w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anti-biofilm
activity
against
Staphylococcus
aureus
MRSA
and
MSSA
of
neolignans
and
extract
of
Piper
regnellii
Lara
Z.S.
Brambilla
a,
Eliana
H.
Endo
a,
Diógenes
A.G.
Cortez
b,
Benedito
P.
Dias
Filho
a,∗aLaboratóriodeInovac¸ãoTecnológicanoDesenvolvimentodeFármacoseCosméticos,DepartamentodeFarmácia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil
bLaboratóriodePesquisasemProdutosNaturaiseBiotecnologia,DepartamentodeFarmácia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16February2016 Accepted9August2016 Availableonline13October2016
Keywords:
Biofilm Conocarpan
Dichloromethaneextract Eupomathenoid-5
Staphylococcusaureus
a
b
s
t
r
a
c
t
Manyinfectionsworldwideareassociatedwithbacterialbiofilms.Theeffectsofisolatedneolignans (conocarpanandeupomathenoid-5)andthedichloromethaneextractofPiperregnellii(Miq.)C.DC., Piperaceae,weretestedagainstisolatesofmethicillin-resistantStaphylococcusaureusand methicillin-sensitiveS.aureusbiofilmsandS.aureusplanktoniccells.Thedichloromethaneextractpresentedbetter resultsthanisolatedneolignansagainstallofthebiofilmstested,withaminimuminhibitory concen-tration<400g/mlforpreformedbiofilmsandminimalbiofilminhibitoryconcentrationof15.6g/ml forbiofilmformation.Theminimuminhibitoryconcentrationtoplanktoniccellswas<12.5g/ml.These resultsindicateagoodeffectofthedichloromethaneextractagainstmethicillin-resistantS.aureusand methicillin-sensitiveS.aureusbiofilmsandefficientprophylaxis.
©2016PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeFarmacognosia.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
Introduction
Infectionsthatareassociatedwithantimicrobialresistanceare a primary challengein publichealth, resulting in highrates of morbidityandmortality,increasedlengthofhospitalization,and higher healthcare costs (Neidellet al., 2012). According tothe
NationalInstitutesofHealth(2002),approximately80%ofall
infec-tionsworldwideareassociatedwithbiofilms,especiallythosethat involvebiomaterials.
Biofilmsarecommunitiesofmicroorganismsthatare embed-dedinanextracellularmatrixthatiscomposedofproteins,lipids, polysaccharides,andnucleicacids.Themembersofabiofilmare protectedfromenvironmentalfactors (e.g., ultravioletlight and dehydration)andhostimmunecells(e.g.,neutrophilsandother phagocytes)(Hall-Stoodleyetal.,2004).Biofilm-associated bacte-riaarealsomuchmoreresistanttoantimicrobialagents(Stewart
andCosterton,2001).
Studies that investigate biofilm physiology are important. Searchingfornewstrategiestocontrolthiscomplexmodeof bac-teriallifeisextremelychallenging(Trentinetal.,2013).Plantswith antibacterialactivity,suchasthegenusPiper,haveshownpromise.
∗ Correspondingauthor.
E-mail:bpdfilho@uem.br(B.P.DiasFilho).
Piperregnellii(Miq.)C.DC.,popularlyknowninBrazilas “pari-paroba,” is an herbaceous plant found to exist in tropical and subtropicalregionsoftheworld(Cronquist,1981).Leavesandroots areusedascrudeextracts,infusionsorplaterstotreat wounds, reductionofswellingsandskinirritations(Corrêa,1984).Extracts oftheleavesofP.regnelliihaveshown goodantibacterial activ-ityagainstStaphylococcusaureusandBacillussubtilis(Pessinietal., 2003)andantifungalactivityagainsttheyeastsCandidaalbicans,
Candidakrusei,andCandidaparapsilosis(Pessinietal.,2005).The extract of the leavesand neolignan eupomatenoid-5have also shownactivityagainstmethicillin-resistantS.aureus(Marc¸aletal., 2010).NeolignansthatareisolatedfromtheleavesofP.regnelliialso haveactivityagainsttheparasitesTrypanosomacruzi(Luizeetal., 2006)andLeishmaniaamazonensis(Vendramettoetal.,2010).
BecauseofthereportedeffectofP.regnelliiagainstS.aureus,the aimofthepresentstudywastotestadichloromethaneextractofP. regnelliiandisolatedneolignans(conocarpanand eupomathenoid-5)againstmethicillin-resistantS.aureusbiofilmsand methicillin-sensitive S. aureus biofilms that were obtained from clinical isolates.
Materialsandmethods
Plantmaterial
Leaves from Piper regnellii (Miq.) C. DC., Piperaceae, were collectedinJune2012intheProfa.IreniceSilvaMedicinalPlant
http://dx.doi.org/10.1016/j.bjp.2016.08.008
GardenonthecampusoftheUniversidadeEstadualdeMaringá andidentifiedbyMariliaBorgooftheBotanyDepartmentofthe UniversidadeFederaldoParaná.Avoucherspecimen(HUM8392) wasdepositedintheHerbariumoftheUniversidadeEstadualde Maringá,Paraná,Brazil.
HPLCanalysis
The analyses were carried out using a Waters Binary HPLC Pump1525,equippedwithUV-VISdetector2489,anautosampler 2707witha20lloop,andcontrolledbyBreeze2Software.
Chro-matographicseparationswerecarriedoutinaPhenomenexODS (C18)Lunacolumn,5m,250×4.6mm,maintainedatroom
tem-perature,inanisocraticsystem,usingacetonitrile–wateracidified with2%aceticacid(70:30,v/v)withaflowrateof1ml/min.The detectionwascarriedoutat280nmandtheruntimewas20min. Conocarpan(1),eupomathenoid-6(2)and eupomathenoid-5(3) werequantifiedbyexternalstandardization,inmethodpreviously validatedbeinglinear,preciseandaccurate.Sampleswerediluted inmethanol,1000g/ml.
HO HO
2 R=H 3 R=OCH3
O
O R
1
Isolationoftheconstituents
DriedandpowderedleavesofP.regnellii(300g)wereextracted bymacerationwithethanol:water(9:1)atroomtemperaturein aleaf:solventratioof1:10(w/v).Thesolventwasthenremoved undervacuumat40◦Ctogiveanaqueousextractanddarkgreen
residuethatwaswashedwithdichloromethane,yielding24gof thedichloromethane extract (DE), and dried atroom tempera-ture,withyieldof8.2%.TheDE(12g)wassubjectedtovacuum column chromatography with silica gel (230–400 mesh) and elutedwithhexane(1000ml),dichloromethane(1400ml),ethyl acetate(1000ml),acetone(700ml),andmethanol(1000ml).The hexanefractionresultedintheisolationofeupomathenoid-5(3, 1.05g).Thedichloromethanefraction(6g)waschromatographed bycolumnchromatographyonsilicagel60(230–400mesh)with hexane:dichloromethane:ethyl acetate (12:7:1, v/v/v) to yield conocarpan(1,0.53g)andeupomathenoid-6(2),butinverysmall
quantity, 0.002g. The column chromatography procedure was monitoredbythinlayerchromatography (TLC)andusedasthe mobilephase,hexane:dichloromethane:ethylacetate(12:7:1)and vanillin sulfuric 2%. Structureswere identified by spectroscopy (UV,1HNMR,13CNMR,H–HCOSY,gNOE,HETCOR,HMBC) and
comparisonswiththeliterature(Achenbachetal.,1987;Chauret
etal.,1996;Snideretal.,1997).
Bacterialstrains
Theorganismsusedinthisstudywereobtainedoflaboratory collectionandwereoriginatedandidentifiedinUniversityHospital ofMaringá.Tenclinicalisolatesofmethicillin-resistantS.aureus
(MRSA),strains72,73,74,76,77,78,79,81,83and90(withstrains 72and73obtainedfrombloodandstrains74,76,77,78,79,81, 83and90obtainedfromsecretion),andthreeclinicalisolatesof andmethicillin-sensitiveS.aureus(MSSA),strains97,170,and212, obtainedfromurine(Marc¸aletal.,2010).ItwasalsousedS.aureus
ATCC25923.Teststrainswerepreservedinglycerol10%at−80◦C
andwereculturedonnutrientagarandincubatedfor24hat37◦C
priortodeterminationoftheminimuminhibitoryconcentration (MIC).
Antibacterialsusceptibilitytesting
The MICof conocarpan,eupomathenoid-5, and theDE were determined by a microdilution method in sterile flat-bottom microplatesaccordingtoCLSI(2012)usingMueller-Hintonbroth (MerckS.A.,SãoPaulo,Brazil).Inoculateswerepreparedinthesame mediumatadensitythatwasadjustedtoa0.5McFarlandturbidity standard(108colony-formingunits[CFU]/ml)anddiluted1:10for
thebrothmicrodilutionprocedure.Conocarpan, eupomathenoid-5 and DE were dilutedand transferredinto the first well,and serialdilutions1:2wereperformedsothatconcentrationsinthe rangeof100–1.56g/mlwereobtained.Vancomycinwasusedas
thereferenceantibacterialcontrolintherangeof50–0.8g/ml.
Positivecontrolofstrains(withoutpresenceofdrugs)and nega-tivecontrol(withmediumsolely)wasperformed.Microtitertrays wereincubatedat37◦C, andtheMICswererecordedafter24h
of incubation. Threesusceptibilityendpoints wererecorded for each isolate. TheMIC was defined asthe lowest concentration of thecompounds atwhich the microorganismdidnot exhibit visible growth. Theminimum bactericidal concentration (MBC) wasperformedinMueller-Hintonagar,incubatedat37◦Cduring
24h. MBCwasdefinedasthelowest concentrationthatyielded negativesubculturesoronlyonecolony.Theinvitroresultsfor thedrugswereclassifiedasthefollowing.MIC<100g/ml(good
antibacterialactivity),MIC=100–500g/ml(moderate
antibacte-rialactivity),MIC=500–1000g/ml(weakantibacterialactivity),
andMIC>1000g/ml(noactivity)(Pessinietal.,2003).
Invitropreformedbiofilmassay
BiofilmsofS.aureusATCC25923,10clinicalisolatesofMRSA, andthree clinicalisolates ofMSSAwereformedonpolystyrene 96-well microtiter plates. A 100l suspension that contained
108cells/mlinTSBmediumwith1%glucosewasseededinwells
andincubatedat37◦Cfor24h.Thewellcontentwasdischarged,
andthewellswerewashedwithphosphate-bufferedsaline(PBS). Severaldilutions of theDE,conocarpan, eupomathenoid-5, and vancomycin (standard reference drug) (15.6–1000g/ml) were
then addedtoeach well.Afterincubationat 37◦C for 24h, the
wells wererinsedwithPBS. TheMTT reductionassaywas per-formedtoevaluatetheviabilityofthebiofilms.Threeindependent assayswereperformed.TheMTTreductionassaywasaslight mod-ification of themethod reported by Schillaci et al. (2008).The MTTsolution(50l;2mg/mlinPBS)wasaddedtoeachwell,and
theplateswereincubatedat37◦Cfor2h.Afterstaining,theMTT
solutionwasremovedfromeachwell,and100lof
dimethylsulf-oxide(DMSO)wasaddedtodissolvetheMTTformazanproduct. TheDMSO(100l)wastransferredtoanewplate,andthe
opti-caldensity wasmeasuredat 570nmusinga microplatereader. Positivecontrolofbiofilmformation(withouttreatment)and neg-ativecontrol(withmediumsolely)wasperformed.Theresultsare expressedastheMIC50,atwhich50%ofthesessileS.aureuscells
wereinhibitedcomparedwiththecontrol(withouttreatment)for theMTTassays.
Inhibitingbiofilmformationinvitro
This assay investigated the drugs ability to preventbiofilm formation. Briefly, 100l of drug (DE, conocarpan and
eupomathenoid-5)atdifferentconcentrations(15.6–1000l/ml)
andstandarddrug,vancomycininconcentration7.8–500l/mlin
a96-wellmicrotiterplate,seededwith100lofasuspensionthat
contained108cells/ml, andincubated for24hat 37◦C toallow
biofilmformation.Thecontentsofthewellswereaspiratedand washedthreetimesinsterilePBS.Theextentofbiofilmformation wasassessedbytheMTTreductionassayasdescribedabove.The resultsareexpressedastheminimalbiofilminhibitory concen-tration(MBIC)comparedwiththecontrol(withouttreatment)for theMTTassays.
Scanningelectronmicroscopy
Scanningelectronmicroscopy(SEM)wasperformedonglass coverslipsbydispensing400lofthestandardizedcell
suspen-sionsthatcontained1.0×108cells/mlofTSBsupplementedwith
1%glucose intothe wellsof 24-well flat-bottomedpolystyrene plates.Toviewpreformedbiofilm,theplateswereincubatedat 37◦C for 24h. AfterwashingwithPBS,conocarpan (110
g/ml)
andtheDE(370g/ml)wereaddedtopreformedbiofilms,andthe
plateswereincubatedfor24hat37◦C.Toviewbiofilmformation,
standardizedcellsuspensionsanddrug(15.6g/ml conocarpan,
31.5g/mleupomathenoid-5,15.6g/mlDE,and7.8g/ml
van-comycin)wereaddedandincubatedat37◦Cfor24h.Thecoverslips
werethenwashedtwicewithPBSandfixedwith2.5% glutaralde-hydeovernightat4◦C.Thecoverslipswerethenwashedtwicewith
0.1Mcacodylatebufferfor15minanddehydratedbyreplacingthe bufferwithincreasingconcentrationsofethanol (30,50, 70,80, 90,95,and100%)for10mineach.Aftercritical-point-dryingand coatingwithgoldsputter,thesampleswereexaminedwitha scan-ningelectronmicroscope,inShimadzuSS-550(Probe4.0andAccV 15.0V).
Resultsanddiscussion
Isolationoftheconstituents
TheextractionofP.regnellii(Miq.)C.DC.resultedinan8.2% yield of dichloromethane extract. From this extract, theuse of silicagelchromatographiccolumnsand agradient systemwith increasingdegreesofpolarityallowedtheisolationoftwomajor compoundsthatwerepresentintheextract:conocarpan(1)and eupomathenoid-5(3),andeupomathenoid-6(2)inminoramount,
notallowing usein biological assays. Conocarpan,with stereo-chemistry(+)-conocarpanisthemostabundantinnature,produced naturallyinplantsofseveralfamilies,andthesemoleculesexhibit atrans-dihydrobenzofuranheterocycleasakeystructuralelement
(ChenandWeisel,2013).Thesesubstanceswereisolatedand
iden-tifiedbycomparingspectroscopicdataof1Hand13CNMRspectra
withthosepreviouslypublished(Achenbachetal.,1987;Chauret
etal.,1996;Snideretal.,1997).
HPLCanalysis
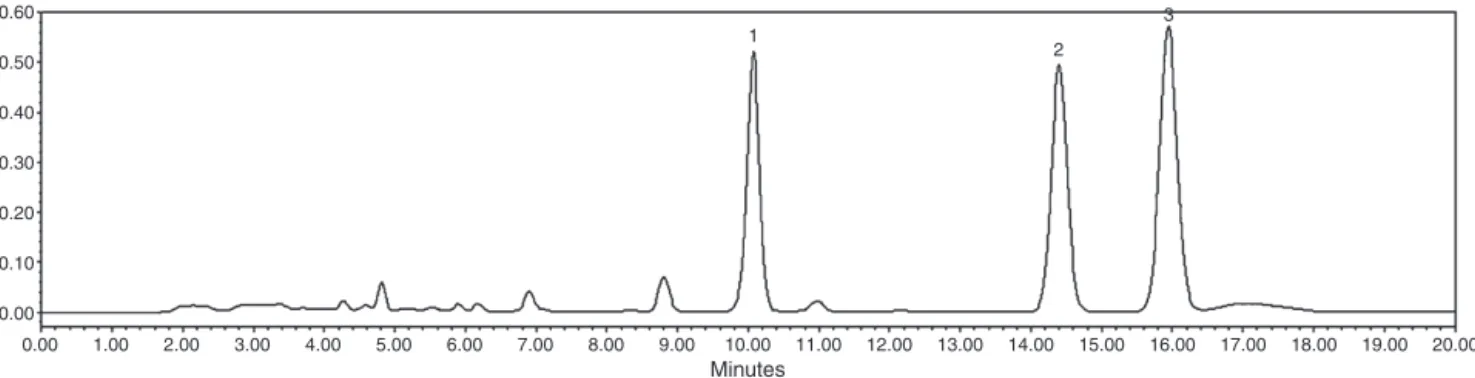
Fig. 1 shows dichloromethane extract chromatogram. HPLC analysisallowedtheneolignansquantification.Dichloromethane extract DE was standardized in 135.32g/ml of conocarpan
(1), 91.90g/ml of eupomathenoid-6 (2) and 106.98g/ml of
eupomthenoid-5(3).
Antibacterialeffectinplanktoniccells
TheMICresultsobtainedinthestudyareshown inTable1. ThedichloromethaneextracthadgoodactivityagainstMRSAand MSSA, with MIC <15g/ml and MBC <30g/ml. These values
wereverysimilartothosereportedbyMarc¸aletal.(2010). Cono-carpanalsohadgoodactivityagainstMRSAandMSSA(Table1),
withamaximumMIC=50g/mlandmaximumMBC=100g/ml.
Eupomathenoid-5hadaMIC≤6.25g/mlandMBC<25g/ml.
TheDEandisolatedneolignansweretestedagainstthestandard strain of S. aureus, with a MIC <15g/ml and MBC <30g/ml
(Table1).Thesevaluesaresimilartothosereportedintheliterature
(Pessinietal.,2003;Felipeetal.,2008).
Antibacterialeffectinpreformedbiofilm
Resistance mechanisms of biofilms are multifactorial and dependoneach organism.Althoughnotfullyunderstood,these mechanismscanbeattributedtosuchfactorsasareduction of the penetration of antibiotics through the biofilm matrix, the presenceofslow-growingornon-growingcells inthebiofilm,a heterogeneousbacterialpopulationwiththepresenceof pheno-typicsubpopulations withdifferentlevelsofresistance,andthe persistentpresenceofcells(MahandO’Toole,2001;Harrisonetal.,
2005).
Table1shows goodactivityoftheisolateneolignansagainst
planktoniccells,buttheactivityagainstthebiofilmwasnotthe
same(Table2).Euphomatenoid-5showednoactivityagainstS.
aureus ATCC 25923 biofilm. However, conocarpan and the DE hadasatisfactoryMIC50againstthebiofilm,110and370g/ml,
respectively(Table2).TheseMTTassayresultsdemonstratethe metabolicactivityofthecellsinthebiofilm,whichwasconfirmed bySEM(Fig.2).
ThephotomicrographsinFig.2Ashowthecontrolbiofilmwith alargeamountofadheredcellsandanextracellularmatrix.The treatedbiofilms(Fig.2BandC)presentedcellularlysisregionsand biofilmdetachment,suggestingthegoodactionofconocarpanand theDEagainstpreformedbiofilms.
Althoughtheconcentrationofconocarpan thatwasrequired tocombatthebiofilmwaslessthantheconcentrationoftheDE, the isolated substance showed no activity against clinical iso-latesofMRSAandMSSAbiofilms(Table2).Thedichloromethane extractwaseffectiveagainstallofthetestedclinicalisolatesof MRSAand MSSAbiofilms(MIC50<400g/ml). Inmany partsof
theworld, MRSA is responsible for a highrate of community-and hospital-acquired S. aureus infections. The most important classesofantibioticsthatareusedtopreventandtreatS.aureus
infectionareineffective(Köcketal.,2010).Inthepresentstudy, dichloromethaneextractofP.regnelliihadbetteractivityagainstS. aureusbiofilms.
Antibacterialeffectininhibitionbiofilmformation
Analternativetothecontrolofbiofilmformationistoprevent colonization.Thus,we evaluatedtheabilityofdifferent concen-trationsoftheDE,conocarpan, andeupomathenoid-5toinhibit theformationofbiofilmswhenaddedtothemediumatthesame timeasthecells.Theresultswerecomparedwithvancomycin.Ifan agentisaddedatthebeginningoftheexperiment,thentheagent mightactbeforethebiofilmisformedandinhibititsdevelopment. Thiscouldbeofinterestforcombatingrecalcitrantinfections(Khan
andAhmad,2012).Concentrationsaslowas15.6g/mlresultedin
asignificantreductionofthemetabolicactivityofadherentcells, with>95%inhibitionofMRSAandMSSAbiofilmformation com-paredwithuntreatedcontrolcells(Table3).TheDEhad results thatwereveryclosetovancomycinfortheinhibitionofS.aureus
biofilmformation.
TheseresultswereconfirmedbySEM.Fig.3showsthepositive controlwithalargenumberofcellsthatadheredtoeachotherand thepresenceanextracellularmatrix(Fig.3A).Photomicrographs ofcellsthatweretreatedwithconocarpan(15.6g/ml;Fig.3B),
0.00 0.10 0.20 0.30
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 1
2
3
Minutes
AU
11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00 0.40
0.50 0.60
Fig.1. ChromatographicprofileofdichloromethaneextractofPiperregenelliat1000g/ml.(1)Conocarpan;(2)eupomatenoid-6;(3)eupomatenoid-5.Conditions:mobile
phase–acetonitrile:waterwith2%aceticacid(70:30);flow:1ml/min,detectionat280nm.
Table1
MICandMBCofPiperregnelliidichloromethaneextract,eupomathenoid-5,conocarpanandVancomycininS.aureusplanktoniccells.
MIC(MBC)g/ml
Strains Dichloromethaneextract Eupomathenoid-5 Conocarpan Vancomycin
72 12.5(12.5) 6.25(25) 50(100) 1.56(1.56)
73 12.5(12.5) 6.25(25) 50(100) 1.56(1.56)
74 6.25(12.5) 6.25(6.25) 25(25) 1.56(1.56)
76 6.25(25) 6.25(12.5) 25(25) 1.56(1.56)
77 12.5(25) 6.25(12.5) 25(25) 1.56(1.56)
78 6.25(12.5) 3.13(6.25) 12.5(100) 1.56(1.56)
79 12.5(25) 3.13(6.25) 25(25) 1.56(1.56)
81 12.5(25) 3.13(6.25) 25(50) 1.56(1.56)
83 6.25(12.5) 3.13(6.25) 25(100) 1.56(1.56)
90 6.25(25) 3.13(6.25) 25(100) 1.56(1.56)
97 12.5(25) 6.25(6.25) 12.5(12.5) 1.56(1.56)
170 12.5(25) 6.25(12.5) 12.5(12.5) 1.56(1.56)
212 12.5(25) 6.25(12.5) 12.5(25) 1.56(1.56)
ATCC25923 6.25(12.5) 6.25(12.5) 12.5(25) 1.56(1.56)
TheMICresultswereconsideredgoodantibacterialactivity(MIC<100g/ml).
Table2
MIC50ofPiperregnelliidichloromethaneextract,eupomathenoid-5,conocarpanandvancomycintoS.aureuspreformedbiofilms.
MIC50(g/ml)
Strains Dichloromethaneextract Eupomathenoid-5 Conocarpan Vancomycin
72 300 – >1000 –
73 170 – – –
74 50 – – –
76 360 – – –
77 390 – >1000 –
78 180 – >1000 –
79 190 – >1000 –
81 210 – >1000 –
83 230 – >1000 –
90 130 – >1000 –
97 260 – >1000 –
170 260 – >1000 –
212 370 – >1000 –
ATCC25923 370 – 110 –
MIC50,minimalinhibitoryconcentrationatwhich50%ofthesessileS.aureuscellswereinhibited.
Fig.2.ScanningelectronmicrographsofrandomlychosenareasofS.aureusATCC25923biofilmat24h.(A)Positivecontrol.(B)Biofilmtreatedwith370g/mlof
Table3
Minimalbiofilminhibitoryconcentration(MBIC)valuesforthePiperregnelliidichloromethaneextract,eupomathenoid-5,conocarpanandvancomycintoS.aureusbiofilms formation.
MBIC(g/ml)
Strains Dichloromethaneextract Eupomathenoid-5 Conocarpan Vancomycin
72 15.6 15.6 15.6 7.8
73 15.6 15.6 15.6 7.8
74 15.6 31.2 15.6 7.8
76 15.6 31.2 15.6 7.8
77 15.6 250 125 7.8
78 15.6 15.6 15.6 7.8
79 15.6 15.6 15.6 15.6
81 15.6 15.6 15.6 7.8
83 31.2 31.2 31.2 62.5
90 15.6 15.6 15.6 7.8
97 15.6 15.6 15.6 7.8
170 15.6 15.6 15.6 7.8
212 15.6 15.6 15.6 7.8
ATCC25923 15.6 31.2 15.6 7.8
Fig.3. ScanningelectronmicrographsofrandomlychosenareasofS.aureusATCC25923biofilmformation.(A)Positivecontrol.(B)Biofilmtreatedwithconocarpan (15.6g/ml).(C)Biofilmtreatedwitheupomathenoid-5(31.25g/ml).(D)Biofilmtreatedwithdichloromethaneextract(15.6g/ml),D Biofilmtreatedwithvancomycin
(7.8g/ml).3000magnification.Thisstudywasperformedaccordingtotheinternational,nationalandinstitutionalrulesconsideringclinicalstudiesandbiodiversityrights.
Fig. 3D) showed isolated and fewer cells compared with van-comycin(Fig.3E).
Biofilm-relatedinfectionsareanimportantcauseof healthcare-associatedinfections(Darouiche,2004).Biofilm-embedded bacte-ria are challenging to treat because they display tolerance to antibioticsandthehost’simmunesystem(Johnetal.,2011;Leite etal., 2011).Traditionalantibiotics thatwere developed tokill planktonicbacteriaoftenhavelimitedeffectsonsessilebacteria thatareencasedwithinabiofilm.Additionally,thedevelopment ofantimicrobial resistanceiscommon insessile bacteria. Thus, thereisanurgentneedtodevelopnon-antimicrobialtreatment strategiestopreventortreatbiofilm-associatedinfections(Kuehn,
2011).
Conclusion
Goodresultswereachievedwiththedichloromethaneextract of P.regnellii (Miq.) C. DC. and isolated neolignans conocarpan andeupomathenoid-5againstplanktoniccellsofS.aureusMRSA andMSSA,similartothestandarddrugvancomycin.Similargood
effectswerefoundagainstbiofilmformation.Thedichloromethane extractandisolatedneolignansalsoshowedactivitythatwasclose to vancomycin and appeared to be effective as a prophylactic alternative.TheDEwasalsoeffectiveagainstS.aureusMRSAand MSSApreformedbiofilms.Theseresultssupporttheapplicationof thisextractofP.regnelliiasanalternativeagentagainst biofilm-associatedbacterialinfections.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
Author’scontribution
LZSB responsible for isolation of substances and performed inhibitionsassays,biofilmformationandpreformedbiofilm.EHE performedinhibitions assays, biofilm formation and preformed biofilm.DAGCsupervisedthelaboratoryworkandcontributedto theisolationandidentificationofsubstances.BPDFsupervisedthe laboratoryworkandcontributedtothebiologicalstudies.Allthe authorshavereadthefinalmanuscriptandapprovedits submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Achenbach,H.,Grob,J.,Xorge,A.D.,Cano,G.,Verde,J.S.,Brussolo,L.D.C.,Mu˜noz,G., Salgado,F.,López,L.,1987.Lignans,neolignansandnorneolignansfromKrameria cystisoides.Phytochemistry26,1159–1166.
Chauret,D.C.,Bernad,C.B.,Arnason,J.T.,Durst,T.,1996.Insecticidalneolignansfrom Piperdecurrens.J.Nat.Prod.59,152–155.
Chen,C.,Weisel,M.,2013.Conciseasymmetricsynthesisof(+)-conocarpanand obtusafuran.Synlett24,189–192.
CLSI,2012.MethodsforDilutionAntimicrobialSusceptibilityTestforBacteriathat GrowAerobically.ApprovedStandard,9thed.M07-A9,ClinicalandLaboratory StandardsInstitute,Wayne,PA.
Corrêa,M.P.,1984.DicionáriodasPlantasÚteisdoBrasiledasExóticasCultivadas. MinistériodaAgricultura,InstitutoBrasileirodeDesenvolvimentoFlorestal, Brasília,687pp.
Cronquist,A.,1981.AnIntegratedSystemofClassification.ColumbiaUniversity, NewYork,670pp.
Darouiche,R.O.,2004.Treatmentofinfectionsassociatedwithsurgicalimplants.J. Med.350,1422–1429.
Felipe,D.F.,DiasFilho,B.P.,Nakamura,C.V.,Cortez,D.A.G.,2008.Evaluationofthe antimicrobialactivityofPiperregnellii(Miq.)C.DC.var.pallescens(C.DC.)Yunck. Lat.Am.J.Pharm.27,618–620.
Hall-Stoodley,L.,Costerton,J.W.,Stoodley,P.,2004.Bacterialbiofilms:fromthe naturalenvironmenttoinfectiousdiseases.Nat.Rev.Microbiol.2,95–108.
Harrison,J.J.,Turner,R.J.,Marques,L.R.,Ceri,H.,2005.Biofilms:anewunderstanding ofthesemicrobialcommunitiesisdrivingarevolutionthatmaytransformthe scienceofmicrobiology.Am.Sci.93,508–551.
John,A.K.,Schmaler,M.,Khanna,N.,Landmann,R.,2011.Reversibledaptomycin toleranceofadherentstaphylococciinanimplantinfectionmodel.Antimicrob. AgentsChemother.55,3510–3516.
Khan,M.S.A.,Ahmad,I.,2012.Antibiofilmactivityofcertainphytocompoundsand theirsynergywithfluconazoleagainstCandidaalbicansbiofilms.J.Antimicrob. Chemother.67,618–621.
Köck,R.,Becker,K.,Cookson,B.,vanGemert-Pijnen,J.E.,Harbarth,S.,Kluytmans, J.,Mielke,M.,Peters,G.,Skov,R.L.,Struelens,M.J.,Tacconelli,E.,Navarro,A., Witte,W.,Friedrich,A.W.,2010.Methicillin-resistantStaphylococcusaureus (MRSA):burdenofdiseaseandcontrolchallengesinEurope.EuroSurveill.15, 19688–19690.
Kuehn,B.M.,2011.Scientistsseekantibioticadjuvants.JAMA306,2203–2204.
Leite,B.,Gomes,F.,Teixeiras,P.,Souza,C.,Pizzolitto,E.,Oliveira,R.,2011.Invitro activityofdaptomycin,linezolidandrifampicinonStaphylococcusepidermidis biofilms.Curr.Microbiol.63,313–317.
Luize, P.S.,Ueda-Nakamura,T., Dias-Filho,B.P.,Cortez, D.A.G.,Nakamura,C.V., 2006.ActivityofneolignansisolatedfromPiper regnellii(MIQ.)C.DC.var. pallescens(C.DC.) YunckagainstTrypanosomacruzy. Biol.Pharm.Bull. 29, 2126–2130.
Mah,T.F.,O’Toole,G.A.,2001.Mechanismsofbiofilmresistancetoantimicrobial agents.TrendsMicrobiol.9,34–39.
Marc¸al, F.J.B., Cortez, D.A.G., Ueda-Nakamura, T., Nakamura, C.V., Dias-Filho, B.P., 2010. Activity of the extracts and neolignans from Piper regnellii against methicillin-resistant Staphylococcus aureus (MRSA). Molecules 15, 2060–2069.
NationalInstitutesofHealth,2002.Availablefrom:http://grants.nih.gov/grants/ guide/pa-files/PA-03-047.html(accessedOctober2013).
Neidell,M.J.,Cohen,B.,Furuya,Y.,Hill,J.,Jeon,C.Y.,Glied,S.,Larson,E.L.,2012.Costsof healthcare-andcommunity-associatedinfectionswithantimicrobial-resistant versusantimicrobial-susceptibleorganisms.Clin.Infect.Dis.55,807–815.
Pessini,G.L.,Dias-Filho,B.P.,Nakamura,C.V.,Cortez,D.A.G.,2003.Antibacterial activ-ityofextractsandneolignansfromPiperregnellii(Miq.)C.DC.var.pallescens(C. DC.)Yunck.Mem.I.OswaldoCruz98,1115–1120.
Pessini,G.L.,Dias-Filho,B.P.,Nakamura,C.V.,Cortez,D.A.G.,2005.Antifungalactivity oftheextractsandneolignansfromPiperregnellii(Miq.)C.DC.var.pallescens(C. DC.)Yunck.J.Braz.Chem.Soc.16,1130–1133.
Schillaci, D., Arizza, V., Dayton, T., Camarda, L., Stefano, V.D.,2008. In vitro anti-biofilmactivityofBoswelliaspp.oleogumresinessentialoils.Lett.Appl. Microbiol.47,433–438.
Snider,B.B.,Han,L.,Xie,C.,1997.Synthesisof2,3-dihydrobenzofuransby Mn(Oac)3-basedoxidativecycloadditionof2-cyclohexenoneswithalkenes.Synthesisof (±)-conocarpan.J.Org.Chem.62,6978–6984.
Stewart,P.S.,Costerton,J.W.,2001.Antibioticresistanceofbacteriainbiofilms. Lancet358,135–138.
Trentin,D.S.,Giordani,R.B.,Macedo,A.J.,2013.Biofilmesbacterianospatogênicos: aspectosgerais,importânciaclínicaeestratégiasdecombate.Rev.Liberato14, 113–238.