ABSTRACT
http://dx.doi.org/10.1590/1678-775720150524
7LVVXH GLVVROXWLRQ DQG PRGL¿FDWLRQV LQ GHQWLQ
com posit ion by differ en t sodiu m hy poch lor it e
concent rat ions
Talita TARTARI1, Luciano BACHMANN2, Amanda Garcia Alves MALIZA1, Flaviana Bombarda de ANDRADE1, Marco
Antonio Hungaro DUARTE1, Clovis Monteiro BRAMANTE1
1- Universidade de São Paulo, Faculdade de Odontologia de Bauru, Departamento de Dentística, Endodontia e Materiais Odontológicos, Bauru, SP, Brasil.
8QLYHUVLGDGHGH6mR3DXOR)DFXOGDGHGH)LORVR¿D&LrQFLDVH/HWUDVGH5LEHLUmR3UHWR'HSDUWDPHQWRGH)tVLFD5LEHLUmR3UHWR63%UDVLO
Corresponding address: Talita Tartari - Faculdade de Odontologia de Bauru - Universidade de São Paulo - Al. Octávio Pinheiro Brisolla, 9-75 - 17012-901 - Bauru - SP - Brazil - Phone: +55 14 32358344 - e-mail: talita_t@hotmail.com
6XEPLWWHG1RYHPEHU0RGL¿FDWLRQ-DQXDU\$FFHSWHG)HEUXDU\
S
odium hypochlor it e ( NaOCl) r em ains t he m ost used ir r igat ion solut ion dur ing r oot canal preparat ion because of charact erist ics such as wide- spect rum ant im icrobial act ivit y and or ganic t issue dissolut ion capacit y. How ever, t hese solut ions can alt er dent in com posit ion and t her e is no consensus on t he opt im al concent rat ion of NaOCl t o be used. Obj ect ives: To det er m ine t he or ganic m at t er dissolut ion and changes in dent in chem ical com posit ion prom ot ed by different concent rat ions of NaOCl over t im e. Mat erial and Met hods: Fragm ent s of bovine m uscle t issue w er e w eighed befor e and aft er 5, 10, and 15 m in of im m er sion in t he gr oups ( n= 10) : G1- 0.9% saline solut ion; G2- 1% NaOCl; G3- 2.5% NaOCl; and G4- 5% NaOCl. Bovine dent in fragm ent s w er e subj ect ed t o t he sam e ir r igant s and absor pt ion VSHFWUD ZHUH FROOHFWHG E\ $WWHQXDWHG 7RWDO 5HÀHFWDQFH RI )RXULHU 7UDQVIRUP ,QIUDUHG Spect r oscopy ( ATR- FTI R) befor e and aft er 0,5, 1, 2, 3, 5, 8, and 10 m in of im m er sion in t he solut ions. The rat ios of t he am ide I I I / phosphat e and car bonat e/ phosphat e absor pt ion bands w er e det er m ined. The t issue dissolut ion and car bonat e/ phosphat e rat ios w er e subm it t ed t o t he t w o- way analysis of var iance ( ANOVA) w it h Tukey’s m ult iple- com par ison t est (D< 0.05) and t o t he one- way analysis of var iance w it h Tukey’s (D< 0.05) . The am ide I I I / phosphat e rat io was analyzed by Fr iedm an t est (D< 0.05) and t he Kr uskal- Wallis t est w it h Dunn’s post - hoc (D< 0.05) . Result s: The incr ease in NaOCl concent rat ion and cont act WLPHLQWHQVL¿HGWKHGLVVROXWLRQRIRUJDQLFPDWWHUDQGGHQWLQFROODJHQZLWKUHGXFWLRQLQ WKHDPLGH,,,SKRVSKDWHUDWLR6LJQL¿FDQWGLIIHUHQFHVEHWZHHQDOOJURXSVSZHUH obser ved in t he dissolut ion of or ganic m at t er at 10 m in and in t he am ide I I I / phosphat e rat io bet w een t he saline solut ion and 5% NaOCl at 5 m in. The car bonat e/ phosphat e rat io GHFUHDVHGVLJQL¿FDQWO\LQ**DQG*DIWHUPLQRILPPHUVLRQSEXWPRUH alt erat ions did not occur in t he subsequent per iods ( p> 0.05) . I nt er gr oup differ ences w er e not obser ved in t his rat io ( p> 0.05) . Conclusions: The incr ease in t he exposur e t im e and in t he concent rat ion of NaOCl solut ion lead t o an incr ease in t he t issue dissolut ion and dent in collagen depr ot einat ion. Fur t her m or e, som e car bonat e ions ar e r em oved fr om t he dent in inor ganic phase by t he NaOCl.Ke y w or ds: Dent in. Dissolut ion. Four ier t ransfor m infrar ed spect r oscopy. Or ganic m at t er. Sodium hypochlor it e.
I N TROD UCTI ON
The physical and chem ical effect s of t he irrigat ion so l u t i o n s u sed i n en d o d o n t i cs ar e cr u ci al f o r cleaning and disinfect ion, since st udies have shown t hat a lar ge num ber of r oot dent in walls r em ain
u n t o u ch ed af t er b i o m ech an i cal p r ep ar at i o n2 5.
t issue dissolut ion capacit y29,33. How ever, t her e is
no consensus r egar ding t he ideal concent rat ion of NaOCl t o be used.
An incr ease in t he num ber of m icr oor ganism s w as obser ved w hen int racanal m edicam ent w as n ot u sed b et w een t h e t r eat m en t session s an d t his fact was assigned t o t he or ganic t issue t hat r em ain ed in t h e r oot can al an d pr ov ided ideal con dit ion s f or bact er ial gr ow t h9. Possible w ay s
t o im pr ov e t he t issue dissolut ion by NaOCl ar e t h e in cr ease in t h e pH7, t h e con cen t r at ion an d
t em perat ur e of t he solut ions, ult rasonic agit at ion, an d p r olon g ed w or k in g t im e2 9 , 3 2. How ev er, t h e
incr ease in concent rat ion of NaOCl solut ions can lead t o undesirable effect s such as an incr ease in t oxicit y t o t he per iapical t issues13.
NaOCl solu t ion s can also act in t h e d en t in changing it s chem ical com posit ion19. I n m ineralized
GHQWLQ WKH FROODJHQ ¿EULOV DUH HQFDSVXODWHG E\
apat it e cr yst als, t hus t he dim ensions of m olecules t hat can penet rat e in t he dent in st r uct ur e should be sm aller35. NaOCl m olecules can penet rat e in
t he apat it e- encapsulat ed collagen m at r ix because of t heir low m olecular w eight ( 74.4 Da)35, and as
DQRQVSHFL¿FR[LGL]LQJDQGSURWHRO\WLFDJHQWFDQ
oxidize t he or ganic m at r ix, denat ur e t he collagen, and adver sely affect s t he m echanical pr oper t ies of dent in24,34. The effect s of NaOCl solut ions on t he
collagen of t he dent in organic m at rix m ay also affect t he sealing abilit y and t he adhesion of r esin- based cem ent s and root canal sealers t hat chem ically bond t o t he dent inal collagen17,22.
I n addit ion, wit h t he t echnological advancem ent in en d od on t ics, t h e b iom ech an ical p r ep ar at ion phase is becom ing fast er, and t he use of m or e concent rat ed ir r igant s for adequat e sanit izat ion is pr obably necessar y. Ther efor e, it is im por t ant t o know how m uch t he increase in NaOCl concent rat ion, wit h t he obj ect ive t o enhance sanit izat ion, im proves t h e or gan ic m at t er dissolu t ion w it h ou t cau sin g m u ch u n d esir ab le alt er at ion s of t h e ch em ical com posit ion of t he dent in. The aim of t he pr esent st udy was t o det er m ine t he dissolut ion capacit y of or ganic m at t er and t he chem ical alt erat ions on t he com posit ion of t he dent in sur face pr oduced by differ ent concent rat ions of NaOCl at differ ent exposure t im es. The null hypot hesis t est ed was t hat t he different concent rat ions of NaOCl solut ions have sim ilar capacit y of t issue dissolut ion and effect s on dent in com posit ion and act sim ilar ly over t im e.
M ATERI AL AN D M ETH OD S
I r r iga t ion solu t ion s
Concent rat ed ( 10- 15% ) NaOCl solut ion ( Sigm a-Aldr ich; St . Louis, MO, USA) was dilut ed in dist illed wat er t o produce solut ions w it h 1% , 2.5% , and 5%
FRQFHQWUDWLRQVWKDWZHUHFRQ¿UPHGE\LRGRPHWULF
t it r at ion . Th e solu t ion s ob t ain ed w er e st or ed , pr ot ect ed fr om t he light in air t ight plast ic bot t les in a r efr igerat or at 4°C, and r em oved one hour before t he experim ent s t o reach room t em perat ure. A 0.9% physiological saline solut ion was used as a cont r ol. The pHs of t he solut ions w er e det er m ined before t he experim ent s using a calibrat ed pH m et er.
Tissu e dissolu t ion
Bovine m uscle t issue was acquir ed on t he day of t he exper im ent and kept r efr igerat ed in 100% hum idit y. The m uscle was cut w it h scalpel blades in pieces w it h 2x2x6 m m ( w idt h x t hickness x lengt h) and t he specim ens obt ained w er e w eighed on t he FX- 300 elect r onic balance ( A&D Com pany; Tokyo, Japan ) . To do t h e sam ple calibr at ion , t h e dat a obt ained w er e subm it t ed t o st at ist ical analysis t o ver ify and ensur e t hat all gr oups w er e st at ist ically sim ilar befor e t he beginning of t he ex per im ent .
Next , t he sam ples w er e subm it t ed t o one of t he following solut ions ( n= 10) : G1– 0.9% physiological saline solut ion ( cont rol) ; G2– 1% NaOCl; G3– 2.5% NaOCl; and G4– 5% NaOCl.
Specim ens fr om each gr oup w er e im m er sed for
PLQLQLQGLYLGXDOFRQWDLQHUV¿OOHGZLWKP/RI
t he t est solut ion. All t he cont ainer s w er e placed in an ult rasonic t ub t o agit at e t he irrigant s for 15 s per each m inut e. Next , t he specim ens were subm erged in dist illed wat er for 0,5 m in t o rem ove t he irrigat ion
VROXWLRQV7KH\ZHUHWKHQEORWWHGZLWK¿OWHUSDSHU
an d r e- w eig h ed . Th is p r oced u r e w as r ep eat ed 3 t im es t o obt ain dat a of 5, 10, and 15 m in of im m ersion. The solut ions were renewed before each im m ersion period t o sim ulat e clinical condit ions and t o prevent sat urat ion. All t est procedures were done at r oom t em perat ur e ( 25°C) .
ATR- FTI R
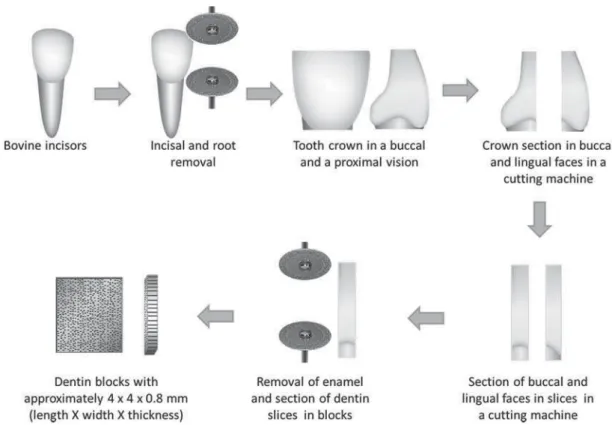
Cr ow ns of bovine t eet h w er e r em oved at t he cem ent oenam el j unct ion using a diam ond disc at low- speed under wat er cooling. Then, t he incisal of t he cr ow ns w er e r em oved in t he sam e way. Each cr ow n w as t h en lon git u din ally sect ion ed in t h e m esiodist al dir ect ion in t he I som et 1000 cut t ing m ach in e ( Bu eh ler Lt d. ; Lak e Blu ff, I L, USA) t o obt ain t he buccal and lingual por t ions. Slices w it h appr oxim at ely 0.8 m m t hicknesses w er e obt ained fr om t hese cr ow n halves. The slices w er e t hen cut again w it h a diam ond disc at low- speed t o r em ove t h e su r r ou n d in g en am el an d t o ob t ain t w en t y specim ens w it h appr oxim at ely 4 m m x 4 m m x 0.8 m m ( lengt h x w idt h x t hickness) ( Figur e 1) .
One sur face of t he dent in specim ens was w et polished w it h 4000 grain silicon car bide abrasive paper s ( Buehler ; Lake Bluff, I L, USA) and alpha al u m i n a su sp en si o n s w i t h 1 an d 0 . 3 m i cr o n s
6WUXHUV%DOOHUXS'HQPDUNXQWLODÀDWDQGVPRRWK
im m er sed in dist illed wat er and ult rasonicat ed for 1 m in t o r em ove any r esidue fr om t he polishing. They w ere t hen dried w it h absorbent paper and t he polished sur face posit ioned on t he diam ond cr yst al
WKDWZDVWKHLQWHUQDOUHÀHFWLRQHOHPHQWIURPWKH
Four ier Transfor m I nfrar ed ( FTI R) Spect r om et er N i co l e t 3 8 0 ( Th e r m o Fi sh e r Sci e n t i f i c I n c. ; Walt ham , MA, USA) and t he absor bance spect ra w ere collect ed by t he t echnique of At t enuat ed Tot al
5HÀHFWLRQ$75EHWZHHQZDYHQXPEHUVRI
and 400 cm- 1 at resolut ion of 1 cm- 1 using 32 scans.
Th e sp ecim en s w er e r an d om ly assig n ed t o t h e p r ev i o u sl y d escr i b ed f o u r g r o u p s ( n = 5 ) . Each sp ecim en w as p laced in sid e a m icr ot u b e cont aining 1.5 m L of t he solut ions for 0,5 m in and ult rasonicat ed for 15 s. Next , t hey were t ransferred t o a m icr ot ube cont aining 1.5 m L of dist illed wat er and rinsed for 1 m in wit h 15 s of ult rasonic agit at ion. They w ere t hen dried w it h absorbent paper and t he ATR- FTI R spect ra r ecor ded again. Specim ens w er e r eplaced in t he solut ions for addit ional 0, 5 m in
follow ing t he sam e prot ocol described t o collect t he new spect ra. This process was sequent ially repeat ed t o obt ain t he spect ra at t im e int er vals of 0, 0.5, 1, 2, 3, 5, 8 and 10 m in. How ever, aft er obt aining t he 1 m in spect r um , t he ult rasonic agit at ion was per for m ed for 15 s per each m inut e of im m er sion in t h e ir r igan t s. To en su r e t h e effect iv en ess of t he solut ions, t hey w er e r enew ed aft er t he t im e int er vals of 2, 5, and 8 m in.
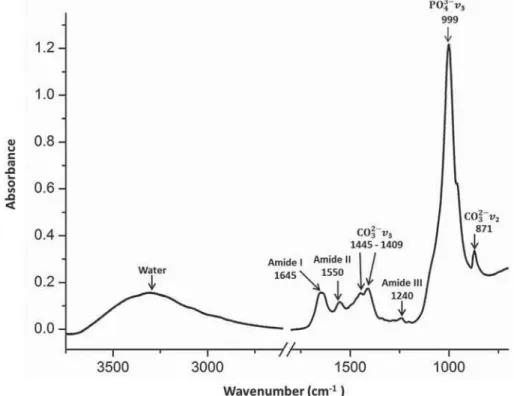
A t ypical absor bance spect r um obt ained fr om a disc of unt r eat ed dent in is show n in Figur e 2. I n t his spect r um t he peak s bet w een 3.750–750
cm- 1ZHUHLGHQWL¿HG7KHDUHDVRIWKHDEVRUSWLRQ
bands of phosphat e ( PO3-) , car bonat e ( CO3-) , and
am id e I I I of each sp ect r u m w er e d et er m in ed . The w av enum ber values em ploy ed for t he ar ea int egrat ions for t he am ide I I I w er e bet w een 1298– 1216 cm- 1 spect ral range, bet w een 888–816 cm- 1
for t he car bonat e, and bet w een 1170–780 cm- 1 for
t he phosphat e. I nside t he phosphat e spect ral range t here is t he carbonat e band at 888–816 cm- 1, whose
value was subt ract ed t o obt ain t he r eal value of t he ar ea of phosphat e band.
To evaluat e t he effect s of NaOCl solut ions on t he chem ical com posit ion of dent in, t w o param et er s w er e calcu lat ed . Th e f ir st w as t h e am id e I I I / phosphat e rat io t hat was used t o det er m ine t he collagen depr ot einat ion by NaOCl. The am ide I I I band was chosen, for in t his r egion of 1298–1216 cm- 1 t her e is no over lapping w it h bands of ot her
dent in com ponent s. I nst ead, in bands of am ides A and B at 3115 and 2860 cm- 1 and am ide I at 1645
cm- 1, over lapping occur s w it h w at er bands, and
in bands assigned t o am ide I I , pr esent at ar ound 1550 cm- 1, t he over lapping occur s w it h car bonat e
bands3. The effect of NaOCl in t he inor ganic phase
of t he dent in was evaluat ed using t he car bonat e/ phosphat e ( PO3-) / ( CO3-) rat io, which was t he second
param et er. The rat ios w er e obt ained by t aking t he quot ient bet w een t he ar eas of t he bands.
The am ide I I I / phosphat e rat io was m easur ed t o evaluat e how t he am ide I I I or t he phosphat e changed w hen im m er sed in NaOCl solut ion. For exam ple, w hen t his rat io decr eases, it m eans t hat t he am ount of am ide I I I ( organic m at t er) decreased
Figure 1- Sample preparation for the ATR-FTIR analysis
4 4
com par ed w it h t he phosphat e ( inor ganic m at r ix) . How ever, w hen em ploying a chem ical agent t hat r em ov es or g an ic m at t er an d in or g an ic m at t er sim ult aneously, t his rat io could st ay unalt er ed. The car bonat e/ phosphat e rat io is em ployed t o evaluat e t he dissolut ion of t he inor ganic m at r ix; t his rat io m easur es t he car bonat e dissolut ion in r elat ion t o t he phosphat e radical.
St a t ist ica l a n a ly sis
Th e collect ed dat a of t issu e dissolu t ion an d c a r b o n a t e / p h o s p h a t e r a t i o s s h o w e d n o r m a l dist r ibut ion, and w er e subm it t ed t o t he t w o- way analysis of variance ( ANOVA) wit h Tukey’s m ult iple-com par ison t est (D< 0 . 0 5 ) t o det ect in t r agr ou p differ ences over t im e and t he one- w ay analy sis of var iance w it h Tukey’s (D< 0.05) t o det ect any differ ences bet w een t he gr oups at t he sam e t im e per iod.
Th e a m i d e I I I / p h o s p h a t e r a t i o e x h i b i t e d abnorm al dist ribut ion. The nonparam et ric Friedm an t est (D< 0 . 0 5 ) w as u sed t o d et ect i n t r ag r o u p differ ences am ong differ ent per iods of im m er sion an d t h e Kr u sk al Wallis t est w it h Du n n ’s p ost
-hoc (D< 0.05) t est was used t o det ect int er gr oup differ ences in t he sam e per iod.
RESULTS
Tissu e dissolu t ion
Table 1 pr esent s t he pHs of t he solut ions, m ean v alu e an d st an dar d dev iat ion of t h e w eigh t of fragm ent s of bovine m uscle t issue and per cent age
d i f f e r e n ce b e t w e e n t h e i n i t i a l w e i g h t o f t h e f r ag m en t s an d t h e w eig h t af t er im m er sion in differ ent solut ions over t im e. The saline solut ion did not alt er t he w eight of fragm ent s bet w een t he per iods analyzed ( p> 0.05) . Tissue dissolut ion was dir ect ly dependent on t he concent rat ion of NaOCl solu t ion s as w ell as t h e im m er sion t im e. Th e
LQWUDJURXSFRPSDULVRQVVKRZHGVLJQL¿FDQWGHFUHDVH
in w eight of t he fragm ent s for all im m er sion t im e per iods in 1, 2.5, and 5% NaOCl ( p< 0.01) . The int er gr oup com par ison show ed t hat t he r educt ion in w eight s w as higher w it h t he incr ease in t he con cen t r at ion of NaOCl. St at ist ical d if f er en ces
EHWZHHQWKHJURXSVZHUHVLJQL¿FDQWSLQ
m in bet w een G4 and all ot her gr oups, t he G3 was equal t o G2 but different from G1, and G2 was equal t o G1. I n 10 and 15 m in of im m ersion, t he int ergroup
GLIIHUHQFHVZHUHLGHQWL¿HGLQWKHIROORZLQJRUGHUIRU
t issue dissolut ion: G4> G3> G2> G1.
ATR- FTI R
Table 2 pr esent s t he r esult s of t he am ide I I I / phosphat e rat io for dent in t r eat ed w it h ir r igant s. The saline solut ion did not alt er t his rat io bet w een t he per iods analyzed ( p> 0.05) . I n G2, G3, and G4, t he collagen was depr ot einat ed by NaOCl solut ions f r o m t h e f i r st p er i o d o f i m m er si o n , r esu l t i n g in decr eases in t h e am ide I I I / ph osph at e r at io.
,QWUDJURXSVLJQL¿FDQWGLIIHUHQFHVSIRUWKH LQLWLDO GHQWLQ FRPSRVLWLRQ ZHUH LGHQWL¿HG DIWHU
m in of im m ersion in all NaOCl concent rat ions. There
ZHUHQRLQWHUJURXSVLJQL¿FDQWGLIIHUHQFHVEHWZHHQ
all NaOCl concent rat ions in all per iods analy zed
( p> 0 . 0 5 ) , h ow ev er, st at ist ical dif f er en ces w er e
LGHQWL¿HG EHWZHHQ WKH 1D2&O DQG WKH VDOLQH
solut ion aft er 5 m in of im m er sion. The effect s of 1% and 2.5% NaOCl in t he am ide I I I / phosphat e rat io w ere low er t han t he effect s of 5% NaOCl, w it h no st at ist ical differ ences ( p> 0.05) for t he saline solut ion.
Regar ding t he car bonat e/ phosphat e rat io, all ir r igant s caused a decr ease in it s init ial pr opor t ion ( Tab le 3 ) . How ev er, on ly t h e NaOCl solu t ion s
SURGXFHGVLJQL¿FDQWLQWUDJURXSFKDQJHVS WKDWZHUHLGHQWL¿HGLPPHGLDWHO\DIWHUPLQRI LPPHUVLRQ6LJQL¿FDQWFKDQJHVLQWKLVUDWLRZHUH
not obser ved bet w een t his t im e int er val and t he subsequent per iods ( p> 0.05) . Alt hough t he NaOCl solut ions caused higher changes in t he car bonat e/ p h o sp h a t e r a t i o t h a n sa l i n e so l u t i o n , i n t h e
LQWHUJURXSFRPSDULVRQVQRVLJQL¿FDQWGLIIHUHQFHV ZHUHLGHQWL¿HGEHWZHHQDOOJURXSVLQWKHSHULRGV
analyzed ( p> 0.05) .
GROUPS pH Initial
weight
Weight after 5 min of immersion
Weight after 10 min of immersion
Weight after 15 min of immersion
X ± SD X ± SD Reduction in
weight of the
IUDJPHQWV
X ± SD Reduction in
weight of the
IUDJPHQWV
X ± SD Reduction in
weight of the
IUDJPHQWV
G1- Saline 6.4 55.8 ± 1.8A,a
53.5 ± 3.1A,a
-4.12 54.5 ±
2.6A,a
-2.3 54.0 ±
2.7A,a
-3.2
G2- 1% NaOCl 11.7 55.9 ± 1.5A,a
50.9 ± 1.8AB,b
-8.9 44.1 ±
1.6B,c
-21.1 36.2 ±
2.1B,d
-35.2
G3- 2.5% NaOCl
12.05 54.7 ± 2.0A,a
48.8 ± 3.0B,b
-10.7 38.9 ±
2.1C,c
-28.8 29.6 ±
3.5C,d
-45.8
G4- 5% NaOCl 12.3 55.9 ± 2.2A,a
36.9 ± 3.0C,b
-33.9 21.8 ±
2.5D,c
-61 12.2 ±
1.5D,d
-78.1
Table 1 - pH of the different irrigation solutions and the mean (X) and standard deviation (SD) in mg of the weights of bovine
muscle tissue fragments before and after different periods of immersion in the irrigators and the reduction in weight of the fragments in percentage.
'LIIHUHQW ORZHUFDVH OHWWHUV LQ URZV LQGLFDWH VWDWLVWLFDOO\ VLJQL¿FDQW LQWUDJURXS GLIIHUHQFHV 7ZRZD\ $QRYD 3 'LIIHUHQWFDSLWDOOHWWHUVLQFROXPQVLQGLFDWHVWDWLVWLFDOO\VLJQL¿FDQWLQWHUJURXSGLIIHUHQFHVLQWKHVDPHWLPHSHULRG2QHZD\ $QRYD3
GROUPS Initial ratio 0.5 min 1 min 2 min 3 min 5 min 8 min 10 min
Med Med Med Med Med Med Med Med
(Min - Max) (Min - Max) (Min - Max) (Min - Max) (Min - Max) (Min - Max) (Min - Max) (Min - Max)
G1 - Saline solution 7.8 (5.3-9.9)A,a 8.2 (5.1-10.7)A,a 7.7 (5.5-11.1)A,a 7.8 (5.6-11.4)A,a 8.7 (5.0-12.0)A,a 8.6 (5.3-11.6)A,a 7.9 (6.0-11.7)A,a 8 (5.7-12.1)A,a
G2 - 1% NaOCl 6.3 (4.9-7.0)A,a 6.2 (4.6-7.0)A,ab 5.1 (3.7-6.9)A,ab 5.1 (3.5-6.5)A,abc 4.6 (3.7-6.0)A,abc 4.3 (3.5-6.1)AB,bc 4.1 (2.6-5.3)AB,bc 3.7 (2.0-5.0) AB,c
G3 - 2.5% NaOCl
4.9 (4.0-9.1) A,a
4.6 (3.7-8.8)Aa
3.9 (3.7-7.5) A,ab
3.5 (3.4-7.8)A,abc 3.7 (3.2-6.9)A,abc 3.5 (2.9-6.3)AB,bc 3 (2.4-6.7)AB,bc 2.4 (2.0-5.9)AB,c
G4 - 5% NaOCl 6.7 (4.4-7.7)A,a 5.9 (3.6-7.0)Aa 5.1 (3.4-6.4) A,ab
4.8 (3.1-5.9)A,abc 4.4 (3.0-5.6)A,abc 3.9 (2.2-4.5)B,bc 2.9 (1.5-3.3)B,bc 1.9 (1.2-2.9)B,c
Table 2- Median (Med), minimum and maximum (Min – Max) values for the ratio of amide III/phosphate in dentin surface
before and after immersion in the irrigation solutions in different periods of time. The ratio values are multiplied by 10-3.
'LIIHUHQW ORZHUFDVH OHWWHUV LQ URZV LQGLFDWH VWDWLVWLFDOO\ VLJQL¿FDQW LQWUDJURXS GLIIHUHQFHV )ULHGPDQ S 'LIIHUHQW FDSLWDOOHWWHUVLQFROXPQVLQGLFDWHVWDWLVWLFDOO\VLJQL¿FDQWLQWHUJURXSGLIIHUHQFHVLQWKHVDPHWLPHSHULRG.UXVNDO:DOOLVDQG
D I SCUSSI ON
I n t h e pr esen t st u dy, t h e t issu e dissolu t ion capabilit y and t he changes in t he dent in chem ical com posit ion by differ ent concent rat ions of NaOCl solut ions w er e assessed. The r esult s dem onst rat ed t h a t Na OCl ca n d i sso l v e t h e o r g a n i c m a t t e r and depr ot einat e t he collagen of dent in in high quant it ies; and ot her w ise, it can cause a sm all r ed u ct ion in t h e car b on at e com p on en t of t h e inor ganic phase of t he dent in.
The null hypot hesis t est ed has t o be r ej ect ed, s i n c e t h e r e w e r e d i f f e r e n c e s b e t w e e n t h e concent rat ions of NaOCl solut ions in t he abilit y of t issue dissolut ion and in t he effect s on dent in com posit ion over t im e.
A concent rat ion and t im e- dependent or ganic t issue dissolut ion capacit y was obser ved for t he NaOCl solut ions ( Table 1) , as pr eviously found in ot her st udies1,7,12,14,291D2&OH[HUWVDQRQVSHFL¿F
non- coagulat ing digest ive effect on vit al and necrot ic t issues14,26 by dir ect cont act bet w een fr ee available
chlor ine m olecules and or ganic m at t er20. The pH
RIWKHVROXWLRQLQÀXHQFHVWKHELRORJLFDOHIIHFWVRI
NaOCl by det er m ining t he equilibr ium of t he fr eely available chlor ine4, i.e., t he sum of concent rat ions
of hypochlorous acid and hypochlorit e anion ( HOCl/ OCl-)4,5. Acid solut ions have a pow er ful bact er icidal
effect because of t he pr evalence of HOCl. The OCl-
has a pow erful oxidat ive effect t hat prom ot e higher t issue dissolut ion and is m or e abundant in alkaline solut ions43UHYLRXVVWXGLHVGLGQRW¿QGGLIIHUHQFHV
in t he t issue- dissolving pr oper t ies of NaOCl at t he sam e concent rat ions and different alkaline pHs of 9 and 127,33. Alt hough t here were differences bet ween
t he pHs of t he solut ions t est ed ( Table 1) t hey w er e
VPDOODQGPD\QRWLQÀXHQFHWKHWLVVXHGLVVROXWLRQ
capabilit y of t he ir r igant s.
Tissu es f r om d if f er en t sou r ces w er e u sed
in st udies about t he t issue dissolv ing abilit y of ir r igat ion solut ions1, 7, 8, 12, 14, 29. Bov ine m uscle w as
ch osen b ecau se of t h e av ailab ilit y an d easier st andar dizat ion of t he specim ens29,30. To pr event
t he confounding fact or s in t he dissolut ion analysis, t he specim ens were prepared wit h sim ilar m ass and sur face ar eas. The sam e t em perat ur e and volum e of t he solut ions w er e used for all gr oups, and t o
VLPXODWHWKHVROXWLRQÀRZLQWKHURRWFDQDOGXULQJ
t h e r oot can al pr epar at ion , t h e solu t ion s w er e agit at ed in an ult rasonic t ub.
The bovine incisor dent in has a sim ilar st r uct ur e and num ber of t ubuli of hum an m olar dent in28 and
per m it s t he achievem ent of a m or e st andar dized subst rat e for analy ses. Ther e ar e no differ ences b et w een t h e m i n er al m at r i ces o f h u m an an d bovine dent in, and fr om t he bovine collagen and dem ineralized hum an dent in, only differ ences in int ensit ies of absor pt ion bands ar e obser v ed3 , 6.
The sam ples w er e pr epar ed as slices t o m aint ain t h e n at u r al st r u ct u r e an d t o p r ev en t ch an g es i n t i ssu e co m p o si t i o n , b e ca u se t h e g r i n d i n g pr ocesses can cause wat er loss t o t he am bient , shift t he wavenum ber, and alt er t he int ensit y of t he absor pt ion bands3.
I n t he dent in vibrat ional spect r um , it is possible t o observe bands relat ed t o wat er, t o hydroxyapat it e, t hat or iginat e fr om t he car bonat e and phosphat e gr oups and t o t he or ganic m at r ix fr om t he gr oups pr esent in t he collagen such as am ides I , I I , and I I I3. Th e t r eat m en t of den t in sh ow ed t h at t h e
NaOCl leads t o concent rat ion- dependent collagen deplet ion ( Table 2) . Alt hough t here are no st at ist ical differences bet ween t he G2, G3, and G4 groups, t he
UHPRYDORIWKHRUJDQLFSKDVHIURPWKHVXSHU¿FLDO
subsur face of m ineralized dent in was considerably
PRUH VHYHUH IRU WKH 1D2&O ZLWK VLJQL¿FDQW
differ ences for t he saline solut ion fr om t he 5 m in of im m er sion . Th is low er am id e I I I / p h osp h at e
GROUPS Initial ratio 0.5 min 1 min 2 min 3 min 5 min 8 min 10 min
(X ± SD) (X ± SD) (X ± SD) (X ± SD) (X ± SD) (X ± SD) (X ± SD) (X ± SD)
G1 - Saline solution
19.3 ± 1.5A,a 19.3 ± 1.4A,a 19.3 ± 1.6A,a 19.0 ± 1.7A,a 18.8 ± 1.2A,a 19.0 ± 1.3A,a 18.9 ± 1.4A,a 19.1 ± 1.2A,a
G2 - 1% NaOCl
18.9 ± 1.7A,a 18.2 ± 1.5A,b 17.7 ± 1.7A,b 17.7 ± 1.6A,b 17.7 ± 2.0A,b 17.8 ± 1.7A,b 17.8 ± 1.6A,b 17.7 ± 1.6A,b
G3 - 2.5% NaOCl
19.1 ± 1.8A,a 18.1 ± 2.0A,b 17.4 ± 2.1A,b 17.4 ± 1.6A,b 17.4 ± 1.5A,b 17.4 ± 1.9A,b 17.5 ± 1.5A,b 17.9 ± 2.1A,b
G4 - 5% NaOCl
19.2 ± 0.9A,a 17.7 ± 1.0A,b 17.5 ± 1.1A,b 17.2 ± 1.0A,b 17.1 ± 0.8A,b 17.2 ± 1.3A,b 17.9 ± 1.0A,b 17.8 ± 1.4A,b
Table 3- Mean (X) and standard deviation (SD) values for the ratio of carbonate/phosphate in dentin surface before and
after immersion in the irrigation solutions in different periods of time.
'LIIHUHQWORZHUFDVHOHWWHUVLQURZVLQGLFDWHVWDWLVWLFDOO\VLJQL¿FDQWLQWUDJURXSGLIIHUHQFHV7ZRZD\$QRYDDQG7XNH\ post-hoc3'LIIHUHQWFDSLWDOOHWWHUVLQFROXPQVLQGLFDWHVWDWLVWLFDOO\VLJQL¿FDQWLQWHUJURXSGLIIHUHQFHVLQWKHVDPHWLPH
rat io was also obser ved for higher concent rat ions of NaOCl in ot her st udies2,15,35. The NaOCl act s on
t he dent in cr eat ing depr ot einat ion channels t hat leads t o a non- unifor m effect10, leaving unbound
hydr ox yapat it e and an apat it e- r ich and collagen sp ar se d en t in su b su r f ace1 0 , 1 1. Th e d est r u ct ion
of t h e den t in collagen m at r ix r esu lt s in a less t ough and m or e br it t le subst rat e10, 18 t hat m ight
facilit at e t he fat igue crack propagat ion during cyclic st resses16,34 and increase t he suscept ibilit y of crown
or r oot fract ur e34. The dest r uct ive effect of NaOCl
on t he dent in is ir r ever sible and if t he chelat ing agent is subsequent ly em ployed, it r em oves t he collagen- deplet ed apat it e phase and exposes t he under lying dest r uct ion caused by NaOCl, w hich is m or phologically per ceived as canal wall er osion35.
I n t he pr esent st udy, a t im e- dependent effect in t he r educt ion of t he am ide I I I / phosphat e rat io
ZDV LGHQWL¿HG LQ DOO 1D2&O FRQFHQWUDWLRQV 7DEOH
2 ) . Th e r esu lt s in dicat e t h at t h er e w as a slow an d co n t i n u o u s d eg r ad at i o n o f co l l ag en f r o m t h e den t in su r f ace an d t h ey ar e in accor dan ce w it h pr evious st udies t hat also obser ved t hat t he r em oval of t he or ganic phase fr om t he dent in is t im e- d ep en d en t3 4 , 3 5. Ot h er st u d ies r ep or t ed an
init ial r educt ion in t he collagen w it h a plat eau in dent in depr ot einat ion r eached over t im e for t he sam e NaOCl concent rat ion2,10,15,21. The plat eau was
not obser ved in t his r esear ch; how ever, t her e was a r educt ion in t he rat e of depr ot einat ion over t im e. This r educt ion m ay be r elat ed t o t he fact t hat t he collagen pr esent on t he dent in sur faces is quickly hy dr oly zed and r em oved, and aft er t he pr ocess it r ever t s t o t he deeper and unexposed collagen t hat is encapsulat ed by hydr oxyapat it e, being less vulnerable t o t he dest r uct ive effect s of NaOCl and show ing lit t le changes over t im e10,15.
Car bonat e gr oups m ay occupy phosphat e and h y d r ox y l ion s sit es in b on e an d t eet h ap at it e. These subst it ut ions affect t he cr yst allinit y of t he apat it es and can accelerat e t he dissolut ion pr ocess of t he t oot h st r uct ur e23,31. I n t he pr esent st udy, a
VLJQL¿FDQW UHGXFWLRQ LQ WKH FDUERQDWHSKRVSKDWH
rat io occur r ed in all NaOCl concent rat ions t est ed aft er 0 , 5 m in of im m er sion, but a plat eau w as obser ved aft er t his im m er sion per iod ( Table 3) . Since t he solut ions w er e r enew ed t o ensur e t heir effect iveness, t his plat eau probably occurs aft er t he rem oval of carbonat e from t he surface, because t he accessibilit y t o t he gr oups t hat ar e in subsur faces layer s of dent in m akes t hem less suscept ible t o t he act ion of t he NaOCl solut ions. These r esult s
FRQ¿UPHGWKDWFDUERQDWHJURXSVDUHPRUHVROXEOH
t han phosphat e gr oups23,31 and ar e in accor dance
w it h a pr evious st udy t hat also obser ved t hat t he NaOCl t r eat m ent r em ov es som e car bonat e ions fr om t he inor ganic dent in st r uct ur e, w hile at t he sam e t im e it depr ot einat es t he or ganic m at t er27.
Th is is con sist en t w it h t h e v er y low solu b ilit y expect ed for apat it e m ineral in alkaline solut ion11
and m ay be pot ent iat ed by t he ult rasonic agit at ion. The design of t he present st udy does not direct ly
UHÀHFWWKHFOLQLFDOFRQGLWLRQVEXWDOORZVTXDQWLWDWLYH
evaluat ions r egar ding t he differ ent concent rat ions and t im e exposur e of NaOCl solut ions. This st udy
FRQ¿UPHGWKHDGYDQWDJHRIXVLQJDORQJHUFRQWDFW
t i m e a n d h i g h e r co n ce n t r a t i o n s o f Na OCl t o pr om ot e t issue dissolut ion. How ever, it incr eases t h e alt er at ion s in d en t in com p osit ion an d t h e r isks of per iapical t issue dam age fr om inadver t ent ext rusion. Based on t hese result s, t he use of NaOCl at low er con cen t r at ion s, su ch as 1 an d 2 . 5 % , dem onst rat es t o be effect ive in prom ot ing a suit able dissolut ion of or ganic t issue pr esent in t he r oot canal syst em and prevent ing a pronounced dam age t o t he dent in st r uct ur e.
CON CLUSI ON S
Th e f i n d i n g s o f t h i s st u d y i n d i ca t e d t h a t t h e in cr ease in t h e ex p osu r e t im e an d in t h e concent rat ion of NaOCl solut ion lead t o an incr ease i n t h e t i ssu e d i sso l u t i o n an d d en t i n co l l ag en depr ot ein at ion . Mor eov er, som e car bon at e ion s ar e r em oved fr om t he dent in inor ganic phase by t he NaOCl.
ACKN OW LED GEM EN TS
This st udy was suppor t ed by t he St at e of São Paulo Research Foundat ion, FAPESP ( 2013/ 19789- 3 and 2012/ 02460- 6) .
REFEREN CES
1- Alm eida LH, Leonar do NG, Gom es AP, Giar dino L, Souza EM, Pappen FG. Pulp t issue dissolut ion capacit y of sodium hypochlorit e com bined w it h cet r im ide and polypr opylene glycol. Braz Dent J. 2013; 24: 477- 81.
$WDEHN'%RGXU+<DOoLQ*.DOD\FLù(IIHFWVRIR[LGDWLYH LUULJDQWV RQ URRW GHQWLQ VWUXFWXUH $WWHQXDWHG 7RWDO 5HÀHFWLRQ
Four ier Transfor m I nfrar ed Spect r oscopy st udy. Oral Healt h Dent Manag. 2014; 13: 753- 6.
3 - Bach m an n L, Dieb old er R, Hib st R, Zezell DM. I n f r ar ed absor pt ion bands of enam el and dent in t issues fr om hum an and bovine t eet h. Appl Spect r osc Rev. 2003; 38: 1- 14.
4- Baker RW. St udies on t he react ion bet ween sodium hypochlorit e and pr ot eins: 1. Phy sico- chem ical st udy of t he cour se of t he r eact ion. Biochem J. 1947; 41: 337- 42.
%ORRP¿HOG6)0LOHV*$7KHDQWLEDFWHULDOSURSHUWLHVRIVRGLXP
dichloroisocyanurat e and sodium hypochlorit e form ulat ions. J Appl Bact er iol. 1979; 46: 65- 73.
6- Bot t a SB, Ana PA, Sant os MO, Zezell DM, Mat os AB. Effect of dent al t issue condit ioners and m at rix m et alloprot einase inhibit ors on t ype I collagen m icr ost r uct ur e analyzed by Four ier t ransfor m in frar ed spect r oscopy. J Biom ed Mat er Res B Appl Biom at er. 2012; 100: 1009- 16.
8- Clar k son RM, Moule AJ, Podlich H, Kellaway R, Macfar lane R, Lew is D, et al. Dissolut ion of por cine incisor pulps in sodium hypochlorit e solut ions of varying com posit ions and concent rat ions. Aust Dent J. 2006; 51: 245- 51.
9- Delany GM, Pat t er son SS, Miller CH, New t on CW. The effect
RI FKORUKH[LGLQH JOXFRQDWH LUULJDWLRQ RQ WKH URRW FDQDO ÀRUD RI
fr eshly ext ract ed necr ot ic t eet h. Oral Sur g Oral Med Oral Pat hol. 1982; 53: 518- 23.
10- Di Renzo M, Ellis TH, Sacher E, St angel I . A phot oacoust ic
)7,56 VWXG\ RI WKH FKHPLFDO PRGL¿FDWLRQV RI KXPDQ GHQWLQ
sur faces: I I . Depr ot einat ion. Biom at er ials. 2001; 22: 793- 7. 11- Dr iscoll CO, Dow ker SE, Ander son P, Wilson RM, Gulabivala K. Ef f ect s of sod iu m h y p och lor it e solu t ion on r oot d en t in e com posit ion. J Mat er Sci Mat er Med. 2002; 13: 219- 23.
12- Dum it r iu D, Dobr e T. Effect s of t em perat ur e and hypochlor it e con cen t r at ion on t h e r at e of collag en d issolu t ion . J En d od . 2015; 41: 903- 6.
13- Gernhardt CR, Eppendorf K, Kozlow ski A, Brandt M. Toxicit y of concent rat ed sodium hypochlor it e used as an endodont ic ir r igant . I nt Endod J. 2004; 37: 272- 80.
14- Hand RE, Sm it h ML, Har r ison JW. Analysis of t he effect of dilut ion on t he necr ot ic t issue dissolut ion pr oper t y of sodium hypochlor it e. J Endod. 1978; 4: 60- 4.
15- Hu X, Peng Y, Sum CP, Ling J. Effect s of concent rat ions and exposur e t im es of sodium hypochlor it e on dent in depr ot einat ion:
DWWHQXDWHGWRWDOUHÀHFWLRQ)RXULHUWUDQVIRUPLQIUDUHGVSHFWURVFRS\
st udy. J Endod. 2010; 36: 2008- 11.
16- Kr uzic JJ, Rit chie RO. Fat igue of m ineralized t issues: cor t ical bone and dent in. J Mech Behav Biom ed Mat er. 2008; 1: 3- 17. 17- Lisboa DS, Sant os SV, Gr iza S, Rodr igues JL, Far ia- e- Silva AL. Dent in depr ot einizat ion effect on bond st r engt h of self- adhesive r esin cem ent s. Braz Oral Res. 2013; 27: 73- 5.
18- Marending M, Paqué F, Fischer J, Zehnder M. I m pact of irrigant sequence on m echanical propert ies of hum an root dent in. J Endod. 2007; 33: 1325- 8.
19- Mar shall GW Jr, Mar shall SJ, Kinney JH, Balooch M. The dent in subst rat e: st r uct ur e and pr oper t ies r elat ed t o bonding. J Dent . 1997; 25: 441- 58.
2 0 - Moor er WR, Wesselin k PR. Fact or s pr om ot in g t h e t issu e d i sso l v i n g cap ab i l i t y o f so d i u m h y p o ch l o r i t e. I n t En d o d J. 1982; 15: 187- 96.
21- Mount ouris G, Silikas N, Eliades G. Effect of sodium hypochlorit e t reat m ent on t he m olecular com posit ion and m orphology of hum an cor onal dent in. J Adhes Dent . 2004; 6: 175- 82.
1HHODNDQWDQ36KDUPD66KHPHVK+:HVVHOLQN35,QÀXHQFH
of ir r igat ion sequence on t he adhesion of r oot canal sealer s t o dent in: a Four ier t ransfor m infrar ed spect r oscopy and push- out bond st r engt h analysis. J Endod. 2015; 41: 1108- 11.
23- Ot suka M, Papangkor n K, Baig AA, Higuchi WI . Chem om et r ic evaluat ion of physicochem ical pr oper t ies of car bonat ed- apat it ic preparat ions by Fourier t ransform infrared spect roscopy. J Biom ed Mat er Res A. 2012; 100: 2186- 93.
24- Pascon FM, Kant ovit z KR, Sacram ent o PA, Nobr e- dos- Sant os M, Puppin- Ront ani RM. Effect of sodium hypochlor it e on dent ine m echanical pr oper t ies. A r eview. J Dent . 2009; 37: 903- 8. 25- Pet er s OA, Schönenber ger K, Laib A. Effect s of four Ni-Ti preparat ion t echniques on root canal geom et ry assessed by m icro com put ed t om ography. I nt Endod J. 2001; 34: 221- 30.
26- Rosenfeld EF, Jam es GA, Bur ch BS. Vit al pulp t issue r esponse t o sodium hypochlor it e. J Endod. 1978; 4: 140- 6.
27- Sakae T, Mishim a H, Kozawa Y. Changes in bovine dent in m i n er a l w i t h so d i u m h y p o ch l o r i t e t r ea t m en t . J D en t Res. 1988; 67: 1229- 34.
28- Schilke R, Lisson JA, Bauss O, Geur t sen W. Com par ison of t he num ber and diam et er of dent inal t ubules in hum an and bovine dent ine by scanning elect r on m icr oscopic invest igat ion. Ar ch Oral Biol. 2000; 45: 355- 61.
29- St oj icic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolu t ion by sodiu m hy poch lor it e: ef f ect of con cen t r at ion , t em perat ur e, agit at ion, and sur fact ant . J Endod. 2010; 36: 1558-62.
30- Tar t ar i T, Guim ar ães BM, Am oras LS, Duar t e MA, Silva ES, Bram ant e CM. Et idr onat e causes m inim al changes in t he abilit y of sodium hypochlor it e t o dissolve or ganic m at t er. I nt Endod J. 2015; 48: 399- 404.
31- Yao F, LeGer os JP, LeGer os RZ. Sim ult aneous incor porat ion o f car b o n at e an d f l u o r i d e i n sy n t h et i c ap at i t es: ef f ect o n cr yst allographic and physico- chem ical pr oper t ies. Act a Biom at er. 2009; 5: 2169- 77.
32- Zehnder M. Root canal ir r igant s. J Endod. 2006; 32: 389- 98. 33- Zehnder M, Kosicki D, Luder H, Sener B, Walt im o T. Tissue-d issolv in g cap acit y an Tissue-d an t ib act er ial ef f ect of b u f f er eTissue-d an Tissue-d unbuffer ed hypochlor it e solut ions. Oral Sur g Oral Med Oral Pat hol Oral Radiol Endod. 2002; 94: 756- 62.