.MushtaqT,AhmedSF.Theimpactofcorticosteroidsongrowth andbonehealth.ArchDisChild.2002;87:93---6.
13.GulatiS,GodboleM,SinghU,GulatiK,SrivastavaA.Arechildren withidiopathicnephroticsyndromeatriskformetabolicbone disease?AmJKidneyDis.2003;41:1163---9.
14.Niaudet P,Boyer O. Idiopathic nephrotic syndrome in child-hood: clinicalaspects.In: AvnerED, HarmonWE,NiaudetP, YoshikawaN,editors.Pediatricnephrology.6thed.Berlin Hei-delberg:Springer-Verlag;2009.p.667---92.
15.Bakr AM. Bone mineral density and bone turnover mark-ers in children with chronic renal failure. Pediatr Nephrol. 2004;19:1390---3.
16.BishopN,ArundelP,ClarkE,DimitriP,FarrJ,JonesG,etal. Fracturepredictionandthedefinitionofosteoporosisin chil-drenandadolescents:theISCD2013PediatricOfficialPositions. JClinDensitom.2014;17:275---80.
17.Lestari N, Nurani N, Julia M. Corticosteroids and obesity insteroid-sensitiveandsteroid-resistantnephroticsyndrome. PaediatrIndones.2015;55:194---8.
18.Kos¸anC,AyarG,OrbakZ.Effectsofsteroidtreatmentonbone mineral metabolism in children withglucocorticoid-sensitive nephroticsyndrome.WestIndianMedJ.2012;61:627---30. 19.Pa´nczyk-TomaszewskaM,AdamczukD,KisielA,SkrzypczykP,
PrzedlackiJ,GórskaE,etal.Markersofbonemetabolismin childrenwithnephroticsyndrometreatedwithcorticosteroids. AdvExpMedBiol.2015;840:21---8.
20.EsmaeeiliM,AzarfarA,HoseinalizadehS.Calciumandvitamin Dmetabolisminpediatricnephrotic syndrome;anupdateon theexistingliterature.IntJPed.2015;3:103---9.
21.AcetoG,D’AddatoO,MessinaG,CarboneV,CavalloL,Brunetti G,etal.Bonehealthinchildrenandadolescentswith steroid-sensitivenephroticsyndromeassessedbyDXAandQUS.Pediatr Nephrol.2014;29:2147---55.
22.YangL,GreyV.Pediatricreferenceintervalsforbonemarkers. ClinBiochem.2006;39:561---8.
23.CanalisE.Mechanismsofglucocorticoid-inducedosteoporosis. CurrOpinRheumatol.2003;15:454---7.
24.vanStaaTP,CooperC,LeufkensHG,BishopN.Childrenandthe riskoffracturescausedbyoralcorticosteroids.JBoneMiner Res.2003;18:913---8.