w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
article
Alkaloids
and
biological
activity
of
beribá
(
Annona
hypoglauca
)
Maria
V.N.
Rinaldi
a,
Ingrit
E.C.
Díaz
b,
Ivana
B.
Suffredini
b,∗,
Paulo
R.H.
Moreno
caProgramadePós-graduac¸ãoemFármacoeMedicamento,FaculdadedeCiênciasFarmacêuticas,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
bNúcleodePesquisasemBiodiversidade,LaboratóriodeExtrac¸ão,UniversidadePaulista,SãoPaulo,SP,Brazil
cInstitutodeQuímica,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received18May2016 Accepted4August2016
Availableonline22September2016
Keywords:
Annonahypoglauca
Antibacterialactivity Cytotoxicity Isoquinolinealkaloids Isoboldine
Actinodaphnine
a
b
s
t
r
a
c
t
AnnonahypoglaucaMart.,Annonaceae,popularlyknownas“beribá”,wascollectedinfloodedareasofthe AmazonianRainForest.Thecrudeextractobtainedfromthisspecieswasfoundtobecytotoxicagainst humancancercells.ChemicalinformationonA.hypoglaucaisscarce.So,thepresentworkaimedthe isolationandidentificationofitsalkaloidsandtotesttheircytotoxicactivity.Alkaloidswereobtained fromstembyacid–basepartitioningandtheremainingalkaloid-freeextractwaspartitionedwithorganic solvents.Gaschromatography–massspectrometryGC/MSanalysisoftotalalkaloidsallowedthe iden-tificationoffouraporphinealkaloids:actinodaphnine,anonaine,isoboldineandnornuciferine.Total alkaloidswerefractionatedbycolumnchromatographyandwerepurifiedbypreparative thin-layer-chromatography,whichallowedtheisolationoftwoaporphinealkaloids,actinodaphnineandisoboldine, characterizedbyNMRandCG–MSanalyses.Thisisthefirstreportfortheoccurrenceofactinodaphnine inAnnonaspecies.Allthesamplesweretestedincytotoxicandantibacterialassays.Totalalkaloidextract anditsfractionsshowedantimicrobialactivityagainstStaphylococcusaureusandEnterococcusfaecalis. Inthecytotoxicityassay,thecrudeextractshowedalethaleffectagainstbreastandcoloncancercells. Isoboldine-containingFA5andactinodaphnine-containingFA6showedactivityagainstbreastcancercell line,whilethealkaloid-freefractionsdidnotshowsignificantactivityagainstcancercelllines.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Annonaceaeisapantropicalfamilycontainingapproximately 130generaand2300speciesoftreesandshrubs(Heywood,1993; Cordelletal.,2001).ThetropicalAmericangenusAnnonaL.is rep-resentedby 140species, which arerecognized mainlyby their ediblefruits,suchasA.muricata(graviola),A.squamosa (fruta-do-conde),A.coriaceae(araticum)andA.cherimolia(cherimoya)plants belongingtothegenusAnnonaarealsousedinfolkmedicineas antitumor,anti-parasiteand antidiarrhea(Pimenta etal.,2003), antiprotozoal (Siqueira et al., 2011), anti-inflammatory (Siebra etal.,2009),cytotoxicandanti-ulcerogenicagents(Hamidetal., 2012).Some species have also been pharmacologically studied for theirplateletanti-aggregationand antiulcerogenicactivities (Villaretal.,1997;Padmaetal.,1998).ThegenusAnnonaisarich sourceofisoquinoline–particularlyaporphinealkaloids(Leboeuf etal.,1982;Rabêloetal.,2014)–andacetogenins(Bermejoetal., 2005).Thestructuraldiversityoftheisoquinolinealkaloidsisas wideastherangeofitsbiologicalactivities,whichcanbeusedas
∗ Correspondingauthor.
E-mail:extractlab@unip.br(I.B.Suffredini).
antimicrobial(Simeónet al.,1990), cytotoxic(Wu et al.,1993), antitumoral(SonnetandJacobson,1971),antiprotozoal(Tempone etal.,2005),antiviral(Montanhaetal.,1995)besidesmanyother applications, especially in demonstrating antimicrobial activity againstGram-positivebacteria(Villaretal.,1987;Abbasogluetal., 1991;Pauloetal.,1992).
AnnonahypoglaucaMart.occursinthefloodedareas(igapós)of thenorthernAmazonianforests,aswellasinterrafirmeforests. Thespeciescanbefoundasatreeupto10mheightwhen occur-ringinterrafirmeorasalianawhenoccurringintheigapóswhere itispopularlyknownasberibá(Gottsberger,1978),orbiribá,in theBrazilianAmazonregion,orasguanábanahuascaandtortuga blancabyotherSouthAmericancommunities,oraswildsoursop, itsEnglishname.Itsfruitsareeatenbylocalsandtheteamadewith thebarkisusedasmedicineagainstparasites,anemiaandchronic diarrhea(Revilla,2002).A.mucosa,A.sylvatica,Duguetia marcgra-vianaandFusealongifoliaarealsoknownasbiribá,inBrazil(Reflora, 2016).
Previousbiologicalscreeningaimingtheidentificationof cyto-toxicity of 1277 Amazon plant extracts against human breast cancercelllines(Suffredinietal.,2007a),prostatecancercelllines (Suffredinietal.,2006a),colon,lung,centralnervoussystemand leukemiacancercelllines(Suffredinietal.,2007b)havebeenmade,
http://dx.doi.org/10.1016/j.bjp.2016.08.006
togetherwiththeevaluationoftheantimicrobialactivityofthe extractsagainstsomepathogenicmicroorganismsas Staphylococ-cusaureus(Suffredinietal.,2004),Enterococcusfaecalis(Castilho et al., 2013, 2014), Streptococcus mutans (Barnabéet al., 2014) andEscherichiacoli(CamargoandSuffredini,2014).Fromthe pre-viousscreening,thecrude extractobtainedfromthestemof A. hypoglaucaMart.,Annonaceae,theso-calledEB1109,hasshowna significantactivityagainstbreastcancercelllineMCF-7(Suffredini etal.,2007a)andagainstS.mutans(Barnabéetal.,2014).Despite thesefirstresults,littleisknownaboutthechemicalcomposition andbiologicalactivitiesofA.hypoglauca,despiteawideliterature consideringotherAnnonaspecies.
Thepresentstudyaimstoreporttheisolationofaporphine alka-loids fromthestems of A. hypoglauca,as wellasto reportthe cytotoxicactivityofEB1109 and itsfractions. Asliteraturealso indicatesaputativeantimicrobialactivityrelatedtotheAnnona
alkaloids,allsampleswerealsotestedagainstthepathogenic bacte-ria.
Materialsandmethods
Plantmaterial
Thestem ofAnnona hypoglaucaMart.,Annonaceae,was col-lected in water-flooded forests (named igapó forest), Amazon rain forest, Manaus, (Lat. 2◦58′ and Long. 60◦27′), Anavilhanas Ecological Station). A voucher specimen was deposited under identificationnumber [AAOliveira,3577 (UNIP Herbarium] and wasidentifiedbyDr.MateusL.B.Paciencia.Plantcollectionwas doneunder Brazilian Governmentplant collectlicense number MMA/ICMBio/SISBIO#14895andlicensetoaccessgeneticmaterial Ibama/MMA/CGen#012A-2008.
Extractionprocedures
Crudeextract:Thestemsoftheplant(1.074kg)wereair-dried at40◦Cinanair-circulatingoven.Plantmaterialwasgroundina hammermillbeforebeingsubmittedto24hmacerationwith10l ofamixturecontainingdichloromethane:methanol(1:1,v/v).The resultingorganicextractwasconcentratedtodryness(46.14g)and yielded4.3%ofEB1109(Younesetal.,2007).
Totalalkaloidfraction(TA):EB1109(5g)wereextractedwith 30ml0.1Mphosphoricacidunderagitationfor30min.Extraction wasrepeatedfourtimes.Acidsolutionswerefilteredandreunited tothesamefunnel.Acidsolutionwasthreetimesextractedwith 50ml hexane in order tohave non-polar compoundsremoved. TheacidsolutionwasbroughttopH∼9withammonia
hydrox-idesolution(25%,w/w)andwaspartitionedwithportionsof50ml chloroform,untilnegativetoDragendorff’sreagent.Theorganic phasewasdriedwithanhydroussodiumsulphateandconcentrated togivethetotalalkaloid(TA)fraction(3.1g;0.28%).TAyieldwas obtainedconsideringthecrudeplantmaterial.
Alkaloid-freeextracts: Thecake, consideredas theremaining insolublematerialresultedfromthealkaloidextraction,wasdried and the remainingsolids were dissolved in a series of solvent systems. Hexane (30ml) was added to the cake and the sys-temremainedunderagitationfor30min.Afterthat,thesystem was decanted and filtered. This procedure was repeated three timesunderthesameconditions.Thecombinedhexanesolutions were evaporated and originated fraction hexane (FHex,2.64g; 0.25%).Aseconddissolutiondoneinasimilarwaywasperformed withamixtureofdichloromethane(DCM)andmethanol(MeOH) (1:1, v/v), resulting fraction DCM/MeOH (DCM/MeOH; 1.71g; 0.16%).Thethirddissolutionwasmadewithethylacetate, result-ingin fractionFEAC (0.21g;0.02%).Finally, theremainingcake
material waspartitioned with a 20% ethanol:H2O solution and extractedwiththreeportionsof250mleachofbutanol,originating thefractionbutanol(FBuOH;0.65g;0.06%).Fractionsyieldswere obtainedconsideringthecrudeplantmaterial.
Isolationofalkaloids
TA(2g)wasfractionatedbycolumnchromatography (70cm lengthx40mmdiameter)on50gnormalphasesilicagel60.Elution wasmadewiththefollowingsolventmixtures:DCMandMeOH in order ofincreasing polarity,as follows: 100% DCM(200ml), DCM:MeOH9:1(400ml),DCM:MeOH8:2(400ml),DCM:MeOH 7:3(400ml),DCM:MeOH1:1(400ml)and100%MeOH(200ml). Theelutionresultedinthefollowingfractions:100%DCMyielded fraction1(FA1, 7.6mg;0.0011%);DCM:MeOH9:1(v/v)yielded fractions2(FA2,0.4mg;0.00005%)and3(FA3,738.4mg;0.1055%); DCM:MeOH8:2(v/v)yieldedfractions4(FA4,161.4mg;0.0231%) and5(FA5,176.1mg;0.0252%);DCM:MeOH7:3(v/v)yielded frac-tions6(FA6,395.8mg;0.0565%)and7(FA7,43.4mg;0.0062%); andfinallyfractions8(FA8, 26.9mg;0.0038%),9(FA9,22.1mg; 0.0031%) and 10 (FA10, 16.0mg; 0.0023%) were eluted with DCM:MeOH1:1(v/v).Fractionsyieldswereobtainedconsidering thecrudeplantmaterial.
Fractions FA4, FA5 and FA6 were again fractionated using preparative thin layer chromatography (PTLC) precoated with 1mmofsilicagel60F254,withoutbeingactivatedandasolvent mix-tureofCHCl3:MeOH(92:8)asmobilephase.Spotswereobserved underU.V.light(=366nm)andrevealedafterreactionwith Dra-gendorff’sreagent.Bandsrelatedtoeachisolatedcompoundwere elutedwithDCMandwerethensubmittedtoRMNanalysis. Frac-tionFA4originatedfourfractions,FA5originatedthreefractions, andFA6originatedsixfractions.Compound1wasthenisolated fromFA5.2usingPTLCasabrownamorphoussolid(3.7mg). Com-pounds2and3wereanalyzedby1HNMRasamixture.Lastly, compound4wasisolatedfromFA6.2,obtainedfrompreparative TLC,asalightbrownamorphoussolid(7.8mg).Fouralkaloidswere identified.
Compound1,isoboldine:(1HNMR,300MHz,CDCl
3,TMS inter-nalstandard):ı7.94(1H,s,H-11),6.74(1H,s,H-8),6.47(1H,s,H-3), 3.84–3.85(6H,s,br,OMe-10andOMe-2),2.49(3H,s,N-CH3)(Soares etal.,2015;JacksonandMartin,1966);13CNMR(75MHz,CDCl
3): ı145.74(C-2),144.90(C-9),144.43(C-10),140.47(C-1),129.7 (C-7a),124.4(C-11a),119.7(C-1a),113.87(C-11),111.55(C-8),108.64 (C-3),62.51(C-6a),56.16(C-2OMe),56.09(C-10OMe),53.36(C-5), 43.80(C-6N-CH3),33.9(C-7),28.79(C-4))(Jackmanetal.,1979). MS/EI (M+)RT=27.99:327(M+), 326(M+-1),310(M+-17), 284 (M+-43),269(M+-58),253(M+-74).
Compound 2, nornuciferine (present in fraction FA5.3): (1H NMR, 200MHz, CDCl3, TMS internal standard): ı 8.32 (1H, d,
J=7.8Hz,H-11),7.28(1H,m,H-8,H-9,H-10),6.58(1H,s,H-3),3.59 (3H,s,2-OCH3),3.32(3H,s,1-OCH3)(Dutraetal.,2012;Hasratetal., 1997).MS/EI(M+)RT=20.12:281(M+),280(M+-1),266(M+-15), 250(M+-31),237(M+-43),221,178,165,152.
Compound3,anonaine(presentinfractionFA5.3):(1HNMR, 200MHz,CDCl3,TMSinternalstandard):ı8.00(1H,d,J=7.8Hz; H-11),7.28–7.14(1H,m,H-8,H-9,H-10),6.68(1H,s,H-3),5.89 (1H,d,J=0.8Hz,1-OCH2O-2)and6.28(1H,d,J=0.8Hz,1-OCH2 O-2,)(Costaetal.,2012;Hasratetal.,1997).MS/EI(M+)RT=21.32: 265(M+),254(M+-1),236(M+-29).
(300MHz,CDCl3):CH:ı114.4;110.1;106.9;CH3:56.1;53.3;CH2: 100.6;42.55;35.4;28.3.13C NMR(75MHz,CDCl
3):ı146.99 (C-2),145.65(C-10),145.35(C-9),141.70(C-1),128.09(C-7a),125.98 (C-1b),125.65(C-3a),122.74(C-11a),116.57(C-1a),114.41(C-8), 110.11(C-11),106.96(C-3),100.62(OCH2O), 56.08(C-10OMe), 53.27(C-6a),42.56(C-5),35.37(C-7),28.32(C-4))(Stévignyetal., 2002).MS/EI(M+)RT=27.48:311(M+),310(M+-1),279,251,181.
NCH
3H
3CO
HO
OH
H
3CO
1
NH
O
O
NH
H
3CO
H
3CO
2
NH
OH
H
3CO
O
O
4
3
Spectroscopicanalysis
NMRspectraof1Hand13CwereobtainedonaBruker spectrom-eter,operatingat200MHzorat300MHz(1H)andat75MHz(13C). GC–MSwasperformedinanAgilentSeries6890chromatograph, equippedwithaHP-5column(30m×0.25mm,filmof0.25m), theinjectorwassetto290◦C,thecarriergas(He)flowwasadjusted to1ml/min,theinitialtemperaturewas95◦Cfor2min,followedby anincrementof8◦C/minuntil310◦C,thetemperaturewas main-tainedfor2min.TheMSdetectoroperatedintheelectronimpact (EI;70eV),wasusedtoqualitativeevaluatethealkaloidcontents.
Biologicalassays
Samplespreparation
Samplepreparationforcytotoxicassay:allthesampleswere diluted to 2mg/ml in 50% dimethylsulfoxide (DMSO) in water beforebeingevaluatedinasingle-concentrationassay(finaltest concentration 100g/ml). Sample preparation for antibacterial assay:samplesweredilutedinDMSO50%tothefollowing concen-trationsof200,180,160,140,120,100,80,60,40and20mg/ml, inordertoproportionatefinaltestconcentrationsof100,90,80, 70, 60, 50, 40, 30, 20 and 10g/ml. Vehicle wastested for its antimicrobialactivity,aswellasdoxorubicin(Adriamicin®)ata concentrationof25mMwasusedasstandarddruginthe cytotox-icityassayandgentamycin andtetracyclinepreparedatvarious concentrationswereusedasstandarddrugsintheantibacterial assay.AntibioticfinaltestconcentrationsforMIC/MBCassayranged from120g/mldownto0.16g/ml.
Antibacterialmicrodilutionbrothassay
Testswereperformedin asepticconditions(Suffredinietal., 2015). Bacteria inoculum was prepared at the concentra-tion of 1.5×102CFU/ml, starting from a 0.5 McFarland (or
1.5×108CFU/ml), as follows (Younes et al., 2007). S. aureus
(ATCC29213),Escherichiacoli(ATCC25922)andE.faecalis(ATCC 29212) were the bacterial strains tested. Bacteria inoculum of eachstrainwasobtainedfromfresh24-hculturecoloniesgrown onMüeller-Hinton agarplates. Eachstrain wasinoculated into 5ml of Müeller–Hinton broth in order to obtain a concentra-tionof1.5×108CFU/ml(0.5McFarland),whichwasdetermined
by a turbidimeter (Oxoid) adjusted to the 0.5 McFarland con-centration.Eachinoculumwasthendilutedinbrothmediumto 1.5×102CFU/ml.Onehundredandninetymicrolitersofthis
sus-pensionwastransferredtoeachmicroplatewell.Tenmicrolitersof eachtreatmentwereaddedtothemicroplatewellsandincubated at35◦Cfor18–20h.Treatmentsthathavevisuallyinhibited bacte-riafromgrowweresubculturedinMüeller-Hintonagarplatesthat wereincubatedat35◦Cfor18to20h.Observationsofbacteriagrow orlackofbacteriagrowdeterminedwhichtreatmentwaseffective. Allsamplesthatshowedeffectivenessinthesingle-concentration assayweresubmittedtothedeterminationofMICandMBC.
Determinationofminimalinhibitoryconcentrationandminimal bactericidalconcentration
Minimalinhibitoryconcentration(MIC)andminimal bacteri-cidalconcentration(MBC)weredeterminedforthetreatmentsthat showedtotalgrowthinhibitioninthesingle-concentrationassay usingtheprotocoldescribedabove.Thesameprotocolwasused todetermineMICandMBC.Sampleswerepreparedin concentra-tionsof0.2upto2mg/ml,ina0.200mg/mlfold(testconcentrations rangingfrom10to100g/ml,in10g/mlfold)wereevaluated. Turbidity wasobserved and those identified ashaving a visual lackofturbidityweresubculturedinMüeller–HintonagarPetri dishes,whichwerethenincubatedat35◦Cfor18–20h.Afterthe incubation, bacteriagrowthwasdeterminedand supportedthe establishmentofMIC’sandMBC’sasfollows:thelower concentra-tionobservedashavingalackofturbiditybutpresentingbacteria growthwasdeterminedastheMICandthelowerconcentration havingalackofturbidityanddidnotpresentanybacteriagrowth aftersubculturewasconsideredastheMBC.
Cytotoxicityassay
treatment(extract,fractions,isolatesordoxorubicin)wasadded to thewells in sextuplicates. Also, in order to obtain a better experiment control,two wellshaving only medium plus treat-mentwere added totheplates, as wellas16 wells containing mediumpluscellswithouttreatment,inordertoestablisha reg-ularcellgrow(positivecontrol).Plateswerekeptincubatingfor 48hbeforeevaluation.Afterthat,plateswerealsofixedwithTCAat thepre-stipulatedconcentration.TCAwasremovedwithwaterand sulforodamineB(SRB)colorimetricassaywasperformed. Accord-ingtoVoigt(2005),theproteindyeSRBassayiscurrentlyused formeasuringdrug-inducedcytotoxicityandcellproliferationfor bindingelectrostaticallyandpHdependenttoproteinbasicamino acidresiduesofTCA-fixedcellsinalinearcorrespondencetothe amountofcells.So,timezero(T0),cellcontrol(C)andtreatment(T) opticaldensitiesat515nmwereusedtocalculatethepercentage ofcellgrowinhibitionor,asfollows:[(T−T0)/(C−T0)×100=%of
growth].Resultswereinterpretedbythecomparisonofcellgrowth aftertreatment(T)topositivecontrolcells(C),bothconsideringcell growthatTimeZero(T0).If100>T>T0wasobservedthepresence ofcellgrowthinhibition;ifT=T0=0wasobservedthatcellsdonot growinrelationtothecelldensityatT0;ifT<T0,itmeansthat treatmentiseffectiveenoughtocausecelllethality.Treatments wereevaluatedataspecifictestconcentrationof100g/ml.
Resultsanddiscussion
Thealkaloid yieldfromA. hypoglaucastems was0.28%. Pre-viousstudies(Debourgesetal.,1987)relateyieldsrangingfrom 0.3%and 0.2%for Duguetia species, while (Fischeret al., 2004) describesyieldsof0.5% for Annona.The yieldof total alkaloids hereobtainedfromA.hypoglaucaissomewhatamongtheexpected values.
TAfractionwasanalyzedbygasGC–MS,andthepresenceoffour aporphinealkaloidscouldbedetermined:isoboldine(1)isolated fromFA5.2,nornuciferine(2)andanonaine(3),identifiedas mix-tureinfractionFA5.3andactinodaphnine(4),isolatedfromFA6.2. Compoundswerecharacterizedbythecomparisonoftheir frag-mentationpatterninthemassspectra(MS)tothelibraryfromthe equipment(Wiley25)andtotheliteraturedata.Majorcompound (Rt20.12min)showedthefollowing fragmentationpattern:m/z
281(M+.),280(M+-1),266(M+-15),250(M+-30),252(M+-29),237, 221,178,165,152whichiscompatibletonornuciferine(2).The peakatm/z280with100%intensityischaracteristicofaporphines, whicheasilylosethehydrogennexttotheNHgroupatC-6a(Ohashi etal.,1963).Nornuciferine(2)wasisolatedfromA.hayesiistemas
thesecondmajorcompound(Rasamizafyetal.,1987).This alka-loidwasalsoreportedtooccurinAnnonaandinotherAnnonaceae genus,suchasEnantia,Guatteria,Isolona,PseudovariaandXylopia
(Lebouefetal.,1982;Lúcioetal.,2015).ThecompoundhavingRt
21.32minwascharacterizedasanonaine(3),bycomparisonofits fragmentationpattern(m/z265(M+.), 264(M+-1),236(M+-29), withtheliterature(JacksonandMartin,1966;Bhakunietal.,1972). CompoundshowingRt27.48min,m/z 311(M+.), 310(M+-1), 282(M+-29),279(M+-31),266(M+-15),251,181wasidentifiedas actinodaphnine(4),whichfragmentationpatternisinaccordance topreviousreport(McLaffertyandStauffer,1989).Isoboldine(1) wascharacterizedatRt27.9minandbyitsfragmentationpattern,
m/z327(M+.),326(M+-1),310(M+-17),284(M+-43),269(M+-58), 253(M+-74)(JacksonandMartin,1966).Isoboldine(1)previously isolatedfromfiveAnnonaspecies:A.cherimola,A.glabra,A. mon-tana,A.salzmanniandA.senegalensis(Lebouefetal.,1982;Simeón etal.,1990;Philipovetal.,1994).Inthepresentwork,both isobol-dineandactinodaphninewerealsoisolatedfromTA,asdescribed below.
Thealkaloidfractions(FA5andFA6),originatedfromcolumn chromatography(CC),werepurifiedbyPTLCandyielded isobol-dine (1)as a brownamorphous solid.Isoboldine structure was proposed based on its molecular weight (M 327Da), that was determinedbyGC–MS, andit iscompatiblewiththemolecular formulaC19H21O4N.Moreover,1Hand13CNMRchemicalshift val-ueswereinaccordancetothosereportedintheliterature(Jackson andMartin,1966),thesingletsat6.47and6.74ppmweredueto thehydrogensincarbons3and8;respectively;abroadsingletat 3.83–3.84ppm(6H)relativetothemethoxygroupsfoundcarbons 2and10andasingletat2.49ppm,relativetothehydrogensinthe NCH3group.Jackmanetal.,1979;Soaresetal.,2015haveassigned thecarbonssignalsintheNMRspectraforthiscompound,andit ispossibletoverifyagainthelevelofaccordancebetweenthedata hereobtainedandtheliteratureresults.Isoboldine(1)was pre-viouslyisolatedfromA.cherimola(Simeónetal.,1990),A.glabra,
A.montanaandfromseveralotherAnnonaceaespecies.However, actinodaphnine(4)hasbeenexclusivelyisolatedfromGuatteria scandens(Hocquemilleretal.,1983),anditisbeingherereported as novelty for the Annona genus. This alkaloid was reported to occur ubiquitously in Hernandiaceae and Lauraceae species (Guinaudeauetal.,1983;Hocquemilleretal.,1983;Sulaimanetal., 2011).
Nornuciferine(2)andanonaine(3)(Hasratetal.,1997)hadtheir structuresproposedbasedonchemical shiftvalues recordedin a200MHzspectrometer,once theywerefoundasamixturein
Table1
Minimalinhibitoryconcentration(MIC)andminimalbactericidalconcentration(MBC)fromthecrudeextractobtainedfromthestemofAnnonahypoglaucaMart.andits alkaloidfractionsagainstthreebacteriastrains.
Extractsandfractions Staphylococcusaureus ATCC29213
EnterococcusfaecalisATCC29212 Escherichiacoli ATCC25922
MIC(g/ml) MBC(g/ml) MIC(g/ml) MBC(g/ml) MIC(g/ml)
Crudeextract >100 >100 >100 >100 >100
FHex >100 >100 >100 >100 >100
DCM/MeOH >100 >100 >100 >100 >100
FEAC >100 >100 >100 >100 >100
FBuOH >100 >100 >100 >100 >100
Totalalkaloids 60 70 50 50 >100
FA4.4 NT NT NT NT NT
FA5a 70 70 40 40 >100
FA6a 70 80 40 40 90
Gentamycin 0.20 0.20 8 8 0.40
Tetracyclin 0.50 0.50 32 32 2
MIC,minimalinhibitoryconcentration;MBC,minimalbactericidalconcentration,NT,nottested.FHex,fractionhexane,DCM/MeOH,fractiondichloromethaneandmethanol, FEAC,fractionethylacetate,FBuOH,fractionbutanol.
fractionFA5.3notallhydrogenscouldbeassigned.So, nornucifer-ineshowedadoubletat8.32ppm(J=7.8Hz)correspondingtotheH atcarbon11,asingletat6.58ppmfortheHatcarbon3,andsinglets at3.59and3.32ppmrelativetothetwomethoxygroups1-and 2-OCH3.Theotheralkaloid,anonaine,showedadoubletat8.00ppm (7.8Hz)relativetotheHatcarbon11,asingletat6.68relativeto theHatcarbon3andtwodoubletsat5.89and6.28belongingto themethylenedioxyatcarbons1and2(1-O-CH2-O-2).
Actinodaphnine(4)wasisolatedfromFA6.2asa lightbrown amorphous solid. The molecularweight(M 311Da)was deter-minedbyGC–MSanditwascompatiblewiththemolecularformula C18H17O4N. The structure of actinodaphine (4) was attributed basedonthecomparisonofNMRspectraldatawithvaluesreported intheliterature(Stévignyetal.,2002).Itispossibletoobservethe presenceoftwoapparentsingletsat5.86and6.01ppmthatwere attributedtothehydrogensinthemethylenedioxyinposition1,2. Thepresenceofasingletreferentto3Hin3.85ppmmaycorrespond toamethoxygroupasasubstituentatcarbon10.Theabsenceof asingletwithintegrationto3Hnextto2.82ppmeliminatesthe possibilityofaNCH3group,confirmingthatcompound4isa nora-porphine.Withtheseobservations,itwaspossibletoattributethat compound4isactinodaphnine.TheattributionsoftheJvalueswere comparedtoresultsfoundintheliterature(Stévignyetal.,2002). Thepuritygradeofthesamplepermitteditsanalysiswithdifferent carbonNMRexperimentstoconfirmthestructure.Actinodaphnine wasfirstisolated fromAnnonaceaespecies suchasG. scandens
asabrowncrystal(Guinaudeauetal.,1983;Hocquemilleretal., 1983).
EB1109, hexane fraction, DCM/MeOH fraction, ethyl acetate fraction butanol fraction (Table 1) did not show a significant antibacterialactivity,consideringthatcrudeextractsandfractions shouldhaveantibacterialactivity<100g/ml(Padmaetal.,1998) tobeconsideredasapotentialantimicrobialnaturalproductagent. Ontheotherhand,TA,FA5(majorcompoundisoboldine(1),other compoundsnornuciferine(2)andanonaine(3))andFA6 (actin-odaphnine(4))showedamoderateactivityagainstGram-positive bacteria(S.aureusandE.faecalis),MICaslowas70and40g/ml, respectively,andgivesusaprospectionofgoodantibacterial activ-itytoactinodaphnine. Almostallsampleswereinactiveagainst theGram-negativeorganism(E.coli), onlyFA6(MIC>90g/ml) showedsomeactivity.Previousreportsshowedthatisoboldine(1) wasnot consideredashavingantibacterialactivity(Villaretal., 1987; Bermejo etal., 2005)whereas anti-S. aureusactivity has beenconfirmedtoactinodaphnine(4)(Hoetetal.,2004), suppor-tingthepresentfindings.Accordingtothepresentachievements, thefractionationofEB1109uptoFA5andFA6ledtoan improve-mentoftheantibacterialactivityandconfirmedactinodaphnineas apotentialantibacterialagent.Theantimicrobialactivityagainst Gram-positivebacteriaofdifferentisoquinolinecompoundshave alreadybeenstudied,regardingtheirstructure–activity relation-ships. In these studies, aporphines demonstrated lower or no activity.Onlynor-aporphinesbearinga1,2-methylendioxygroup couldbeconsideredactive.A.hypoglaucaaccumulatedatleasttwo aporphinealkaloids,anonaine(3)andactinodaphnine(4),that con-tainthestructuralrequirementslikelytobetheresponsibleforthe resultsobserved.
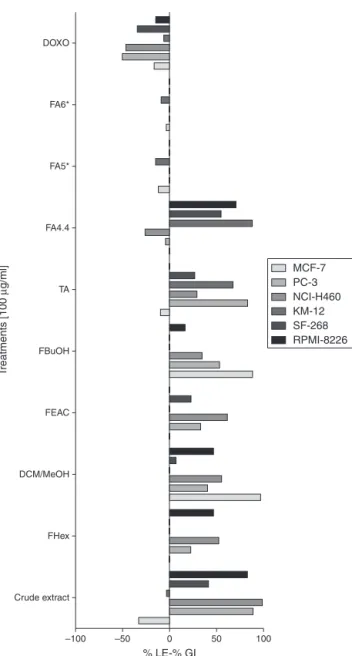
Resultsshown inFig.1 andTable2 describethepercentage ofgrowthand lethalityobservedforalltreatmentsagainst can-cercelllines.EB1109showedcytotoxicityagainstbreast(MCF-7) andcolon (KM-12)cancercelllines.Thepercentageoflethality foundforbreastcelllineswas−32.80%.Thisnegativevalueshall
beinterpretedbasedonthetimezero(T0)opticaldensityrelated tocellgrowthbeforetreatmentaddition,soEB1109notonlykilled 100%ofthesecells(whencomparedtoacellgrowthcontrol with-outtreatment), but alsokilleda number ofthe cellsthat grew beforetreatment.EB1109hasalsoshownlethalityagainstKM-12
Percentage of growth inhibition
DOXO
FA6*
FA5*
FA4.4
TA
FBuOH
T
reatments [100
µ
g/ml]
FEAC
DCM/MeOH
FHex
Crude extract
–100 –50 50
% LE-% GI
100 0
MCF-7 PC-3 NCI-H460 KM-12 SF-268 RPMI-8226
Fig.1.Cytotoxicactivityobservedaftertreatmentswithcrudeextract,total alka-loids(TA),alkaloid-freefractionsdescribedashexanefraction(FHex),butanol fraction(FBuOH),dichloromethane/methanolfractionandethylacetatefraction (FEAC),from stemofAnnona hypoglauca againstsixhuman tumorcelllines. MCF-7:breastcarcinoma;PC-3:prostatecarcinoma;NCI-H460:non-smallcells lungcarcinoma;KM-12:colonadenocarcinoma;SF-268:glioblastomaand RPMI-8226:multiplemyeloma.*FA4.4,FA5andFA6:columnfractionsobtainedfrom TAcontainingasmajorcomponentsisoboldineandactinodaphnine,respectively. NI:noinhibition;NT:nottested.Graphicis interpretedasthepercentageof growthobservedforthesixcellsafterbeingtreated.Calculationsusetheformula [{(T−T0)/(C−T0)×100}−100=%ofgrowthinhibition].Resultsareinterpretedby
thecomparisonofcellgrowthaftertreatment(T)tothecellgrowthatTimeZero (T0).If100>T>T0itmeansthattreatmentpreventedapercentageofcellsfrom grow;ifT=T0=0itmeansthattreatmentiseffectivetoavoidcellsfromgrow,in relationtoT0;ifT<T0itmeansthattreatmentiseffectiveenoughtocauselethality.
(−2.5%)andcytotoxicityagainstcentralnervoussystemcellline
SF-268(41.5%growthinhibition),prostate cancercelllinePC-3 (88.6%ofgrowthinhibition),leukemiacelllineRPMI-8226(82.7% ofgrowthinhibition)andlungcancercelllineNCI-H461(98.7%of growthinhibition).Totalalkaloidfractionshowedexpressive cyto-toxicityagainstMCF-7(−8.90%oflethality)andhasalsoshown
Table2
PercentageofgrowthofcancercelllinesMCF-7(breast),PC-3(prostate),NCI-H460(lung),KM-12(colon),SF-268(centralnervoussystem)andRPMI-8226(leukemia),after treatmentwithorganicextractofstemofAnnonahypoglaucaanditsfractions.NegativenumbersmeanlethalitytocelllinesinrelationtocellgrowthcontrolandTimezero growthcontrol(cellgrowthbeforetreatmentaddition).
MCF-7 PC-3 NCI-H460 KM-12 SF-268 RPMI-8226
Crudeextract −32.80 11.4 1.3 −2.5 58.5 17.3
FHex NI 77.4 48.1 NI NI 53.5
DCM/MeOH 2.90 59.6 44.9 NI 93.7 53.4
FEAC Nottested 67.1 38.6 NI 77.4 NI
FBuOH 11.90 46.6 65.6 NI NI 83.8
TA −8.90 17.04 71.7 32.4 73.3 NI
FA4.4 Nottested −3.8 −26.0 11.7 45.4 29.7
FA5a
−11.60 Nottested Nottested −14.4 Nottested Nottested
FA6a
−3.10 Nottested Nottested −8.4 Nottested Nottested
DOXO −16.31 −50.0 −46.2 −5.1 −34.0 −14.3
FHex,fractionhexane;DCM/MeOH,fractiondichloromethaneandmethanol;FEAC,fractionethylacetate;FBuOH,fractionbutanol;TA,totalalkaloidfraction;DOXO, doxorubicin.
aFA5andFA6arecolumnfractionsobtainedfromthetotalalkaloidscontainingasmajorcomponentsisoboldineandactinodaphnine,respectively.
inhibitedbreastcancercelllines,andthatmayindicatethatthe alkaloidsareresponsible tothecytotoxicityagainstbreast can-cercellline.Noneofthefractionsobtainedfromthecakeshowed lethality,althoughhaveshowncytotoxicity,asDCM/MeOH frac-tion, which showed growth inhibition of 97.1% against breast cancercelllineandBuOHfraction, whichinhibitedin88.1%the growthofthesamecell.FA5andFA6weretestedonlyagainst MCF-7andKM-12.Itwasobservedalethalityof11.6%againstMCF-7 breastcancercelllineand14.4%againstcoloncancercellline KM-12forfractionFA5.Isoboldineisthemajorcompoundoffraction FA5,whichcontainsyetnornuciferineandanonaine. Nornucifer-ineshowedleishmanicidalactivityagainst Leishmania mexicana
andL.panamensis(Montenegroetal.,2003)Anonaine,asisolated fromdiverseAnnonaceaeandMagnoliaceaespecies isfoundto beactiveasantiplasmoidal,antimicrobial,antioxidant,anticancer, antidepressantandvasorelaxant(Lietal.,2013).Insilico analy-sisofanonaineledtotheidentificationofitspotentialtoinhibit topoisomeraseII,oneofthecrucialtargetsagainstcancers(Singh etal.,2016).FA6hasalsoshownalethaleffect,althoughweaker, againstthecelllinesofbreastcancerMCF-7(−3.1%)andcolon
can-cerKM-12(−8.4%).Actinodaphnine(4)wasfoundinthisfraction.
Aporphinoidalkaloidsbearinga methylenedioxygroup,suchas actinodaphnine(4),exhibitedahighaffinitytotheDNAmolecule demonstratingacytotoxicactivityinvitroandanon-specific inhi-bitionoftheTopoisomeraseIactivitythroughDNAintercalation (Hoetetal.,2004).Inourfindings,fractionFA5,which contains isoboldine(1),nornuciferine(2)andanonaine(3),wasmore effi-cient thanFA6,the fractioncontainingactinodaphnine. Further studiesrelatedtostructure–activityrelationshipareneededto elu-cidatedifferencesinbiologicalactivityrelatedtoAnnonaalkaloids. Moreover,fractionFA4.4,obtainedfromfractionFA4byCCD,was testedandresultsshoweditssignificantlethalityagainstlung can-cercelllineNCI-H460(−26.0%)andprostatecancerPC-3(−3.8%),
andshowingagrowthinhibitionagainstthecelllinesofCNS can-cerSF-268(54.6%),leukemiaRPMI-8226(70.3%)andcoloncancer KM-12(88.3%).Itisclearthatthetotalalkaloidfractionandtheir fractionsFA5andFA6haveshownanexcellenttumorcelllethality incomparisontoanyofthefractionsobtainedfromthecake,and clearly,thealkaloidsareresponsibleforthesignificantcytotoxic activity,butmaybethepresenceofothercompoundsmayinduce somesynergismobservedintheextremegoodactivityofthecrude extract.Also,fractionFA4.4hasshownadifferentcytotoxicity pro-file,becauseitwastheonlyfractionamongalltestedthatrevealed anextremegoodactivityagainstlungcancercellline,althoughno alkaloidhasbeenidentifiedfromthatfractionsofar.Thestronger effectobservedforthecrudeextractmightbeexplainedbya syn-ergismamongthealkaloidsandtheothermetabolitespresentin theextract.
Conclusions
ThepresentresultsdemonstratedthatA. hypoglaucashowed animprovementofitsantibacterialactivity,asfractionationwas performed,maybeduetothepresenceofactinodaphnine. Actin-odaphnine,thealkaloidpresentinFA6,mayalsobetheresponsible forthefractioncytotoxicity,butthepresenceofisoboldine, nornu-ciferineandanonainemayhavesynergisticallycontributedtothe expressivelethalitytobreastcancercells,ifcomparedtoFA6.This isthefirstreportfortheoccurrenceofactinodaphnineinAnnona
species.Also,EB1109,TA,FA5andFA6showedsignificant cyto-toxicactivityagainstbreastandcoloncancercelllines.Finally,the identificationofsuchenthusiasticresultsledustopursuenew evi-dencesofbiologicalactivitiesrelatedtoA.hypoglauca,aswellasto achieveresultsonitstoxicactivitiesinthefuture.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authorscontributions
MVNRexecutedalltheexperiments;IECDmadeNMRanalyses; IBSexecuted/designedcytotoxicityassays andmanuscript writ-ingandtranslation;PRHMwriting/translationofthemanuscript, designedthechemicalexperiments,conductedasasupervisor.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsareindebtedtoFAPESP(#99/05904-6; #08/58706-8)forthefinancialsupportandtheCNPqforresearchgrant.
References
Barnabé,M.,Saraceni,C.H.C.,Dutra-Correa,M.,Suffredini,I.B.,2014.Theinfluence ofBrazilianplantextractsonStreptococcusmutansbiofilm.J.Appl.OralSci.22, 366–372.
Bermejo, A., Figadere, B., Zafra-Polo, M.C., Barrachina, E.E., Cortes, D., 2005. AcetogeninsfromAnnonaceae:recentprogressinisolation,synthesisand mech-anismsofaction.Nat.Prod.Rep.22,269–303.
Bhakuni,D.S.,Tewari,S.,Dhar,M.M.,1972.AporphinealkaloidsofAnnonasquamosa. Phytochemistry11,1819–1822.
Camargo,L.R.P.,Suffredini,I.B.,2014.Atividadeanti-Escherichiacolideextratosde plantasbrasileiras.Novastendênciasempesquisaveterinária.Arq.Bras.Med. Vet.Zootec.66,617–620.
Castilho,A.L.,daSilva,J.P.C.,Saraceni,C.H.C.,Díaz,I.E.C.,Paciencia,M.L.B.,Varella, A.D.,Suffredini,I.B.,2014.InvitroactivityofAmazonplantextractsagainst
Enterococcusfaecalis.Braz.J.Microbiol.45,769–779.
Castilho,A.L.,Saraceni,C.H.C.,Diaz,I.E.C.,Paciencia,M.L.B.,Suffredini,I.B.,2013. Newtrendsindentistry:plantextractsagainstEnterococcusfaecalis.Theefficacy comparedtochlorhexidine.Braz.OralRes.27,109–115.
Cordell,G.A.,Quinn-Beattie,M.L.,Farnsworth,N.R.,2001.Thepotentialofalkaloids indrugsdiscovery.Phytother.Res.15,183–205.
Costa,E.V.,Sampaio,M.F.C.,Salvador,M.J.,Nepel,A.,Barison,A.,2012.Chemical constituentsofthestembarkofAnnonapickelii(Annonaceae).Quim.Nova38, 769–776.
Debourges,D.,Roblot,F.,Hocquemiller,R.,Cavé,A.,1987.AlkaloidesdesAnnonacées 77.AlcaloidesdesDuguetiaspixiana.J.Nat.Prod.50,664–673.
Dutra,L.M.,Costa,E.V.,Moraes,V.R.S.,nogueira,P.C.L.,Vendramin,M.E.,Barison, A.,Prata,A.P.N.,2012.ChemicalconstituentsfromtheleavesofAnnonapickeii
(Annonaceae).Biochem.Syst.Ecol.41,115–118.
Fischer,D.C.H.,Gualda,N.C.A.,Bachiega,D.,Carvalho,C.S.,Lupo,F.N.,Bonotto,S.V., Alves,M.O.,Yogi,A.,DiSanti,S.M.,Avila,P.E.,Kirchgatter,K.,Moreno,P.R.H., 2004.Invitroscreeningforantiplasmodialactivityofisoquinolinealkaloidsfrom Brazilianplantspecies.ActaTrop.92,261–266.
Gottsberger,G.,1978.SeeddispersalbyfishininundatedregionsofHumaita, Ama-zonia.Biotropica10,170–183.
Guinaudeau,H.,Leboeuf,M.,Cavé,A.,1983.Aporphinealkaloids,III.J.Nat.Prod.46, 761–835.
Hamid,R.A.,Foong,C.P.,Ahmad,Z.,Hussain,M.K.,2012.Antinociceptiveand anti-ulcerogenicactivitiesoftheethanolicextractofAnnonamuricataleaf.Rev.Bras. Farmacogn.22,630–641.
Hasrat,J.A.,Pieters,L.,deBacker,J.P.,Vauquelin,G.,Vlietnick,A.J.,1997.Screening ofmedicinalplantsfromSurinamefor5-HT1Aligands:bioactiveisoquinoline alkaloidsfromthefruitofAnnonamuricata.Phytomedicine4,133–140. Heywood,V.H.,1993.FloweringPlantsoftheWorld.BTBatsfordLtd,London. Hocquemiller,R.,Rasamizafy,S.,Cavé,A.,1983.AlcaloïdesdesAnnonaceesXXXVII:
AlcaloïdesduGuatteriascandens.J.Nat.Prod.46,335–341.
Hoet,S.,Stévigny,C.,Block,S.,Opperdoes,F.,Colson,P.,Baldeyrou,B.,Lansiaux,A., Bailly,C.,Quetin-Leclercq,J.,2004.AlkaloidsfromCassythafiliformisandrelated aporphines:antitrypanosomalactivity,cytotoxicity,andinteractionwithDNA andtopoisomerases.PlantaMed.70,407–413.
Jackman,L.M.,Thewella,J.C.,Moniot,J.L.,Shamma,M.,Stephens,R.L.,Wenkert, E.,1979.Thecarbon-13NMRspectraofaporphinealkaloids.J.Nat.Prod.42, 437–449.
Jackson,A.H.,Martin,J.A.,1966.Stericeffectsinthemassspectraofaporphine alkaloids.J.Chem.Soc.(C)23,2181–2183.
Leboeuf,M.,Cavé,A.,Bhaumilk,P.K.,Mukherjee,R.,1982.Thephytochemistryofthe Annonaceae.Phytochemistry21,2783–2813.
Li,H.T.,Wu,H.M.,Chen,H.L.,Liu,C.M.,Chen,C.Y.,2013.Thepharmacological activ-itiesof(−)-anonaine.Molecules18,8257–8263.
Lúcio,A.S.S.C.,Almeida,J.R.G.S.,Cunha,E.V.L.,Tavares,J.F.,Barbosa-Filho,J.M.,2015. AlkaloidsoftheAnnonaceae:occurrenceandacompilationoftheir biologi-calactivities.TheAlkaloids:ChemistryandBiology,74.,1sted.Elsevier,pp. 233–409.
McLafferty,F.W.,Stauffer,D.B.,1989.TheWiley/NBSRegistryofMassSpectralData. Wiley-Interscience,NewYork.
Monks,A.,Scudiero,D.,Skehan,P.,Shoemaker,R.,Paull,K.,Vistica,D.,Hose,C., Langley,J.,Cronise,P.,Vaigrowolff,A.,Graygoodrich,M.,Campbell,H.,Mayo,J., Boyd,M.,1991.Feasibilityofahigh-fluxanticancerdrugscreenusingadiverse panelofculturedhumantumorcelllines.J.Natl.CancerInst.83,757–766. Montanha,J.A.,Amoros,M.,Boustie,J.,Girre,L.,1995.Anti-herpesvírusactivityof
aporphinealkaloids.PlantaMed.61,416–424.
Montenegro,H.,Gutiérrez,M.,Romero,L.I.,Ortega-Barría,E.,Capson,T.L.,Rios, L.C.,2003.AporphinealkaloidsfromGuatteriaspp.withleishmanicidalactivity. PlantaMed.69,677–679.
Ohashi,M.,Wilson,J.M.,Budzikiewicz,H.,Shamma,M.,Slusarchyk,W.A.,Djerassi, C.,1963.MassspectrometryinstructuralandstereochemicalproblemsXXXI. Aporphinesandrelatedalkaloids.J.Am.Chem.Soc.85,2807–2810.
Padma,P.,Chansouria,J.P.N.,Khosa,R.L.,1998.Effectofsomeindigenousdrugson coldimmobilizationstressinducedgastriculcer.Phytother.Res.12,127–128. Paulo,M.Q.,Barbosa-Filho,J.M.,Lima,E.O.,Maia,R.F.,Barbosa,R.C.B.B.C.,Kaplan,
M.A.C., 1992. Antimicrobial activity of benzilisoquinoline alkaloids from
AnnonasalzmaniiD.C.J.Ethnopharmacol.36,36–41.
Pimenta,L.P.S.,Pinto,G.B.,Takahashi,J.A.,Silva,L.G.F.,Boaventura,M.A.D.,2003. Bio-logicalscreeningofAnnonaceusBrazilianmedicinalplantsusingArtemiasalina
(BrineShrimpTest).Phytomedicine10,209–212.
Rabêlo,S.V.,Araújo,C.S.,Costa,V.C.O.,Tavares,J.F.,Silva,M.S.,Barbosa-Filho,J.M., Almeida,J.R.G.S.,2014.OccurrenceofalkaloidsinspeciesofthegenusAnnonaL. (Annonaceae):areview.NutraceuticalsandFunctionalFoods:NaturalRemedy, vol.1.,1ed.NovaSciencePublishers,NewYork,UnitedStates,pp.41–60. Rasamizafy,S.,Hocquemiller,R.,Cassels,B.K.,Cavé,A.,1987.AlkaloidsfromAnnona
hayesii.J.Nat.Prod.50,759–761.
Reflora. 2016. http://reflora.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/ BemVindoConsultaPublicaConsultar.do?invalidatePageControlCounter=7& idsFilhosAlgas=%5B2%5D&idsFilhosFungos=%5B1%2C11%2C10%5D&lingua=& grupo=5&familia=null&genero=&especie=&autor=&nomeVernaculo=birib%C3% A1&nomeCompleto=&formaVida=null&substrato=null&ocorreBrasil= QUALQUER&ocorrencia=OCORRE&endemismo=TODOS&origem=TODOS& regiao=QUALQUER&estado=QUALQUER&ilhaOceanica=32767& domFitogeograficos=QUALQUER&bacia=QUALQUER&vegetacao=TODOS& mostrarAte=SUBESP VAR&opcoesBusca=TODOS OS NOMES&loginUsuario= Visitante&senhaUsuario=&contexto=consulta-publica(Accessedin26.07.16). Revilla,J.,2002.PlantasúteisdaBaciaAmazônica,II.INPA,Manaus,pp.444. Siebra,C.A.,Nardin,J.M.,Florão,A.,Rocha,F.H.,Bastos,D.Z.,Oliveira,B.H.,
Weffort-Santos,A.M.,2009.PotencialantiinflamatóriodeAnnonaglabraAnnonaceae. Rev.Bras.Farmacogn.19,82–88.
Simeón,S.,Ríos,J.L.,Villar,A.,1990.AntimicrobialactivityofAnnonacherimoliastem barkalkaloids.Pharmazie45,442–443.
Singh, S.,Das, T., Awasthi,M., Pandey,V.P., Pandey,B., Dwivedi,U.N., 2016. DNAtopoisomerase-directedanticancerousalkaloids:ADMET-basedscreening, moleculardocking,anddynamicssimulation.Biotechnol.Appl.Biochem.63, 125–137.
Siqueira,C.A.T.,Oliani,J.,Sartoratto,A.,Queiroga,C.L.,Moreno,P.R.H.,Reimão,J.Q., Tempone,A.G.,Fischer,D.C.H.,2011.Chemicalconstituentsofthevolatileoil fromleavesofAnnonacoriaceaandinvitroantiprotozoalactivity.Rev.Bras. Farmacogn.21,33–40.
Soares,E.R.,Silva,F.M.A.,Almeida,R.A.,Lima,B.R.,SilvaFilho,F.A.,Barison,A.,Koolen, H.H.F.,Pinheiro,M.L.B.,Souza,A.D.L.,2015.DirectinfusionESI-IT-MSnalkaloid
profileandisolationoftetrahydroharmanandotheralkaloidsfromBocageopsis pleiospermaMaas(Annonaceae).Phytochem.Anal.26,339–345.
Sonnet,P.E.,Jacobson,M.,1971.TumorinhibitorsII:cytotoxicalkaloidsfromAnnona purpurea.J.Pharm.Sci.60,1254–1256.
Stévigny,C.,Block,S.,Pauw-Gillet,M.C.,Hoffmam,E.,Llabres,G.,Adjakidjé,V., Quetin-Leclercq,J.,2002.CytotoxicaporphinealkaloidsfromCassythafiliformis. PlantaMed.68,1042–1044.
Suffredini, I.B.,Paciencia,M.L.B.,Frana,S.A.,Varella, A.D.,Younes,R.N., 2007a.
InvitrobreastcancercelllethalityofBrazilianplantextracts.Pharmazie10, 798–800.
Suffredini,I.B.,Paciencia,M.L.B.,Varella,A.D.,Younes,R.N.,2007b.Invitrocytotoxic activityofBrazilianplantextractsagainstahumanlung,colonandCNSsolid cancersandleukemia.Fitoterapia78,223–226.
Suffredini,I.B.,Paciencia,M.L.B.,Varella,A.D.,Younes,R.N.,2006a.Invitroprostate cancercellgrowthinhibitionbyBrazilianplantextracts.Pharmazie61,722–724. Suffredini,I.B.,Varella,A.D.,Younes,R.N.,2006b.Cytotoxicmoleculesfromnatural sources.TappingtheBrazilianbiodiversity.AnticancerAgentsMed.Chem.6, 67–75.
Suffredini,I.B.,Sader,H.S.,Gonc¸alves,A.G.,Reis,A.O.,Gales,A.C.,Varella,A.D.,Younes, R.N.,2004.Screeningofantibacterialactiveextractsobtainedfromplantsnative toBrazilianAmazonrainforestandAtlanticforest.Braz.J.Med.Biol.Res.37, 379–384.
Suffredini,I.B.,Saraceni,C.H.C.,Díaz,I.E.C.,2015.Canmouthwashescontaining chlorhexidine0.12%beusedassynonymofawatersolutionofchlorhexidine 0.12%?Braz.J.Pharm.Sci.51,367–372.
Sulaiman,S.N.,Mukhtar,M.R.,Hadi,A.H.,Awang,K.,Hazni,H.,Zahari,A.,Litaudon, M.,Zaima,K.,Morita,H.,2011.Lancifoliaineanewbisbenzylisoquinolinefrom thebarkofLitsealancifolia.Molecules16,3119–3127.
Tempone,A.G.,Borborema,S.E.T.,Andrade,H.F.J.,AmorimGualda,N.C.,Yogi,A., Carvalho,C.S.,Bachiega, D.,Lupo, F.N.,Bonotto,S.V.,Fischer,D.C.H.,2005. AntiprotozoalactivityofBrazilianplantextractsfromisoquinoline alkaloid-producingfamilies.Phytomedicine12,382–390.
Villar,R.,Calleja,J.M.,Morales,C.,Cáceres,A.,1997.Screeningof17Guatemalan medicinalplantsforplateletantiaggregantactivity.Phytother.Res.11,441–445. Villar,A.,Mares,M.,Rios,J.L.,Canton,E.,Gobernado,M.,1987.Antimicrobialactivity
ofbenzylisoquinolinealkaloids.Pharmazie42,248–250.
Voigt,W.,2005.SulforhodamineBassayandchemosensitivity.MethodsMol.Med. 110,39–48.
Wu,Y.C.,Chang,G.Y.,Duh,C.Y.,Wang,S.K.,1993.CytotoxicalkaloidsofAnnona montana.Phytochemistry33,497–500.