w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antiproliferative
effects
of
pinostrobin
and
5,6-dehydrokavain
isolated
from
leaves
of
Alpinia
zerumbet
Walter
A.
Roman
Junior
a,b,∗,
Denise
B.
Gomes
a,
Barbara
Zanchet
a,
Amanda
P.
Schönell
b,
Kriptsan
A.P.
Diel
b,
Thais
P.
Banzato
c,
Ana
L.T.G.
Ruiz
c,
João
E.
Carvalho
c,d,
Angelita
Neppel
e,
Andersson
Barison
e,
Cid
Aimbiré
M.
Santos
faProgramadePós-graduac¸ãoemCiênciasdaSaúde,UniversidadeComunitáriadaRegiãodeChapecó,Chapecó,SC,Brazil
bGrupodePesquisaemFitoquímicaeFarmacologiadeProdutosNaturais,UniversidadeComunitáriadaRegiãodeChapecó,Chapecó,SC,Brazil
cCentroPluridisciplinardePesquisasQuímicas,BiológicaseAgrícolas,DivisãodeFarmacologiaeToxicologia,UniversidadeEstadualdeCampinas,Campinas,SP,Brazil dFaculdadedeCiênciasFarmacêuticas,UniversidadeEstadualdeCampinas,Campinas,SP,Brazil
eDepartamentodeQuímica,UniversidadeFederaldoParaná,Curitiba,PR,Brazil
fLaboratóriodeFarmacognosia,DepartamentodeFarmácia,UniversidadeFederaldoParaná,Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14April2017 Accepted30May2017 Availableonline29July2017
Keywords:
Medicinalplants Antiproliferativeeffects 5,6-Dehydrokavain Pinostrobin Antitumoragents
a
b
s
t
r
a
c
t
Naturalproductsareamajorsourceofdrugsforthetreatmentofcancer.ThespeciesAlpiniazerumbet
(Pers.)B.L.Burtt&R.M.Sm,Zingiberaceae,iswidelydistributedinBrazilwhereitisknownas“colônia”. Theleavesarecommonlyusedinthetreatmentofhypertensionanddyspepsia,however,theeffectsof
A.zerumbetextractsandisolatedsubstancesonhumancancercellsremaintobeelucidated.Thisstudy wasdesignedtoidentifythechemicalconstituentsofhydroalcoholicanddichloromethaneextractsfrom
A.zerumbetleavesandtoinvestigatetheirinvitroantiproliferativeactivity.Theisolatedphytochemicals includedkaempferol,dihydro-5,6-dehydrokavain,5,6-dehydrokavain,andpinostrobin.The hydroalco-holicextractinhibitedcellularproliferationonlyathighconcentrations,whilethedichloromethane extractshowedamoderateantiproliferativeeffectagainstleukemiaandlungtumorcelllines. 5,6-Dehydrokavainshowedpotentcytostaticactivityagainstglioblastomacellsandamoderateeffectonall othertumorcelllines.Pinostrobinshowedpotentactivityagainstleukemiaandbreasttumorcelllines andmoderatecytostaticeffectagainstovariancell.Furthermore,thisisthefirstreportontheisolationof kaempferolandpinostrobinfromA.zerumbetleaves.Moreover,thepurificationprocessdescribedinthis studywaseffective.TheseresultssuggestthatA.zerumbetleavesareapromisingsourceofanticancer compounds.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cancerisaseriouspublichealthproblemrepresentingthe sec-ondlargestcauseofdeath(Siegeletal.,2016).Overthenexttwo decades,approximately22 millioncases per yearare expected worldwide(McGuire,2016).Canceristhenamegiventoasetof morethan 100diseases thathave incommontheuncontrolled growthof(malignant)cellsthatinvadetissuesandorgansandcan spread(metastasize)tootherpartsofthebody.Dividingrapidly, thesecellstendtobeveryaggressiveanduncontrollable,causing
∗ Correspondingauthor.
E-mail:romanwa@unochapeco.edu.br(W.A.Junior).
theformationoftumorsormalignantneoplasms(Almeidaetal., 2005;Elkadyetal.,2016).
Thetreatmentofcancerisbasedmainlyonsurgicalresection of the tumor mass and/or administration of radiotherapy, immunotherapy,and/or chemotherapy.However,many cancers stillexhibitonlymodestclinicalresponsestoprotocolsdeveloped foreitherprimarytumorsormetastases(Costa-Lotufoetal.,2010). Moreover,manyanticanceragentshavehighratesofadverse reac-tionsandtoxicityaswellasalowselectivityfortumorcells(Prakash etal.,2013;TopculandCetin,2014).Therefore, withthe objec-tiveoffindingmoreeffectiveandsafetreatments,pharmacological studieswithsubstancesisolatedfromplants,aswellassynthetic derivativesbasedonthesenatural compounds,haveintensified (Harveyetal.,2015;NewmanandCragg,2016).
http://dx.doi.org/10.1016/j.bjp.2017.05.007
Naturalproductshave beenreportedtoact directlyor indi-rectlyviamultiplecellsignalingpathways.Thus,thecombination oftraditionalchemotherapeuticdrugswithextractsand/orisolated substancescouldprovideaneffectivealternativeforcancer treat-mentorovercomechemoresistance(Apayaetal.,2016).Among naturalproducts,phenoliccompounds,includingflavonoids,have beenshowntohaveanarrayofpharmacologicalactivities(Kristo etal.,2016)suchasanti-inflammatory,cancerchemopreventive, andchemotherapeutic(García-Lafuenteetal.,2009;Georgeetal., 2017).
Alpiniazerumbet(Pers.)B.L.Burtt&R.M.Sm,Zingiberaceae,is
nativetoChinaandJapanandcultivatedinBrazilwhereispopularly knownasfalso-cardamomo,pacová,gengibre-concha,andcolônia (Lorenzi and Matos,2002; Lorenziand Souza,2008).Thisplant is herbaceous,tropical perennial, rhizomatoza,with stemshort thatcanreachupto3mtall.Itbearsfunnel-shapedflowersand theleavesarelanceolate,aromatic,andofcoriaceousconsistency (Correaetal.,2010;Sabooetal.,2014).TheA.zerumbetleavesare traditionallyusedindyspepsiatreatmentandasananthelmintic, in addition to theirantimicrobial, anti-inflammatory,and anti-hypertensive properties (Almeida, 1993; Correa et al., 2010). In addition tokava pyrones,dihydro-5,6-dehydrokavain (DDK), and 5,6-dehydrokavain (DK), rutin, kaempferol-3-O-rutinoside, kaempferol-3-O-glucuronide, (+)-catechin, and (−)-epicatechin, have been reported (Mpalatinos et al., 1998; Elzaawely et al., 2007;XuanandTeschke,2015;Kumagaietal.,2016).The phar-macologicalpropertiesattributedtoextractsofA.zerumbetleaves, flowers, seeds, and rhizome include diuretic and hypotensive effects(Laranjaetal.,1991;Mendonc¸aetal.,1991;Lahlouetal., 2003),antioxidant(Elzaawelyetal.,2007),vasodilatory(Pintoetal., 2009;Victórioetal.,2009),hypolipidemic(Linetal.,2008),and antidepressant-likeeffect(Bevilaquaetal.,2016).Further, antineo-plasticeffectsofAlpiniaofficinarumHence(Ghil,2013)andAlpinia
galanga(L.)Willd.(Samarghandianetal.,2014)rhizomeextracts
havebeendescribed.However,therearenoreportsregardingthe antiproliferativeactivityofA.zerumbetextractsandisolated sub-stances.
Inthiscontext,thepresentstudyaimedtoinvestigatethe chem-ical composition and the in vitro antiproliferative effects of A.
zerumbetleafextractsandtheirmajorconstituents.
Materialandmethods
Standardsandchemicals
Allsolventsand reagentswereofanalytical gradeandwater wasdistilledanddeionized.Thesolventsusedwereethylacetate, methylenechloride,ethanol,andhexane(Vetec®,RiodeJaneiro, Brazil).Nuclearmagneticresonance(NMR)experiments(1Hand 13C)wereperformedonaBrukerAvance400spectrometer(400
and100MHz,respectively)usingCDCl3assolvent.Chemicalshifts
of 1H and 13C NMR were expressed in ppm (ı)using TMS at
0.00ppm as aninternal standard, and couplingconstants(J)in Hz.Columnchromatographywasperformedonsilicagel(Merck, Darmstadt,Germany;230–400meshASTM),andanalytical thin layerchromatography(TLC)wasperformedusingsilicagelplates (Kieselgel60F254,Merck).Thespotswerevisualizedusing
ultra-violetlight(366nm)orbysprayingwith10%H2SO4inmethanol
followedbyheatingat110◦C(10min).
Plantmaterial
TheleavesofAlpiniazerumbet(Pers.)B.L.Burtt&R.M.Sm, Zin-giberaceae,werecollectedinChapecó(SC),Brazil(26◦58′36.06′′S and52◦44′27.18′′W).TheplantmaterialwasidentifiedbyOsmar
dosSantosRibas,theherbariumcuratoroftheMunicipalBotanical MuseumofCuritiba(PR),whereavoucherspecimenwasdeposited (MBM#306196).
PreparationofextractsofA.zerumbetandchemicalisolation
The leaves of A. zerumbet were dried at room temperature (25±5◦C), pounded in a knife mill (Ciemlab®, CE430),passed throughsieve(425m;35Tyler/Mesh),identified,andstored
pro-tectedfromlight.Theextractswereproducedbymaceration(5 days)atroomtemperatureusingdry-milledleavesofA.zerumbet
(100g)insolvent(1:20,w/v),first,withdichloromethaneand, sub-sequently,70%ethanol.AfterfiltrationthroughBüchnerfunnel,the dichloromethane (DEA)and hydroalcoholic(HEA)extractswere concentratedbyevaporationunderreducedpressure,lyophilized, weighed,andstoredinafreezerat−20◦C.
AsampleofDEAextract(4g)wasdissolvedinhexaneand sub-mittedtocolumnchromatographyusingastationaryphasesilica gel(Merck,Darmstadt,Germany)and eluted withhexane:ethyl acetate(EtOAc)(90:10,v/v)increasinginpolarityto90%EtOAc(v/v) toyieldfoursubfractions.ThesubfractionswereanalyzedbyTLC usinghexane:EtOAc(80:20,v/v)asthemobilephase,visualizedat 366nm,andrevealedwithH2SO4 (10%inmethanol)followedby
heatingat110◦C(10min).Subfraction2(0.032g)wasobservedasa spotbyTLCanalysisandwasidentifiedascompound1.Subfraction 3(0.316g)wasfurtherseparatedusingflashcolumn chromatogra-phywithdichloromethaneasaneluent,producingthreeadditional subfractions.Subfraction3.1(0.042g)wasidentifiedascompound 2.
TheHEAextract(50g)wasdilutedindistilledanddeionized water(500ml)andpartitionedwithEtOAc(fivetimes;500ml).The fractionEtOAc(5.2g)wasfractionatedusingcolumn chromatog-raphy (SephadexLH-20) withMeOH asan eluent.TLC analysis usingDCM:MeOH(90:10,v/v)asthemobilephasewasusedto identifyfivesubfractions.Subfraction4(0.053g)wasidentifiedas compound3.
FreshleavesofA.zerumbet(2kg)wereextractedbydecoctionin distilledwater(10l)for15min.Afterfiltration,theaqueousextract (AEA)wasreducedto1000mlbyevaporationunderreduced pres-surefollowedbyliquidpartitionwithchloroform(1000ml).The chloroformfraction(CFA)wasconcentratedbyevaporationunder reducedpressureandweighed(0.785g).Forrecrystallization,to CFA(0.785g),100mlofdistilledwaterwasaddedat100◦Candthe solutionfilteredthroughaglassfiltrationfunnel.Thefiltratewas immediatelyrefrigeratedat−8◦Cfor48h,afterwhich,the crys-talswerefilteredusingaBüchnerfunnelandstoredinthehood withanhydrousNa2SO4.Thecrystalsobtained(0.045g)were
ana-lyzedbyTLCusingCHCl3:EtOH(9:1,v/v)asaneluentandvisualized
at366nmorbysprayingwith10%H2SO4 inmethanolfollowed
byheatingat110◦C(10min)(Itokawaetal.,1981).Thecrystals isolatedusingthismethodwereidentifiedascompound4.
Antiproliferativeassay
RPMI-1640supplementedwith5%fetalbovineserum(RPMI/FBS 5%)withpenicillin:streptomycin mixture1000U/ml:1000g/ml
(1ml/l;RPMI-1640).
Stocksolutionsofthesamples(5mg)werepreparedinDMSO (50l)followedbysuccessivedilutionsinRPMI/FBS5%togivefinal
concentrationsof0.25,2.5,25,and 250g/ml.Doxorubicinwas
usedasapositivecontrolatfinalconcentrationsof0.25,2.5,25, and250g/ml.
Cells in 96-well plates (100l cells/well, cell densities:
3–7×104cells/ml)wereincubatedwitheachofthefour
concentra-tionsofsamplesolutionordoxorubicin(100l/well)intriplicate
(n=3),for48hat37◦Cand5%ofCO2.Before(T0plate)andafter(T1 plates)sampleaddition,cellswerefixedwith50%trichloroacetic acid(50l well) and stained with sulforhodamine B to
quan-titate cell proliferation using the reading at 540nm. The GI50
(concentrationthatproduces50%cellgrowthorcytostaticeffect) andtheTGI(concentrationthatresultedintotalcellulargrowth inhibition)valuesweredeterminedthroughnon-linearregression appliedtoasigmoidalcurveusingOrigin8.0software(OriginLab Corporation).
Results
ChemicalconstituentsofAlpiniazerumbet
The purification techniques employed allowedthe isolation of four compounds from the A. zerumbet leaf extracts (HEA, DEA, and AEA); thesecompounds were identified by compari-son of theirexperimental spectra (IR, NMR 1H, and 13C) with
thosepreviouslydescribed:DDK(1),DK(2)(Itokawaetal.,1981; Mpalatinosetal.,1998),3,4′,5,7-tetrahydroxyflavone(kaempferol; 3)(Markhametal.,1978),and 5-hydroxy-7-methoxy-2-phenyl-2,3-dihydrochromen-4-one(pinostrobin,PTB;4)(Smolarzetal., 2006;Ramirezetal.,2013).
Diidro-5,6-dehydrokavain(DDK;1):Itwasobtainedascolorless needles,meltingpoint96–98◦C;C14H14O3,IRnmax(cm−1;KBrdisk)
1725(pyronecarbonyl),1650,1530(C C),1238,1129(C O C), 1600,725, 600(benzenering)cm−1; ESI-MS:231.1[M+H]+; 1H
NMR(400MHz,CDCl3)ppm (ı):2.79(2H,t; J=8Hz, 7-H),2.96
(2H,t;J=8Hz,8-H),3.81(3H,s,MeO-),5.51(1H,d;J=2.2Hz,3-H), 5.92(1H,d;J=2.2Hz,5-H),7.19–7.34(5H,m,aromatic);13CNMR
(100MHz,CDCl3)ppm(ı):33.72(C-8),36.13(C-7),56.82(MeO-),
88.21(C-3),101.96(C-5),128.53(C-12),129.44(C-10),129.49 (C-14),129.52(C-11),129.55(C-13),141.23(C-9),166.26(C-6),167.81 (C-2),173.76(C-4).
5,6-Dehydrokavain(DK;2):Itwasobtainedasyellowneedles, melting point 136–138◦C; C14H12O3, IR nmax (cm−1; KBr disk)
1710(pyronecarbonyl),1620,1525(C C),1230,1125(C O C), 1650,700, 620(benzenering)cm−1; ESI-MS:229.1[M+H]+; 1H
NMR(400MHz,CDCl3)ppm(ı):3.87(3H,s,MeO-),5.62(1H,d;
J=2.2Hz,3-H),6.24(1H,d;J=2.2Hz,5-H),6.86(1H,d;J=16Hz, 7-H),7.43(1H,d;J=16Hz,8-H),7.36–7.59(5H,m,aromatic);13CNMR
(100MHz,CDCl3)ppm(ı):56.61(MeO-),89.50(C-3),102.73(C-5),
120,12(C-7),128.10(C-14),128,44(C-10),129,41(C-13),129.52 (C-11),130.23(C-12),136.31(C-9),136.74(C-8),160.55(C-2),166.63 (C-6),173.34(C-4).
3,4′,5,7-Tetrahydroxyflavone(kaempferol;3):Itwasobtained asyellowcrystallinepowder,meltingpoint276–278◦C;C15H10O6, IRnmax(cm−1;KBrdisk)3440(OH),1681(C O),1600(C C),1375
(C C),1335,1303,1267,1165(C O)746,748,705.ESI-MS:286.1 [M+H]+.1HNMR(400MHz,CDCl
3)ppm(ı):6.17(1H,d,J=2.1Hz,
6-H),6.37(1H,d,J=2.1Hz, 8-H),6.89(2H, d,J=9.0Hz,3′,5′-H), 8.07(2H,d,J=9.0Hz,2′,6′-H);13CNMR(100MHz,CDCl
3)ppm(ı):
99.30(C-6),94.56(C-8),104.67(C-10),116.35(C-3′),116.38 (C-5′),123.70(C-1′),130.76(C-6′),130.78(C-2′),137.01(C-3),148.09
Table1
ValuesofGI50andTGI(g/ml)forAlpiniazerumbetleaveshydroalcoholic(HEA)and
dichloromethane(DEA)extractsagainstdifferentcelllines.
Celllines GI50(g/ml) TGI(g/ml)
HEA DEA HEA DEA
U-251 32.73 22.89 222.00 81.82
MCF-7 27.58 9.44 213.60 69.43
NCI/ADR-RES a 7.79 a a
786-0 202.53 24.51 a 52.80
NCI-H460 12.17 5.85 228.88 62.35
PC-3 226.33 23.05 a 78.34
OVCAR-3 18.39 2.05 a 69.17
HT-29 45.13 23.68 a 60.51
K-562 66.98 6.12 a 55.69
HaCat 0.93 4.44 133.31 74.34
Note:Human tumorcelllines: glioblastoma (U-251),breast (MCF-7), ovarian expressingtheresistancephenotype(NCI/ADR-RES),786-O(kidney),non-small cellslung(NCI-H460),prostate(PC-3),ovarian(OVCAR-3),colon(HT-29),leukemia (K-562);humanimmortalizedkeratinocyte(HaCat).GI50=50%growthinhibition;
TGI,totalinhibitionofgrowth.
aEffective concentration higher than the highest tested concentration
(250g/ml).
(C-2),158.35(C-9),160.67(C-4′),162.54(C-5),165.64(C-7),177.41 (C-4).
5-Hydroxy-7-methoxy-2-phenyl-2,3-dihydrochromen-4-one (pinostrobin(PTB);4):Itwasobtainedasyellowneedles,melting point 96–98◦C;C
16H14O4,IRnmax (cm−1; KBrdisk) 3432(OH),
1635(C O),1620(C C),1390(C C),1350,1311,1301,1245,1178 (C O)759, 749,700.ESI-MS:271.0[M+H]+;1HNMR(400MHz,
CDCl3)ppm(ı):2.72(1H,dd,J=16.6,3.2Hz,3-H),2.98(1H,dd,
J=16.6, 12.7Hz, 3-H), 3.82 (3H, s, 7-OMe), 5.24 (1H, dd, 12.7, 3.2Hz, 2-H), 6.05 (1H, d;J=2.2Hz, 6-H), 6.10 (1H, d;J=2.2Hz, 8-H),7.35–7.42(5H,m,aromatic);13CNMR(100MHz,CDCl
3)ppm
(ı):46.52(C-3),56.31(C-7-OMe),80.20(C-2),94.41(C-8),97.28 (C-6),106.20 (C-10), 127.33(C-5′), 127.39 (C-6′), 129.51(C-3′), 129.78(C-4′), 129,79 (C-2′), 141.13(C-1′), 164.48 (C-7),166.67 (C-5),167.28(C-9),191.88(C-4).
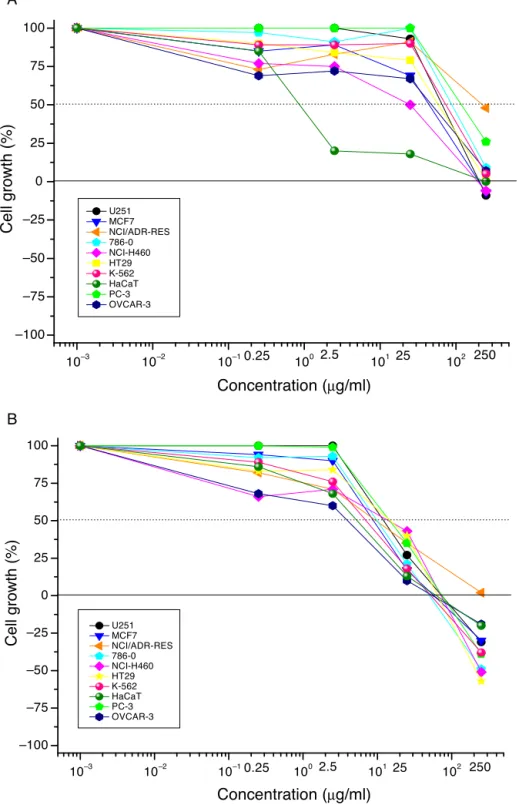
Antiproliferativeeffects
The HEA extract inhibited 50% of the growth of various human tumor cell lines (Fig. 1): immortalized keratinocytes (HaCat; GI50=0.93g/ml), lung (NCI-H460; GI50=12.17g/ml),
ovarian (OVCAR-3; GI50=18.39g/ml), and breast (MCF-7;
GI50=27.58g/ml); however,theHEA extract couldnot induce
totalgrowthinhibition(TGI>200.00g/ml)(Table1).Incontrast,
10–3 10–2 10–1 100 101 102 –100
–75 –50 –25 0 25 50 75 100
A
Ce
ll g
ro
w
th
(
%)
Concent
ration
(
µ
g/ml)
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
0.25 2.5 25 250
B
Concent
ration
(
µ
g/ml)
10–3 10–2 10–1 100 101 102
–100 –75 –50 –25 0 25 50 75 100
C
el
l g
ro
w
th
(
%)
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
0.25 2.5 25 250
Fig.1.InvitroantiproliferativeeffectsofAlpiniazerumbetleavesextracts.(A)Hydroalcoholicextract(HEA);(B)dichloromethaneextract(DEA);concentrationrange: 0.25–250g/ml;expositiontime:48h;humantumorcelllines:glioblastoma(U-251),breast(MCF-7),ovarianexpressingtheresistancephenotype(NCI/ADR-RES),786-O
(kidney),non-smallcellslung(NCI-H460),prostate(PC-3),ovarian(OVCAR-3),colon(HT-29),andleukemia(K-562);humanimmortalizedcellline:keratinocytes(HaCat).
ovarian(OVCAR-3;GI50=2.05g/ml)andlungtumorcells
(NCI-H460;GI50=5.85g/ml)inadditiontototalgrowthinhibitionof
allcelllinesatconcentrationsfrom52.80to81.82g/ml(Fig.1
andTable1).
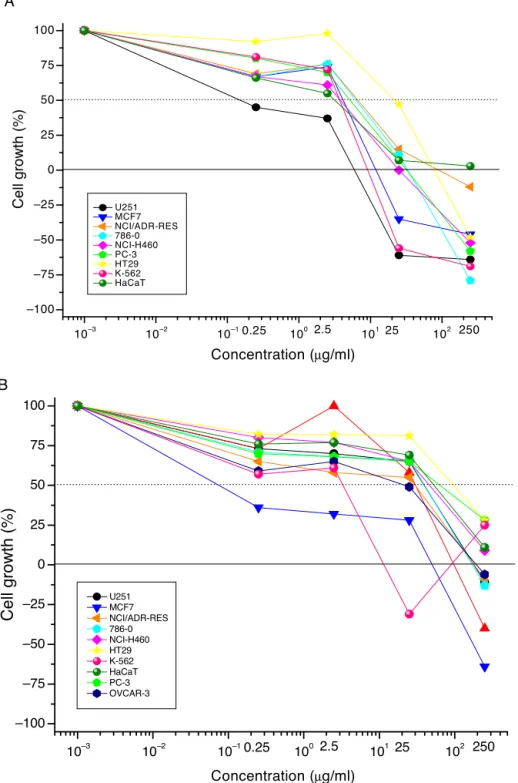
Amongtheisolatedcompounds,DK(2)andPTB(4)were eval-uatedintheantiproliferativeassay(Fig.2).DK(2)showedamore potentantiproliferativeeffectthandidPTB(4)andshowed cyto-staticeffectsagainstalmostallthecelllinestestedwithaGI50from
0.25to5.03g/ml(Table2).Interestingly,PTB(4)wasmoreactive
againstthebreastcancercellline(MCF-7)withGI50<0.25g/ml
andtheleukemiacellline(K562;GI50=0.91g/ml)(Table2).
Discussion
ThefamilyZingiberaceaeisarichsourceofsubstanceshavinga therapeuticvalue,suchasflavonoids,whichhavebeendetectedin severalspecies(Iwashina,2000)andareconsideredphytochemical markersoftheorderZingiberales(Pugiallietal.,1993).Previous studiesonAlpiniarhizomeandseedhavereportedtheisolation of alpinetinfrom Alpiniaspeciosa (Krishna andChaganty,1973; Itokawaetal.,1981);pinocembrinandPTBfromAlpiniarafflesiana
10–3 10–2 10–1 100 101 102 –100
–75 –50 –25 0 25 50 75 100
Cell
grow
th
(
%
)
Conc
entration
(
µg/ml)
Conc
entration
(
µg/ml)
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 PC-3 HT29 K-562 HaCaT
0.25 2.5 25 250
A
10
–310
–210
–110
010
110
2–10
0
–75
–50
–25
0
25
50
75
100
Ce
ll gr
ow
th
(%
)
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
0.25
2.5
25
250
B
Fig.2. Invitroantiproliferativeeffectsof5,6-dehydrokavain(A)andpinostrobin(B)isolatedfromAlpiniazerumbetleaves.A:5,6-dehydrokavain;B:pinostrobin;concentration range:0.25–250g/ml;expositiontime:48h;humantumorcelllines:glioblastoma(U-251),breast(MCF-7),ovarianexpressingtheresistancephenotype(NCI/ADR-RES),
786-O(kidney),non-smallcellslung(NCI-H460),prostate(PC-3),ovarian(OVCAR-3),colon(HT-29),andleukemia(K-562);humanimmortalizedcellline:keratinocytes (HaCat).
kaempferol-3-O-glucuronide,andkaempferol-3-O-rutinosidehave beenisolated fromA.zerumbetleaves(Mpalatinos etal., 1998; Victórioetal.,2009).
Inthisstudy,fourcompoundswereisolatedfromA.zerumbet
leaves,namelyDDK(1),DK(2),kaempferol(3),andPTB(4).DDK (1),andDK(2),whicharecommonconstituentsofthegenusAlpinia
(Pugiallietal.,1993;Iwashina,2000),havebeenidentifiedinthe rootsandleavesofA.zerumbet(Mpalatinosetal.,1998;Kusteretal., 1999;Chompooetal.,2011).However,thisisthefirstreportof kaempferol(3)andPTB(4)isolationfromA.zerumbetleavesand,
thus,contributestothechemotaxonomicinformationrelatedto
Alpiniaspecies.
According to the literature, alcoholic extracts from other
Alpinia species have shown antiproliferative effects against
MCF-7 cells. A methanolic extract of A. officinarum rhizomes (85g/ml) inhibited MCF-7 cell proliferation by inducing
pro-grammed celldeathand arrestingthecell cycleat theSphase (Ghil, 2013). Moreover, a 48h treatment with an ethanolic extract ofA. galanga (L.) Willd. rhizomesat 250g/ml reduced
Table2
ValuesofGI50andTGI(g/ml)for5,6-dehydrokavain(DK)andpinostrobin(PTB)
isolatedfromAlpiniazerumbetleavesagainstdifferentcelllines.
Celllines GI50(g/ml) TGI(g/ml)
DK PTB DK PTB
U-251 0.25 27.33 4.43 228.40
MCF-7 2.85 <0.25 17.28 17.67
NCI/ADR-RES 4.95 4.35 106.64 a
786-0 5.03 12.11 28.08 221.63
NCI-H460 2.04 27.41 25.96 a
PC-3 4.19 34.81 31.26 a
OVCAR-3 a 3.97 a
HT-29 24.77 89.70 79.03 a
K-562 2.81 0.91 9.79 235.62
HaCat 1.53 30.76 150.60 a
Note:Human tumorcelllines: glioblastoma (U-251),breast (MCF-7), ovarian expressingtheresistancephenotype(NCI/ADR-RES),786-O(kidney),non-small cellslung(NCI-H460),prostate(PC-3),ovarian(OVCAR-3),colon(HT-29),leukemia (K-562);humanimmortalizedkeratinocyte(HaCat).GI50,50%growthinhibition;
TGI,totalinhibitionofgrowth.
aEffective concentration higher than the highest tested concentration
(250g/ml).
phosphatidylserineresidueexposureandplasmamembrane per-meation(Samarghandianetal.,2014).
Here,basedontheantiproliferativeactivity,wedemonstrated that successive extraction with an increasingly polar solvent resultedin theconcentrationof bioactive compoundsfromthe DEAextractbutnotfromtheHEAextractofA.zerumbetleaves.A possiblereasonisthatthemorelipophilicchemicalconstituents present in extracts of lower polarity have greater affinity and greatereaseof permeationacrosscellularmembranes (Lee and Houghton,2005).UndertheconditionsdescribedbyFoucheetal. (2008),theHEAextractshowedaweakcytostaticeffectagainst lung (NCI-H460, GI50=12.17g/ml) and ovary tumor cell lines
(OVCAR-3,GI50=18.39g/ml).However,inourexperiments,the
HEA extractactivelyinhibitedtheproliferationof immortalized keratinocytes(HaCat,GI50=0.93g/ml),suggestingpossibletoxic
effectsonnon-tumortissues.Inaddition,theDEAextractshowed moderateantiproliferativeeffectsagainstleukemia(K-562),ovary (OVCAR-3andNCI-ADR/RES),lung(NCI-H460),andbreasttumor celllines(MCF-7)andagainstimmortalizedhumankeratinocytes (HaCat)(Table1).
Amongtheisolatedcompounds,DKandPTBwereevaluated againsttumor and non-tumorcell lines(Table2).Accordingto Fouche et al. (2008), compounds with TGI values<6.25g/ml
show potent antiproliferative activity. Thus, DK had a potent antiproliferativeeffectagainstglioblastomacells(U-251)withTGI value=4.43g/ml,whichwasabout33-foldhigherthanthatfor
theimmortalizedkeratinocytecellline(HaCat;TGI=150.60),and withtwicethepotencyofdoxorubicinusedasapositivecontrol, indicatingpossibleselectivity.ThemainmetabolicpathwayofDK inratsandhumansisthehydroxylationoftheC-12inthearomatic ringtoproducep-hydroxy-5,6-dehydrokavain.Thismetabolitewas detectedinbloodandurineinthefirstfewhoursafterabsorption, producingbydesmethylationofthe4-methoxygroupofthe lac-toneringthecompoundo-desmethyl-hydroxy-5,6-dehydrokavain. Inrats,approximately50–75%ofadministeredkavalactonesare excretedintheurine, mostly asglucuronideand sulphate con-jugates.Approximately15%is excretedin thebile(Roweetal., 2011).
WithregardtotheGI50results,PTBshowedapotentcytostatic
effect(GI50<0.25g/ml)againstbreast carcinomacells(MCF-7)
anda219-foldselectivitywhencomparedtothatforimmortalized keratinocytes(HaCat,GI50=30.76g/ml).Accordingtothe
litera-ture,PTBdemonstratedastrongantitumoractivityinmammary carcinomacells,whichwaspartiallyexplainedbytopoisomerase
Iinhibition (Sukardimanet al.,2000).Thisenzymeis responsi-ble for DNAstrandbreak repair, allowingthebroken strandto rotateontheintactoneandreducingthetorsionaltensionofthe molecule(Brandãoetal.,2010)duringtheprocess.The antiprolif-erativeeffectofPTBonmammarytumorscouldalsobeattributed toananti-aromataseeffectratherthanantiestrogenicactivity,as demonstratedbyLeBailetal.(2000).Becausearomataseis respon-siblefortheconversionoftestosteronetoestrogen,inhibitionofthe enzymecouldreduceestrogenlevels,andthereforetheprobability ofhormone-relatedcancer(LeBailetal.,2000).
In addition, PTB presented a potent cytostatic effect on the leukemiacellline(K-562,GI50=0.91g/ml)(Table2),whichisin
agreementwiththeresultsdescribedbySmolarzetal.(2006).PTB alsoinhibitedendothelialcellproliferation,probablybypromoting membrane celldepolarization,suggestinga potential antiangio-genic effect (Siekmann et al., 2013). Due to its lipolyphilicity (LogP=3.1),PTBinratsshowedhighabsorptionwithamaximum concentrationtime of about2h. A largevolumeof distribution has been observed, and the metabolism appears to be essen-tiallyhepatic.Basedonclearancevaluesthiscompoundismainly excretedvianon-renal,withserumhalf-lifeofapproximately7h (Sayreetal.,2012,2015).
Cancerchemotherapycanoftenprolonglife,andprovide tem-poraryrelieffromsymptomsandoccasionallycompleteremission. Asuccessfulanticancermoleculeshouldkillorincapacitatecancer cellswhilehavingreducedtoxicitytowardnormaltissues(Sharma etal.,2016).Inthisstudy,DKandPTBshowedhighselectivityand potentantiproliferativeorcytostaticeffectsagainstglioblastoma andbreastcancercelllines,implicatingapotentialrolein inhibi-tingcancerprogression.Theseresultsaddtotheliteratureshowing thatanumberofherbalcompoundsarecytostaticandinducecell cyclearrest,therebyshowinganimportantmechanismusefulfor developmentofnewantineoplasticdrugs(Sharmaetal.,2014).
Conclusions
DKandPTBisolatedfromtheleavesofA.zerumbethad antipro-liferativeeffectsinvitrowithhighpotencyandselectivityagainst glioblastoma(U-251)andbreastcarcinoma(MCF-7)tumorlines and are potential candidates for development of antineoplasic drugs.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authorscontribution
WARJ,DBG,BZ,APS,andKAPDcontributedinallstepsofthis study.TPB,ALTGR,andJECcontributedtoantiproliferativestudies. AN,andABcontributedtochemicalanalyses.CAMScontributedto designofthestudy.Alltheauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Acknowledgments
ThisworkwassupportedbytheUnochapecó[modalityArt.170 and171–FUMDES].
References
Almeida,E.R.,1993.Plantasmedicinaisbrasileiras:conhecimentospopularese cien-tíficos.Hemus,SãoPaulo.
Almeida,V.L.,Leitão,A.,Reina,L.C.B.,Montanari,C.A.,Donnici,C.L.,Lopes,M.T.P., 2005.Câncereagentesantineoplásicosciclo-celularespecíficoseciclo-celular nãoespecíficosqueinteragemcomoDNA:umaintroduc¸ão.Quim.Nova28, 118–129.
Apaya,M.K.,Chang,M.T.,Shyur,L.F.,2016.Phytomedicinepolypharmacology: can-certherapythroughmodulatingthetumormicroenvironmentandoxylipin dynamics.Pharmacol.Ther.162,58–68.
Bevilaqua,F.,Mocelin, R.,Grimm Junior,C.,daSilvaJunior,N.S.,Buzetto,T.L., Conterato,G.M.,RomanJunior,W.A.,Piato,A.L.,2016.Involvementofthe cate-cholaminergicsystemontheantidepressant-likeeffectsofAlpiniazerumbetin mice.Pharm.Biol.54,151–156.
Brandão,N.H.,David,J.P.,Couto,R.D.,Nascimento,J.A.P.,David,J.M.,2010.Química efarmacologiadequimioterápicosantineoplásicosderivadosdeplantas.Quim. Nova33,1359–1369.
Chompoo,J.,Upadhyay,A.,Kishimoto,W.,Makise,T.,Tawata,S.,2011.Advanced glycationendproductsinhibitorsfromAlpiniazerumbetrhizomes.FoodChem. 12,709–715.
Correa,A.J.C.,Lima,C.E.,Costa,M.C.C.D.,2010.Alpiniazerumbet(Pers.)B.L.Burtt& R.M.Sm(Zingiberaceae):levantamentodepublicac¸õesnasáreasfarmacológica equímicaparaoperíodode1987a2008.Rev.Bras.Pl.Med.12,113–119. Costa-Lotufo,L.V.,Montenegro,R.C., Alves,A.P.N.N.,Madeira,S.V.F.,Pessoa,C.,
Moraes,M.E.A.,Moraes,M.O.,2010.Contribuic¸ãodosprodutosnaturaiscomo fontedenovosfármacosanticâncer:estudosnoLaboratórioNacionalde Oncolo-giaExperimentaldaUniversidadeFederaldoCeará.Rev.Virtual.Quim.2,47–58. Elkady,A.I.,Hussein,R.A.,El-Assouli,S.M.,2016.Harmalextractinducesapoptosis ofHCT116humancoloncancercells,mediatedbyinhibitionofnuclear factor-(Bandactivatorprotein-1signalingpathwaysandinductionofcytoprotective genes.AsianPac.J.CancerPrev.17,1947–1959.
Elzaawely,A.A.,Xuan,T.D.,Koyama,H.,Tawata,S.,2007.Antioxidantactivityand contentsofessentialoilandphenoliccompoundsinflowersandseedsofAlpinia zerumbet(Pers.)B.L.Burtt&R.M.Sm.FoodChem.104,1648–1653.
Fouche,G.,Cragg,G.M.,Pillay,P.,Kolesnikova,N.,Maharaj,V.J.,Senabe,J.,2008. InvitroanticancerscreeningofSouthAfricanplants.J.Ethnopharmacol.119, 455–461.
García-Lafuente,A.,Guillamón,E.,Villares,A.,Rostagno,M.A.,Martínez,J.A.,2009. Flavonoidsasanti-inflammatoryagents:implicationsincancerand cardiovas-culardisease.Inflamm.Res.58,537–552.
George,V.C.,Dellaire,G.,Rupasinghe,H.P.V.,2017.Plantflavonoidsincancer chemo-prevention:roleingenomestability.J.Nutr.Biochem.45,1–14.
Ghil,S.,2013.AntiproliferativeactivityofAlpiniaofficinarumextractinthehuman breastcancercelllineMCF-7.Mol.Med.Rep.7,1288–1292.
Harvey,A.L.,Edrada-Ebel,R.A.,Quinn,R.J.,2015.There-emergenceofnatural prod-uctsfordrugdiscoveryinthegenomicsera.Nat.Rev.DrugDiscov.14,111–129. Hema,P.S.,Nair,M.S.,2009.Flavonoidsandotherconstituentsfromtherhizomesof
Alpiniacalcarata.Biochem.Syst.Ecol.37,52–54.
Itokawa,H.,Morita,M.,Mihashi,S.,1981.Phenoliccompoudsfromtherhizomesof Alpiniaspeciosa.Phytochemistry20,2503–2506.
Iwashina,T.,2000.Thestructureanddistributionoftheflavonoidsinplants.J.Plant Res.113,287–299.
Krishna,B.M.,Chaganty,R.B.,1973.Cardamoninandalpinetinfromtheseedsof Alpiniaspeciosa.Phytochemistry12,238–242.
Kristo,A.S.,Klimis-Zacas,D.,Sikalidis,A.K.,2016.Protectiveroleofdietaryberries incancer.Antioxidants5,37.
Kumagai,M.,Mishima,T.,Watanabe,A.,Harada,T.,Yoshida,I.,Fujita,K.,Watai,M., Tawata,S.,Nishikawa,K.,Morimoto,Y.,2016.5,6-DehydrokawainfromAlpinia zerumbetpromotesosteoblasticMC3T3-E1celldifferentiationIrecommenda shortmorphologicaldescriptionofA.zerumbet.Biosci.Biotechnol.Biochem.7, 1425–1432.
Kuster,R.M.,Mpalantinos,M.A.,Holanda,M.C.,Lima,P.,Brand,E.T.,Parente,J.P., 1999.Determinationofkava-pyronesinAlpiniazerumbetleaves.J.HighResolut. Chromatogr.22,129–130.
Lahlou,S.,Interaminense,L.F.,Leal-Cardoso,J.H.,Duarte,G.P.,2003. Antihyperten-siveeffectsoftheessentialoilofAlpiniazerumbetanditsmainconstituent, terpinen-4-ol,inDOCA-salthypertensiveconsciousrats.Fundam.Clin. Pharma-col.17,323–330.
Laranja,S.M.R.,Bergamaschi,C.M.,Schor,N.,1991.Evaluationofacute administra-tionofnaturalproductswithpotentialdiureticeffects,inhumans.Mem.Inst. OswaldoCruz86,237–240.
LeBail,J.C.,Aubourg,L.,Habrioux,G.,2000.Effectsofpinostrobinonestrogen metabolismandestrogenreceptortransactivation.CancerLett.156,37–44.
Lee,C.C.,Houghton,P.,2005.CytotoxicityofplantsfromMalaysiaandThailandused traditionallytotreatcancer.J.Ethnopharmacol.100,237–243.
Lin,L.Y.,Peng,C.C.,Liang,Y.J.,Yeh,W.T.,Wang,H.E.,Yu,T.H.,Peng,R.Y.,2008.Alpinia zerumbetpotentiallyelevateshigh-densitylipoproteincholesterollevelin ham-sters.J.Agric.FoodChem.56,4435–4443.
Lorenzi,H.,Matos,F.J.A.,2002.PlantasmedicinaisnoBrasil:nativaseexóticas. Insti-tutoPlantarum,NovaOdessa.
Lorenzi,H.,Souza,H.A.M.,2008.PlantasornamentaisnoBrasil:arbustivas,herbáceas etrepadeiras,4ed.InstitutoPlantarum,NovaOdessa.
Markham,K.R.,Ternai,B.,Stanley,R.,Geiger,H.,Mabry,T.J.,1978.Carbon-13NMR studiesofflavonoids.III.Naturallyoccurringflavonoidglycosidesandtheir acyl-atedderivatives.Tetrahedron34,1389–1397.
McGuire,S.,2016.WorldCancerReport2014.Geneva,Switzerland:WorldHealth Organization,InternationalAgencyforResearchonCancer,WHOPress,2015. Adv.Nutr.7,418–419.
Mendonc¸a,V.L.M.,Oliveira,C.L.A.,Craveiro,A.A.,Rao,V.S.,Fonteles,M.C.,1991. Phar-macologicalandtoxicologicalevaluationofAlpiniaspeciosa.Mem.Inst.Oswaldo Cruz86,93–97.
Mohamad,H.,Abas,F.,Permana,D.,Lajis,N.H.,Alib,A.M.,Sukaric,M.A.,Hinc,T.Y.Y., Kikuzakid,H.,Nakatanid,N.,2004.DPPHfreeradicalscavengercomponents fromthefruitsofAlpiniarafflesianaWall.ex.Bak.(Zingiberaceae).Z.Naturforsch. 59,11–12.
Monks,A.,Scudiero,D.,Skehan,P.,Shoemaker,R.,Paull,K.,Vistica,D.,Hose,C., Langley,J.,Cronise,P.,Vaigro-Wolff,A.,Gray-Goodrich,M.,Campbell,H.,Mayo, J.,Boyd,M.,1991.Feasibilityofahigh-fluxanticancerdrugscreenusingadiverse panelofculturedhumantumorcelllines.J.Natl.CancerInst.83,757–766. Mpalatinos,M.,Moura,R.S.,Parente,J.P.,Kuster,R.M.,1998.Biologicallyactive
flavonoidsandkavapyronesfromtheaqueousextractofAlpiniazerumbet. Phy-tother.Res.12,442–444.
Newman,D.J.,Cragg,G.M.,2016.Naturalproductsassourcesofnewdrugsoverthe period1981–2014.J.Nat.Prod.79,629–661.
Pinto,N.V.,Assreuy,A.M.,Coelho-de-Souza,A.N.,Ceccatto,V.M.,Magalhães,P.J., Lahlou,S.,Leal-Cardoso,J.H.,2009.Endothelium-dependentvasorelaxanteffects oftheessentialoilfromaerialpartsofAlpiniazerumbetanditsmainconstituent 1,8-cineoleinrats.Phytomedicine16,1151–1155.
Prakash,O.,Kumar,A.,Pawan,K.,2013.Anticancerpotentialofplantsandnatural products:areview.Am.J.Pharmacol.Sci.1,104–115.
Pugialli,H.R.L.,Kaplan,M.A.C.,Gottlieb,O.R.,1993.Chemotaxonomyofsuperorder Zingiberiflorae(sensuDahlgren)I.Flavonoids.ActaBot.Bras.7,135–148. Ramirez,J.,Cartuche,L.,Morocho,V.,Aguilar,S.,Malagon,O.,2013.Antifungal
activ-ityofrawextractandflavanonsisolatedfromPiperecuadorensefromEcuador. Rev.Bras.Farmacogn.23,370–373.
Rowe,A.,Zhang,L.Y.,Ramzan,I.,2011.Toxicokineticsofkava.Adv.Pharmacol.Sci. 1,1–6.
Saboo,S.S.,Chavan,R.W.,Tapadiya,G.G.,Khadabadi,S.S.,2014.Anorganized assess-mentofspeciesofplantsofAlpiniagenera,belongingtofamilyZingiberaceae. Am.J.Ethnomed.2,102–108.
Samarghandian,S.,Hadjzadeh,M.A.,Afshari,J.T.,Hosseini,M.,2014. Antiprolifera-tiveactivityandinductionofapoptoticbyethanolicextractofAlpiniagalanga rhizhomeinhumanbreastcarcinomacellline.BMCComplement.Altern.Med. 14,1–9.
Sayre,C.L.,Zhang,Y.,Stephanie,E.,Martinez,S.E.,Takemoto,J.K.,Davies,N.M.,2012. Stereospecificanalyticalmethoddevelopmentandpreliminaryinvivo pharma-cokineticcharacterizationofpinostrobinintherat.Biomed.Chromatogr.27, 548–550.
Sayre,C.L.,Alrushaid,S.,Martinez,S.E.,Anderson,H.D.,Davies,N.M.,2015. Pre-clinicalpharmacokineticandpharmacodynamiccharacterizationofselected chiralflavonoids:pinocembrinandpinostrobin.J.Pharm.Pharm.Sci.4,368–395. Sharma,A.K.,Kumar,S.,Chashoo,G.,Saxena,A.K.,Pandey,A.K.,2014.Cellcycle inhibitoryactivityofPiperlongumagainstA549celllineanditsprotectiveeffect againstmetal-inducedtoxicityinrats.IndianJ.Biochem.Biophys.51,358–364. Sharma,U.K.,Sharma,A.K.,Pandey,A.K.,2016.Medicinalattributesofmajor
phenyl-propanoidspresentincinnamon.BMCComplement.Altern.Med.16,156. Siegel,R.L.,Miller,K.D.,Jemal,A.,2016.Cancerstatistics.CACancerJ.Clin.66,7–30. Siekmann,T.R.,Burgazli, K.M.,Bobrich,M.A., Nöll, G.,Erdogan, A.,2013. The antiproliferativeeffectofpinostrobinonhumanumbilicalveinendothelialcells (HUVEC).Eur.Rev.Med.Pharmacol.Sci.17,668–672.
Smolarz,H.D.,Mendykb,E.,Bogucka-Kockaa,A.,Kocki,J.,2006.Pinostrobin–an anti-leukemicflavonoidfromPolygonumlapathifoliumL.ssp.nodosum(Pers.) Dans.Z.Naturforsch.61,64–68.
Sukardiman,H.,Darwanto,A.,Tanjun,M.,Darmadi,M.O.,2000.Cytotoxic mecha-nismofflavonoidfromTemuKunci(Kaempferiapandurata)incellcultureof humanmammarycarcinoma.Clin.Hemorheol.Microcirc.23,185–190. Topcul,M.,Cetin,I.,2014.Endpointofcancertreatment:targetedtherapies.Asian
Pac.J.CancerPrev.15,4395–4403.
Victório,C.P.,Kuster,R.M.,Moura,R.S.,Lagel,C.L.S.,2009.Vasodilatoractivityof extractsoffieldAlpiniapurpurata(Vieill)K.SchumandA.zerumbet(Pers.)Burtt etSmithculturedinvitro.Braz.J.Pharm.Sci.45,507–514.