ContentslistsavailableatScienceDirect
Veterinary
Parasitology
j o ur na l h o me p a g e:w w w . e l s e v i e r . c o m / l o c a t e / v e t p a r
Immunological
profile
of
resistance
and
susceptibility
in
naturally
infected
dogs
by
Leishmania
infantum
Gleisiane
Gomes
de
Almeida
Leal
a,
Bruno
Mendes
Roatt
a,b,f,
Rodrigo
Dian
de
Oliveira
Aguiar-Soares
a,b,
Cláudia
Martins
Carneiro
a,b,
Rodolfo
Cordeiro
Giunchetti
a,c,
Andréa
Teixeira-Carvalho
d,
Olindo
Assis
Martins-Filho
d,
Amanda
Fortes
Francisco
a,
Jamille
Mirelle
Cardoso
a,
Fernando
Augusto
Siqueira
Mathias
a,
Rodrigo
Correa-Oliveira
e,f,
Mariângela
Carneiro
g,
Wendel
Coura-Vital
b,f,g,
Alexandre
Barbosa
Reis
a,b,f,∗aLaboratóriodeImunopatologia,NúcleodePesquisasemCiênciasBiológicas/NUPEB,UniversidadeFederaldeOuroPreto,OuroPreto,
MinasGerais,Brazil
bLaboratóriodePesquisasClínicas,DepartamentodeAnálisesClínicas,EscoladeFarmácia,UniversidadeFederaldeOuroPreto,Ouro
Preto,MinasGerais,Brazil
cLaboratóriodeBiologiadasInterac¸õesCelulares,DepartamentodeMorfologia,UniversidadeFederaldeMinasGerais,BeloHorizonte,
MinasGerais,Brazil
dLaboratóriodeBiomarcadoresdeDiagnósticoeMonitorac¸ão,CentrodePesquisasRenéRachou,Fundac¸ãoOswaldoCruz-FIOCRUZ,
BeloHorizonte,MinasGerais,Brazil
eLaboratóriodeImunologiaCelulareMolecular,CentroRenéRachou,Fundac¸ãoOswaldoCruz,BeloHorizonte,MinasGerais,Brazil fInstitutoNacionaldeCiênciaeTecnologiaemDoenc¸asTropicais,INCT-DT,Brazil
gP´os-Graduac¸˜aoemInfectologiaeMedicinaTropical,FaculdadedeMedicina,UniversidadeFederaldeMinasGerais,BeloHorizonte,
MinasGerais,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23January2014
Receivedinrevisedform21August2014
Accepted25August2014
Keywords:
Caninevisceralleishmaniasis
Leishmaniainfantum
Clinicalforms
Immunophenotyping Cytokines
a
b
s
t
r
a
c
t
Visceralleishmaniasishasagreatimpactonpublichealth,anddogsareconsideredthemain domesticreservoirofLeishmaniainfantum,thecausalparasite.Inthisstudy,159animals naturallyinfectedbyL.infantumfromanendemicareaofBrazilwereevaluatedthroughan analysisofcellularresponses,usingflowcytometry,andofthehematologicalparameters. Theresultsconfirmedthatdiseaseprogressionisassociatedwithanemiaandreductions ineosinophils,monocytesandlymphocytes.Theinvestigationoftheimmuneresponse, basedontheimmunophenotypicprofileofperipheralblood,showeddeclinesinthe abso-lutenumbersofTlymphocytesCD5+andtheirsubsets(CD4+andCD8+)andadropofB lymphocytesinasymptomaticseropositive(AD-II)andsymptomaticseropositive(SD)dogs. Neutrophils,whenstimulatedwithsolubleantigenofL.infantum,showedhighersynthesis ofinterferon(IFN)-␥+inAD-IIandSDgroups,withdecreasedproductionofinterleukin
∗ Correspondingauthorat:LaboratóriodeImunopatologia,NúcleodePesquisasemCiênciasBiológicas,ICEBII,MorrodoCruzeiro,UniversidadeFederal
deOuroPreto,OuroPreto,MGCEP35400-000,Brazil.Tel.:+55213135591694;fax:+55213135591680.
E-mailaddresses:alexreisufop@gmail.com,alexreis@nupeb.ufop.br(A.B.Reis).
http://dx.doi.org/10.1016/j.vetpar.2014.08.022
(IL)-4+inasymptomaticseronegativedogspositiveforL.infantuminfectionbasedon poly-merasechainreactiontesting(AD-Igroup).IntheAD-IIandSDgroups,subpopulationsof stimulatedlymphocytes(CD4+andCD8+)alsoexhibitedgreatersynthesisofIFN-␥+and IL-4+inculture.TheseresultssuggestthattheanimalsoftheAD-IIandSDgroupsexhibited amixedimmuneresponse(Type1and2)andtheAD-Igrouppresentinganimmuneprofile verysimilartonormalcontrolanimals.
©2014ElsevierB.V.Allrightsreserved.
1. Introduction
Visceral leishmaniasis(VL) caused by theprotozoan
Leishmania(Leishmania)infantum,isoneofthemost
impor-tantzoonoticdiseasesaffectingdogsandhumansinSouth andCentral America,theMediterraneanbasin andparts of Asia (World Health Organization, 2010).Dogs (Canis
familiaris)are the mostimportant reservoir of the
par-asite in urban areas, especially those that have a high parasite burden in the skin and a high prevalence in this environment (Giunchetti et al., 2006; Coura-Vital etal.,2011b).Ithasoftenbeenobservedthatanincrease in caninevisceral leishmaniasis (CVL) cases precedes a rise inhuman cases(Fragaet al., 2012;Grimaldi et al., 2012).
CVL may evolve from a nonapparent infection to a severeandsystemicdisease,whichusuallyculminatesin death. Asymptomaticdogscanrecoveror develop clini-calsymptomaticdisease,ortheymayremaininfectedfor years, evenlifelong,withoutclinicalmanifestation (Reis etal.,2009).Recentlyithasbeenshownthatahigh per-centage ofasymptomaticinfecteddogsarePCR positive butseronegative(Coura-Vitaletal.,2011b).Theseanimals, althoughtheirinfectionstatusisnotdetectedby conven-tionalserology,aremorelikelytoseroconvert(Coura-Vital etal.,2013).Theyalsoapparentlyhaveadifferenttypeof immuneresponsethatseemstoberelatedtoresistance andischaracterizedbyhighproportionsofCD4+T lym-phocytesandCD21+BcellsandhighexpressionofIFN-␥ (Reisetal.,2006b;Coura-Vitaletal.,2011a;Menezes-Souza etal.,2011).
The components of innate and adaptive immunity engageinarangeofinteractionsthatisremarkablydiverse andcomplex(Reisetal.,2009,2010).ThecourseofCVL isinterconnectedwiththehostimmuneresponseandthe persistenceandproliferationoftheparasitesthroughout theskinandvisceralorgans.Theinnateimmuneresponse hasarelevantroleinprotectingagainsttheparasitein addi-tiontoswitchingontheadaptiveresponsethatcancontrol
theLeishmaniainfectionwithoutthedevelopmentofa
spe-cificadaptiveimmunity(MorenoandAlvar,2002).Studies indicatethatthesuccessfulresolutionofLeishmania infec-tions depends on the ability of the host to mount a specificT-cellresponse,withtheactivationofmacrophages mediatedbycytokinesderivedfromTcells(Carrilloand Moreno,2009).Symptomaticdogsthatdevelopsevere dis-easealreadyexhibitclearsuppressionofspecifictypesof cell-mediatedimmunity,particularlyCD8+Tlymphocytes (Pinellietal.,1994).Resistancetoinfectionisassociated witha Type 1response, witha predominance ofIL-12,
IFN-␥,IL-2andtumornecrosisfactor(TNF)-␣,whichwill increase theefficiencyof phagocyticcells and cytotoxic lymphocytes, triggeringa protective immune response. On theother hand, susceptibilityto infectionis associ-atedwithaType2response,withpredominanceofIL-4, IL-5,IL-10, IL-13and TGF-(Pinelliet al.,1995,1999a; Correaetal.,2007;Lageetal.,2007;Menezes-Souzaetal., 2011).
In a previously reported study, our group showed that asymptomatic dogs (seronegative/PCR+ [AD-I] and seropositive[AD-II])appeartohaveadichotomous infec-tion spectrum that influencesthe humoral and cellular immunologicalstatusinCVL(Coura-Vitaletal.,2011a).The aimofthepresentstudywastoinvestigateimmunological eventsinnaturallyinfecteddogsbasedonthedichotomy betweenasymptomatic groups (AD-I and AD-II) and to identifybiomarkersassociatedwithresistanceand suscep-tibilitytoinfectionbyL.infantum.
2. Materialsandmethods
2.1. Experimentaldesign
Thepresentstudyincluded159mongreldogsofboth sexes(81maleand78 female)fromanendemicareaof Brazil.Themeanagewas49.8months(SD37.8),andthe medianwas42months(IQR24;66).Thesampleswere col-lectedfromdomesticdogsattheZoonosesControlCentre ofBeloHorizonte.Serologicaltests(immunofluorescence antibodytest[IFAT]andELISA)wereperformedfollowing themanufacturer’sinstructions.Dogs withanIFAT titer <1/40wereconsideredseronegative,anddogswithIFAT titer≥1/40wereconsideredseropositiveandinfectedwith
Leishmaniaspp. Theserologicaltestswereperformedin
theLaboratoryof ZoonosisattheBeloHorizonteHealth Department.Becauseofthehighprevalenceof seroneg-ative/PCR+ dogsintheendemic area(Coura-Vital et al., 2011b),theseronegativedogsweresubjectedto molec-ular testing. Molecular testing (PCR) was performed in buffycoatsampleswithprimersfromaconservedregion
of the Leishmania kDNA minicircle (P150–152) (Passos
2.2. Serologicalassays
TheELISAwasperformedusingtheEIE-LVC®kit
(Bio-Manguinhos/Fiocruz,Rio deJaneiro,Brazil) accordingto themanufacturer’sinstructions.Thereactionswere per-formedandsampleswithopticaldensityabovethecut-off were considered positive. The cut-off was defined on each platebyconsidering themeanof theoptical den-sityofthenegativecontrolsmultipliedbytwo.Theassays were read on an automatic EL 800G ELISA microplate reader.
TheIFAT wasconductedusingtheIFI-LVC® kit
(Bio-Manguinhos/Fiocruz, Rio de Janeiro, Brazil). The tests wereexecutedaccordingtothemanufacturer’sinstruction. The slides were prepared and examined using a fluo-rescentmicroscopewith40×objective(OlympusBX40).
Theresultswereconsideredpositivewhenthefluorescent parasiteswereobservedatserumtiterof1:40ormore.
2.3. Clinicalgroups
Dogs wereclinically classified accordingto serologi-cal(ELISAandIFAT)andmoleculartests(PCR-RFLP)and by clinical features, as described by Coura-Vital et al. (2011a), and were divided into fourgroups. Dogs with noclinicalsigns andnegativeserologicaland molecular resultscomposedthecontrolgroup(CD;n=44). Seroneg-ativedogs withoutclinicalsigns but positive molecular resultsfor L.infantum were classified as asymptomatic dogsI(AD-I;n=53).Dogswithpositiveserologicalresults
for Leishmaniaspp. but noclinical signswere classified
asasymptomaticdogsII(AD-II;n=20).Dogswith clini-calsigns and positiveserologicalresultswere classified as symptomatic dogs (SD; n=38). With regard to gen-der,meanage(inmonths)andstandarddeviationthese groupshave thefollowing characteristics: CD (27♂and 17♀;[52.4;SD38.2]);AD-I(24♂and29♀;[51.0;SD38.4]); AD-II(9♂and11♀;[45.4;SD17.7])andSD(20♂and18♀; [45.5;SD22.8]).
For immunophenotyping and intracytoplasmic cytokine assays, 124 of the 159 dogs were grouped as follows: CD (n=28), AD-I (n=34), AD-II (n=20) and SD (n=42). Alreadythesegroups thegender ageof the animalsandstandarddeviationwere:CD(18♂and10♀; [50.6;SD39.5]);AD-I(17♂and17♀;[50.9;SD35.8]);AD-II (9♂and11♀;[45.4;SD17.7])andSD(21♂and21♀;[46.8; SD22.6]).
2.4. Bloodsamplesandhematologicalevaluation
Peripheral blood (5mL) from the brachiocephalic or jugularveinwascollectedintotubescontainingethylene diaminetetraceticacid(EDTA)atafinalconcentrationof 1mg/mLforthehemogram,bloodfilmand immunologi-calevaluation byimmunophenotyping(exvivo)byflow cytometry.Erythrocytesand leukocyteswere quantified usinganautomaticcellcounter(Model2800Vet,Mindray). Morphologicalcharacteristicsoftheblood cellsand dif-ferentialleukocytecountswereobtainedbybloodsmear analysisafterpriorstainingbyroutinemethods.Toconduct thetestsforevaluatingtheimmuneresponseafterinvitro
stimulation,5mLofaheparinizedperipheralbloodsample fromeachdogwastransferredtosterileheparinizedtubes (BDPharmingen,SanDiego,CA).
2.5. Caninebloodleukocyteimmunophenotyping
Immunophenotyping of peripheral blood by flow cytometry wascarried outusing simple markupof five primary 5-mL polystyrene tubes (Falcon® 2054, Becton
Dickinson, San Diego, USA) with the following mAbs: dilutedphycoerythrin(PE)-labeledanti-canineCD5(1:400, mouseIgG2a,cloneYKIX322.3),anti-canineCD4(1:1000, mouse IgG2a,cloneYKIX302. 9)and dilutedfluorescein isothiocyanate(FITC)anti-canineCD8(1:80,mouseIgG1, cloneYCATE55.9)(Serotec,USA).ThemAbswereusedinan indirectimmunofluorescenceprocedureinwhichpooled normal ratserum(diluted1:6000)wasusedasthe iso-typiccontrol.Amouseanti-humanCD21PE(1:100,mouse IgG1,cloneIOBla;ImmunotechCo.Marseille,France)and a diluted PE/Cy-5 conjugated mouse anti-human CD14 (1:300mL,1:200,IgG2a,cloneTÜK4;Serotec,USA)were alsoused.FiftymicrolitersofbloodcollectedinEDTAwas added to tubes containing 50L of antibody and incu-batedfor30minatroomtemperature(RT).Afterwards,the erythrocyteswerelysedbyadding2mLoflysissolution (FACSbrandlysingsolution;BectonDickinson,SanDiego, CA,USA),whichwasfollowedbyincubationfor10minat RT.Theleukocyteswerethenwashedtwicewith2mLof PBS(phosphatebufferedsaline0.15M,pH7.2)and cen-trifugedat400×gfor10minatRT.Then,thelabeledcells
werefixedfor30minatRTwith200LofFACSFIX solu-tion(10g/Lparaformaldehyde;10.2g/Lsodiumcacodylate and 6.65g/L sodiumchloride, pH 7.2). Flow cytometric measurementswereperformedonaFACScaliburTM instru-ment (Becton Dickinson, Mountain View, CA), and the datawereanalyzedusingFlowJo®software(20,000events
acquiredpersample).Theresultswereexpressedin abso-lute counts (cellnumber/mm3) through the product of the percentage of positive cells (CD5+, CD4+, CD8+ and CD21+)withingatedlymphocytesbyabsolutelymphocyte counts.Theabsolutecountsformonocyteswereobtained throughtheproductsofCD14+cellswithinungated leuko-cytes by the selection of the region of interest, based on morphometric and immunophenotypic graphics of distribution CD14/FL3 versus SSC, to identify the pop-ulation of CD14+High and SSCIntermediate, minimizingthe contaminationof thispopulationwithlymphocytesand neutrophils.
2.6. Antigenproductionforinvitroassays
Soluble L. infantum (MHOM/BR/1070/BH46) antigen (SLAi)waspreparedasdescribedbyReisetal.(2006c)from promastigotes harvested from stationary-phase in liver infusiontryptosecultures.Theconcentrationofproteinin theSLAisolutionwasdeterminedaspreviouslydescribed Lowryetal.(1951)andadjustedto1mg/mL.DilutedSLAi wasdividedintosmallportionsandstoredat−80◦Cuntil
Table1
HematologicalparametersofdogsnaturallyinfectedwithLeishmaniainfantumanduninfecteddogs.
Hematologicalparameters Clinicalgroups
CD AD-I AD-II SD
Erythrocytes(106/mm3) 7.3(6.8–8.2) 7.41(6.6–7.9) 6.2(5.3–6.5)a,b 4.6(3.9–5.5)a,b
Hemoglobin(g%) 15.0(13.6–16.2) 15.6(13.8–17.1) 13.0(11.5–13.9)a,b 9.4(7.5–11.5)a,b,c
Hematocrit(%) 40.8(37.0–45.0) 41.4(39.0–46.3) 40.9(34.5–44.9) 29.8(24.6–34.1)a,b,c
Platelets(103/mm3) 222.0(159.0–321.0) 258.0(171.0–342.0) 199(148.0–240.0) 202.5(114.8–250.0)b
Leucocytes(103/mm3) 11.2(9.0–13.4) 11.1(9.6–14.1) 8.4(7.6–11.3)b 10.8(7.2–12.8)
Totalneutrophils 5.2(4.3–7.0) 5.8(4.5–7.3) 5.4(4.3–7.9) 7.0(4.2–9.6)
Eosinophils 0.5(0.4–0.7) 0.4(0.3–0.7) 0.3(0.2–0.5)a,b 0.3(0.1–0.4)a,b
Monocytes 0.5(0.4–0.7) 0.5(0.4–0.6) 0.3(0.2–0.4)a,b 0.3(0.2–0.4)a,b
Lymphocytes 4.3(3.6–5.5) 4.4(3.6–5.5) 2.7(2.4–3.7)a,b 2.5(2.0–3.4)a,b
Resultsareshownasmedianvaluesandthebracketsfirst(Q1)andthird(Q3)quartile.
CD,controldogs;AD-I,asymptomaticdogsI;AD-II,asymptomaticdogsII;SD,symptomaticdogs.
aStatisticallysignificantdifferencescomparedwithCD.
bStatisticallysignificantdifferencescomparedwithAD-I.
cStatisticallysignificantdifferencescomparedwithAD-II.
2.7. Immunostainingforcellsurfacemarkersand
intracellularcytokines
Bloodsampleswerecollectedinsteriletubes contain-ingsodiumheparinatafinalvolumeof5mLofperipheral blood.Monoclonalantibodiesusedtodetectcellsurface markersincludedanti-canineFITC-CD4 antibody(1:200, mouseIgG2a,cloneYKIX322.3)andanti-canineFITC-CD8 antibody (1:100,mouse IgG2a,clone YKIX302 9). Addi-tionally,mAbscross-reactivewithcaninecytokineswere usedforintracytoplasmicstaining,includinganti-bovine PE-IFN-␥antibody(cloneCC302) andanti-bovine PE-IL-4 antibody (clone CC303), all purchased from Serotec (Oxford,UK).
Two14-mLpolypropylenetubeswerepreparedforeach animalstudied;oneservedasacontroltube(1mLRPMI plus1mLofwholebloodinheparin),andinthestimulated
tubeL.infantumantigenwasaddedatfinalconcentration
of25g/mL.Thetubeswereincubatedfor12handkeptat 37◦Cinanincubatorwith5%CO
2.BrefeldinA–BFA(Sigma, StLouis,MO,USA)wasaddedtoeachtubeatafinal con-centrationof10g/mL,andcultureswerethensubmitted toanadditional 4hofincubationin 5%CO2 humidified incubatorat37◦C.AtubecontainingPMAatafinal con-centrationof25ng/mL wasusedasapositivecontrolat final4hofincubationasBFA.Firststainingwasperformed formonoclonalanti-surfacemolecules(CD4+ andCD8+). Afterresuspensionoftheselabeledcells,weproceededto stainintracytoplasmiccytokines(anti-IFN-␥andanti-IL-4) inU-bottom96-wellplates.Themicrotubeswerekeptat 4◦Cuntiltheacquisitionofcountsontheflowcytometer (FACScalibur–BectonDickinson,SanJose,CA,USA),which evaluatedatleast30,000eventspertube.
Distinct gating strategies were used to select the leukocyte subpopulations. The canine neutrophils were identifiedandselectedbasedontheiruniqueexpression ofCD4cellsurfacemarker,usingsidescatter(SSC)versus FL1/anti-CD4FITCdotplotdistributions,thusminimizing contaminationof theselectedregionby monocytesand eosinophils.Theeosinophilswereidentifiedandselected based ontheir autofluorescence, using nonrelated FL-3 channel versus forward scatter (FSC) dot plot distribu-tions.TheanalysisofthecytokineprofileofCD4+andCD8+
T-cellsubsetswasperformedbyfirstestablishinga scat-teringgateonthelymphocytepopulation,usinglaserSSC versusFL1dotplotdistributions.Afterselectingtheregion ofinterest(R1)dotplotswereconstructedforFSCversus IFN-␥/FL2orIL-4/FL2todeterminethepercentageof
IFN-␥+orIL-4+cellswithinthepopulationofneutrophilsand eosinophilspreviouslyselectedinR1.ToevaluatetheCD4+ andCD8+Tsubsets, dotplotswereusedforCD4/FL1or CD8/FL1versus IFN-␥/FL2 or IL-4/FL2. Theresults were expressedinpercentageofcells(IFN-␥+neutrophils,IL-4+ neutrophils,IFN-␥+eosinophils,IL-4+eosinophils,IFN-␥+ CD4+,IL-4+CD4+,IFN-␥+CD8+andIL-4+CD8+).
2.8. Statisticalanalysis
Statistical analysis was performed using GraphPad Prism5.0.Thenormalityofthedatawasassessedusing theKolmogorov–Smirnofftest.Consideringthe nonpara-metricnatureofalldatasets,Kruskal–Wallistestswere usedtoinvestigatedifferencesbetweenthegroups, fol-lowedbyDunn’stestforpairwisecomparisons.Spearman’s rank correlation was computed to investigate associa-tions between cell immunophenotyping and cytokine-producingcellsandtheclinicalgroups.TheChi-squaretest andKruskal–Wallistestwereusedtoevaluateifthe clin-icalgroupswerehomogeneousinrelationtogenderand agerespectively.Significantdifferenceswereconsideredat
p<0.05.
2.9. Ethicalstatement
3. Results
Tocheckfortheinfluenceofgenderandageofthe ani-malsontheresultsweevaluatedthehomogeneityofthe clinicalgroups.Itwasobservedthatthereisnosignificant differencebetweenthegroupsregardinggenderandageof animals(datanotshow).
3.1. Hematologicalprofile
Evaluationofhematologicalparametersshowedsevere anemia in the SD group, with significant decreases (p<0.0001)inthenumberoferythrocytes,hemoglobinand hematocrit.Decreasederythrocytecountswereobserved intheAD-IIandSDgroupscomparedwiththeCDand AD-Igroups. Furthermore,hemoglobinconcentrations were reducedintheAD-IIgroupcomparedwiththeCDandAD-I groups.IntheSDgroup,hemoglobinandhematocritwere reducedcomparedwithallothergroups.Thewhiteblood cellsanalysisrevealedreductionsin leukocytecountsin theAD-IIgroupcomparedwiththeAD-Igroup(p=0.0052). Absolutevalues of eosinophils,monocytesand lympho-cytesweredecreasedintheAD-IIandSDgroupscompared withtheCDandAD-Igroups(p<0.0001).Plateletcounts werereduced(p=0.0098)intheSDgroupcomparedwith theAD-Idogs(Table1).
3.2. ImmunophenotypingofcirculatingTlymphocytes
andtheirsubsets,Blymphocytesandmonocytes
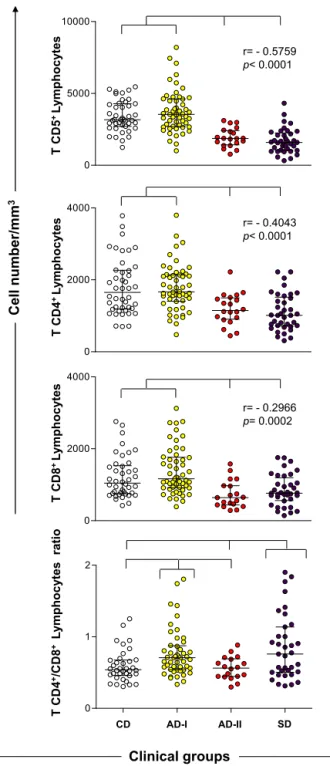
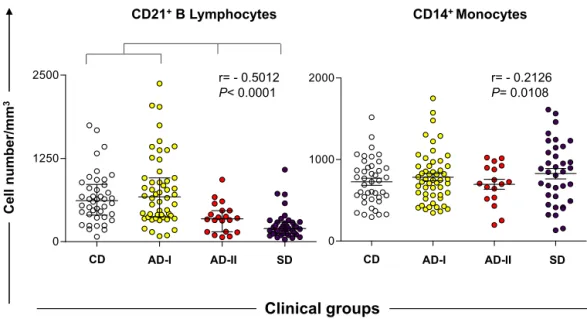
AnimalsfromtheAD-IIandSDgroupsshowed signifi-cantlydecreasedabsolutevaluesofTlymphocytes(CD5+) andtheirsubpopulations(CD4+andCD8+)comparedwith theCD andAD-Igroups(p<0.0001). Anegative correla-tionwasobservedwiththeclinicalstatus.TheCD4+/CD8+ TlymphocyteratiowassignificantlyhigherintheAD-Iand SDgroupscomparedwiththeCDandAD-IIgroups(Fig.1). Inaddition,theAD-IIandSDdogshadlowerB-lymphocyte counts(CD21+)thantheCDandAD-Igroups(p<0.0001). Nodifferencewasobservedinmonocytescomparedwith allgroups.Anegativecorrelationwasalsofoundbetween the number of B lymphocytes, monocytes and clinical groups(Fig.2).
3.3. IntracytoplasmicsynthesisofIFN-+andIL-4+by
eosinophilsandneutrophils,afterinvitroantigen-specific
stimulation
After stimulation with SLAi, neutrophils had higher frequencyofIFN-␥+ intheAD-IIandSD groupsin com-parisonwiththeCD andAD-Igroups.SynthesisofIL-4+ wasreduced intheAD-Igroupcomparedwithallother groups (p<0.0001). In addition, a positive correlation between clinical ongoing CVL and IFN-␥+ or IL4+ neu-trophils was observed, and the IFN-␥+/IL-4+ ratio was increasedintheAD-IIandSDgroupscomparedwiththeCD group(p=0.0002).However,nosignificantchangeswere observedincytokine-producingeosinophilsafterinvitro SLAistimulation(Fig.3).
T
CD
5
+Ly
mphoc
yt
es
T
CD
4
+Ly
mphoc
yt
es
T
CD
8
+Ly
mphoc
yt
es
T
CD
4
+/CD
8
+
Ly
mphoc
yt
e
s
ratio
0 5000 10000
0 2000 4000
0 2000 4000
0 1 2
Cel
l
number/mm
3
Clinical groups
CD AD-I AD-II SD
r= - 0.5759
p< 0.0001
r= - 0.4043
p< 0.0001
r= - 0.2966
p= 0.0002
Fig.1.Immunophenotypicprofileoflymphocytesinperipheralbloodof
dogsnaturallyinfectedbyL.infantumcategorizedbytheirclinicalstatus
andlaboratoryresultsasasymptomatic-I(AD-I),asymptomatic-II
(AD-II)andsymptomatic(SD).Noninfecteddogswereusedascontrols(CD).
Resultsareexpressedasabsolutecellcountsinscatterplots,andbars
rep-resentmedianandinterquartilerange.Significantdifferences(p<0.05)
areindicatedbyconnectinglinesbetweenthegroups.ThePearson
cor-relation(r)andpvalueshowninthegraphicsdemonstratecorrelations
betweenTCD5+lymphocytesandtheTsubsets(CD4+andCD8+)with
CD21
+B Lymphoc
ytes
CD1
4
+Monocytes
CD AD-I AD-II SD
0 1250 2500
CD AD-I AD-II SD
Cel
l
number/mm
3
Clinical groups
0 1000 2000 r= - 0.5012P< 0.0001
r= - 0.2126 P= 0.0108
Fig.2.ImmunophenotypicprofileofBlymphocytesandmonocytesinperipheralbloodofdogsnaturallyinfectedbyL.infantumcategorizedbytheir
clinicalstatusandlaboratoryresultsasasymptomatic-I(AD-I),asymptomatic-II(AD-II)andsymptomatic(SD).Noninfecteddogswereusedascontrols
(CD).Resultsareexpressedasabsolutecellcountsinscatterplots,andbarsrepresentmedianandinterquartilerange.Significantdifferences(p<0.05)are
indicatedbyconnectinglinesbetweenthegroups.ThePearsoncorrelation(r)atp<0.05showedcorrelationbetweenproductionofCD21+Blymphocytes
andCD14+monocyteswithclinicalprogression.
3.4. IntracytoplasmicsynthesisofIFN-+andIL-4+by
lymphocytesubsets(CD4+andCD8+)afterinvitro
antigen-specificstimulation
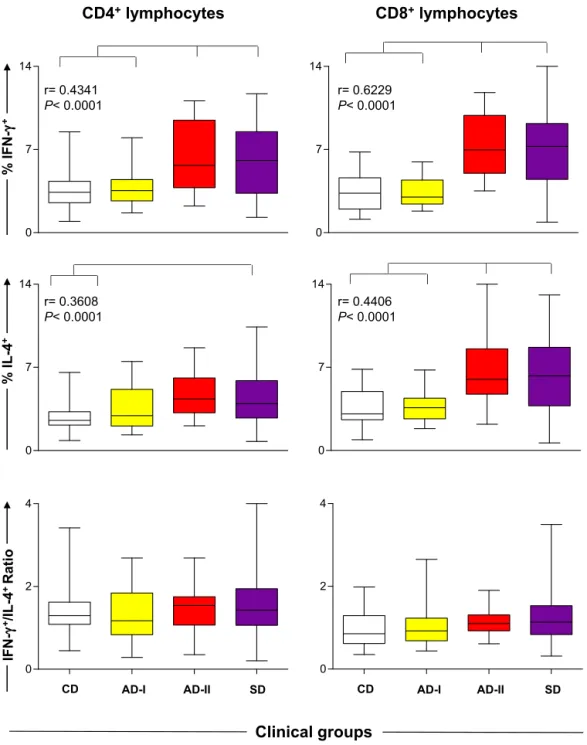
IncreasedpercentagesofIFN-␥producingCD4+orCD8+ lymphocyte subsets wereobservedin theAD-II andSD groupscomparedwiththeCDandAD-Igroups(p<0.0001). Moreover,weobservedanincreasedpercentageofIL-4+ CD4+lymphocytesintheSDgroupascomparedwiththe CDgroup(p=0.0005).ThepercentageofIL-4+CD8+ lym-phocyteswashigherintheAD-IIandSDgroupscompared withtheCDandAD-Igroups,andapositivecorrelationwas foundbetweenthesynthesisofIFN-␥+ orIL-4+bythese cellsandtheclinicalstatus(p<0.0001).Inananalysisof theIFN-␥+/IL-4+CD4+orCD8+cellratio,nodifferencewas observedintheexperimentalgroups(Fig.4).
4. Discussion
Studiesofthehematologicalandimmunological pro-files of dogs naturally infected by L. infantum and presenting different clinical profiles are important in assessingbiomarkersrelatedtothepathogenesisand prog-nosisofCVL(Reisetal.,2009).Thesearchforbiomarkers maybeimportanttobetterpredicttheevolutionofcanine disease,sincetheprogressionofinfectiondependsonthe efficiencyoftheimmuneresponseofthehost.Thus,the identificationandcharacterization ofbiomarkerscanbe importantinthedevelopmentofdiagnosis/prognosistests, vaccinesandtherapiesevaluationsappliedtoCVL.
Inthepresentstudy,diseaseprogressionwasassociated withchanges in varioushematological parameterssuch assignificantreductionoferythrocytes,hemoglobinand hematocrit,whichcharacterizedsevereanemia,especially in symptomaticdogs.Our findingscorroborateprevious
studiesthatidentifiedanemiaasacommon hematologi-caleventinactiveandsevereCVL(Reisetal.,2006a;da Costa-Valetal.,2007;deFreitasetal.,2012).DeLunaetal. (2000)suggested that anemiawould result in impaired erythrocytemembranefluidityinCVL,whichwouldfavor mechanicalsequestrationoferythrocytesintothespleen and/or alter receptor–ligand erythrocyte cytoadherence mechanisms. In addition to anemia, seropositive dogs (AD-IIandSDgroups)presentedeosinopenia, lymphope-nia and monocytopenia as previously documented by Reis et al. (2006b). It has been shown that reduced white blood cell counts in symptomatic dogs may be associatedwithbone marrowdysfunction, withintense parasitism causing decreased hematopoiesis and redi-recting bone marrow function (Tropia de Abreu et al., 2011).Nevertheless, consideringthatthedogsare from endemicarea,thehematologicchangescannotbeentirely attributedtotheCVL,sinceothercaninevector-borne dis-easesthat present similarlaboratory findings werenot ruledout.
0 1 4
0 7 14 0 2 14
IFN-γ
+
/IL-4
+
Ratio
% I
L
-4
+
% I
F
N
-γ
+
Neutrophils
CD AD-I AD-II SD CD AD-I AD-II SD
Clinical groups
Eosinophils
0 7 14
0 7 14
0 2 4
r= 0.5895 p< 0.0001
r= 0.2007 p= 0.0261
Fig.3.PercentageofneutrophilsandeosinophilsproducersofIFN-␥+,IL-4+andratioIFN-␥+/IL-4+instimulatedculture(SLAi)ofdogsnaturallyinfectedby
L.infantumcategorizedbytheirclinicalstatusandlaboratoryresultsasasymptomatic-I(AD-I),asymptomatic-II(AD-II)andsymptomatic(SD).Noninfected
dogswereusedascontrols(CD).Barsrepresentminimumandmaximumvalues;boxesdisplaymedianandinterquartilerange.Significantdifferences
(p<0.05)areindicatedbyconnectinglines.ThePearsoncorrelation(r)andpvalueshowcorrelationbetweenaproductionofIFN-␥+andIL-4+byneutrophils
withclinicalprogression.
populationinsymptomaticdogs(Bourdoiseauetal.,1997; Reisetal.,2006b;Alexandre-Piresetal.,2010), andthis reductionwasassociatedwithuncontrolledparasitismin theseanimals.
TheCD4+ and CD8+ T-cellsubsetsfollowed a similar profileasobservedfortheCD5+Tlymphocytes,with reduc-tionsintheAD-IIandSDgroups.Decreasedfrequencyof theCD4+T-cellsubpopulationinactiveCVLhasbeen con-firmedbymanygroups(Bourdoiseauetal.,1997;Moreno etal.,1999;Reisetal.,2006b;Guerra etal.,2009).This
reduction foundin seropositiveanimalsappearstobea critical factor for parasite replication, indicating a poor prognosisofdiseaseprogression.Furthermore,CD8+Tcells havebeenimplicatedinthecontrolofvisceralizingspecies
ofLeishmania(Tsagozisetal.,2003).Someresearchershave
0 7 14
0 7 14 0 7 14
0 7 14
IFN-γ
+
/IL-4
+
Ratio
% I
L
-4
+
% I
F
N
-γ
+
CD AD-I AD-II SD CD AD-I AD-II SD
Clinical groups
CD4
+lymphocytes
0 2 4
0 2 4
r= 0.4341 P< 0.0001
r= 0.6229 P< 0.0001
r= 0.3608 P< 0.0001
r= 0.4406 P< 0.0001
CD8
+lymphoc
ytes
Fig.4. PercentageofCD4+andCD8+T-lymphocyteproducersofIFN-␥+,IL-4+andtheIFN-␥+/IL-4+ratioinstimulatedculture(SLAi)derivedfromdogs
naturallyinfectedbyL.infantumcategorizedbytheirclinicalstatusandlaboratoryresultsasasymptomatic-I(AD-I),asymptomatic-II(AD-II)and
symp-tomatic(SD).Noninfecteddogswereusedascontrols(CD).Barsrepresentminimumandmaximumvalues;boxesdisplaymedianandinterquartilerange.
Significantdifferences(p<0.05)areindicatedbyconnectinglines.ThePearsoncorrelation(r)andpvalueshowcorrelationbetweenaproductionofIFN-␥+
andIL-4+byCD4+andCD8+Tlymphocyteswithclinicalprogression.
Tcellsmediatetheimmuneresponsebycytotoxic mecha-nismsthatassistinprotectionduringtheearlyphaseofthe infectionsasobservedintheAD-Igroup.However,reduced levelsofthiscelltypeintheAD-IIgroupcannotcontrolthe infection,indicatingapoorprognosisasobservedinthe SDgroup.Therefore,theevaluationofT-cellsubsets(CD4+ andCD8+)representsanimportantbiomarkerofclinical progressioninCVL.
maybeabiomarkerforsusceptibilityand/orsevereCVL (Bourdoiseauetal.,1997;Coura-Vitaletal.,2011a).
Recentstudiesshowedthatneutrophilsplayan impor-tant regulatory role in the early stages of Leishmania
infection(PetersandSacks,2009;Ritteretal.,2009). Stud-ieshavealsoshownthatthesecellshaveadirectimpact onthedeathoftheparasiteandthedevelopmentofa pro-tectiveimmuneresponse againstinfections causedbyL.
donovaniandL.infantum(Rousseauetal.,2001;McFarlane
et al.,2008).Thus, thesecells could provide an impor-tantlinkbetweeninnate andadaptiveimmunityduring parasiteinfection, and theyareon of themresponsible for the visceralization of the parasitism for many lym-phoidorgans(Carvalhoetal.,2012).Inthisstudy,itwas observedforthefirsttimethatcaninebloodneutrophils, whenstimulatedinvitrowithSLAi,wereabletoproduce highlevelsofIFN-␥+ inAD-IIandSDanimalscompared withCDandAD-Idogs.Thesefindingswereconfirmedby thecorrelationobservedwhendogsexhibitactiveCVL.In addition,highlevelsofIFN-␥+neutrophilsareanattempt bytheimmunesystemtodecreasetheparasiteload,but IFN-␥alonecannot protecttheanimalsduringinfection (Chamizoetal.,2005;Lageetal.,2007;Reisetal.,2010). Interestingly,theAD-Igroupshowedreducedsynthesisof IL-4byneutrophils compared withtheother groups.In humanpatientswithactiveVL,increasedsynthesisofIL-4 byneutrophilsstimulatedbysolubleLeishmaniaantigens hasbeenreported(Peruhype-Magalhaesetal.,2005).IL-4 appearstoberelatedtotheseverityofCVL(Quinnelletal., 2001),thusthiscytokinecouldbeanimportantbiomarker ofsusceptibilityincaninesnaturallyinfectedbyL. infan-tum.AlthoughPeruhype-Magalhaesetal.(2005)founda highfrequencyofstimulatedeosinophilIFN-␥+inhuman VL,wedidnotobservethisfindingeitherforneutrophilor eosinophilIL-4+indogsnaturallyinfectedbyL.infantum.
Anevaluationofintracytoplasmiccytokineexpression ininvitroexperimentswithT-cellsubsets(CD4+andCD8+ lymphocytes) showed that both subsets produced high levelsofIFN-␥(mainlybyCD8+lymphocyteswithhigh cor-relation)inseropositiveanimals(AD-IIandSDgroups)in comparisonwiththeCDandAD-Igroups.Previousstudies havereportedthathighexpressionofIFN-␥isassociated withsymptomaticdisease(Lageetal.,2007; Rodriguez-Cortesetal.,2007;Costaetal.,2013).However,Carrillo etal.(2007)foundreducedexpressionofIFN-␥in experi-mentallyinfecteddogspresentingsymptomaticVL.These highlevelsofIFN-␥producedbylymphocytesinAD-IIand SDdogsindicateanattempttocontrolinfectioninthese animals.However,ourresultsalsosuggested thatIFN-␥
wasnotsufficienttopreventdisease,anditcouldnotbe consideredas a marker of resistanceon these animals. Perhaps,successful infectioncontrolcouldnot achieved becausethere wasa balancewithmodulating cytokines suchasIL-10andTGF-thatunderminedclinical improve-mentinanimals.Additionalstudiesevaluatingtheprofile ofcytokinemodulatorswillbeimportanttoclarifythisfact. RegardingtheparticipationofIL-4,manyresearchers demonstrated thatthis cytokine is associated with sus-ceptibilitytodiseaseand isassociated withanincrease in parasitic load in CVL (Pinelli et al., 1999b; Quinnell etal.,2001;Alvesetal.,2009).Strauss-Ayalietal.(2007)
reportedthatearlyexpressionofIL-4measuredinspleen cells playsanimportantroleinthepersistenceof para-sitesdespitehighexpressionofIFN-␥(Strauss-Ayalietal., 2007).BasedonthesefindingswesuggestthatAD-I ani-malshaveextremelylowparasitism,withlittlestimulation oftheIL-4+synthesisbylymphocytes,whichmaybea pro-tectivefactorfortheseanimals.Incontrast,theproduction ofIL-4+ byCD4+ andCD8+ cellsdetectedinseropositive dogs(AD-IIandSD)maycontributetobetterunderstand thepersistenceandparasitereplicationobservedinsevere CVL,aspreviouslydocumentedbyGuerraetal.(2009).
Inconclusion,thisstudyshowedforthefirsttimethat theAD-Igroupdoesnotdifferfromhealthyanimalsinthat nosignificantalterationoccursinthecellpopulation eval-uated and there isnoactivationof theType2 immune response,whicheffectivelyconfersaresistanceprofilefor them.Incontrast,theanimalsfromtheAD-IIandSDgroups exhibitedamixedimmuneprofile(Type1and2)in paral-lelwiththeimmunosuppressionthataffectedboththeT andBcompartments,withaconcomitantpresenceof out-standingIFN-␥+andIL-4+producedbyneutrophilsandT lymphocytes,makingthemunabletocontrolparasite repli-cation.
Acknowledgements
Thisstudywassupportedbythefollowinggrants: Fed-eralUniversity ofOuroPreto;DECIT/MS/CNPq/BR/grant: 576062/2008-1;FAPEMIG/BR/grant:CBB-APQ-3073-4.01/ 07, CNPq/BR/grant: 472554/2007-7; PPSUS/MS/CNPq/ FAPEMIG/SES-MG/grant CBB-APQ-00356-10; FAPEMIG/ PPMandPNPD/Institutional/2011.Thefundershadnorole instudydesign,datacollectionoranalysis,thedecisionto publish,orthepreparationofthemanuscript.ABR,CMC, RCG,ATC,OAMF,andMCaregratefulforCNPqfellowships, WCVisgratefultothePNPD/CAPESfellowshipsandFederal UniversityofOuroPreto.Wealsothankthestaffofthe Sec-retariaMunicipaldeSaúdedeBeloHorizonte,MinasGerais, forcooperation,logisticalsupport,andspecialdedication tothiswork.
References
Alexandre-Pires,G.,deBrito,M.T.,Alguero,C.,Martins,C.,Rodrigues,
O.R.,daFonseca,I.P.,Santos-Gomes,G.,2010.Canineleishmaniasis.
Immunophenotypicprofileofleukocytesindifferentcompartments of symptomatic,asymptomatic andtreated dogs. Vet. Immunol. Immunopathol.137,275–283.
Alves,C.F.,deAmorim,I.F.,Moura,E.P.,Ribeiro,R.R.,Michalick,M.S.,
Kalapothakis,E.,Bruna-Romero,O.,Tafuri,W.L.,Teixeira,M.M.,Melo,
M.N.,2009.ExpressionofIFN-gamma,TNF-alpha,IL-10andTGF-beta
inlymphnodesassociateswithparasiteloadandclinicalformof dis-easeindogsnaturallyinfectedwithLeishmania(Leishmania)chagasi. Vet.Immunol.Immunopathol.128,349–358.
Bourdoiseau,G.,Bonnefont,C.,Magnol,J.P.,Saint-Andre,I.,Chabanne,L.,
1997.Lymphocytesubsetabnormalitiesincanineleishmaniasis.Vet.
Immunol.Immunopathol.56,345–351.
Carrillo,E.,Ahmed,S.,Goldsmith-Pestana,K.,Nieto,J.,Osorio,Y.,Travi,
B.,Moreno,J.,McMahon-Pratt,D.,2007.Immunogenicityofthe
P-8amastigoteantigenintheexperimentalmodelofcaninevisceral leishmaniasis.Vaccine25,1534–1543.
Carrillo,E.,Moreno,J.,2009.Cytokineprofilesincaninevisceral
leishman-iasis.Vet.Immunol.Immunopathol.128,67–70.
Carvalho,L.P.,Petritus,P.M.,Trochtenberg,A.L.,Zaph,C.,Hill,D.A.,Artis,D.,
Scott,P.,2012.LymphnodehypertrophyfollowingLeishmaniamajor
Chamizo, C.,Moreno,J.,Alvar, J.,2005. Semi-quantitativeanalysisof cytokine expression in asymptomatic canine leishmaniasis. Vet. Immunol.Immunopathol.103,67–75.
Correa, A.P.,Dossi,A.C.,deOliveiraVasconcelos,R.,Munari,D.P.,de
Lima,V.M.,2007.Evaluationoftransformationgrowthfactorbeta1,
interleukin-10, andinterferon-gamma inmale symptomatic and asymptomaticdogsnaturallyinfectedbyLeishmania(Leishmania) cha-gasi.Vet.Parasitol.143,267–274.
Costa,D.J.,Carvalho,R.M.,Abbehusen,M.,Teixeira,C.,Pitombo,M.,Trigo,
J.,Nascimento,F.,Amorim,L.,Abreu-Silva,A.L.,doSocorroPiresCruz,
M.,Miranda,J.C.,Fukutani,K.,deOliveira,C.I.,Barral,A.,Barral-Netto,
M.,Brodskyn,C.,2013.Experimentalinfectionofdogswithleishmania
andsalivaasamodeltostudycaninevisceralleishmaniasis.PLOSONE 8,e60535.
Coura-Vital,W.,Marques, M.J.,Giunchetti,R.C.,Teixeira-Carvalho,A.,
Moreira,N.D.,Vitoriano-Souza,J.,Vieira,P.M.,Carneiro,C.M.,
Correa-Oliveira, R., Martins-Filho, O.A., Carneiro, M., Reis, A.B., 2011a.
Humoralandcellularimmuneresponsesindogswithinapparent nat-uralLeishmaniainfantuminfection.Vet.J.190,e43–e47.
Coura-Vital,W.,Marques,M.J.,Veloso,V.M.,Roatt,B.M.,Aguiar-Soares,
R.D.,Reis,L.E.,Braga,S.L.,Morais,M.H.,Reis,A.B.,Carneiro,M.,2011b.
PrevalenceandfactorsassociatedwithLeishmaniainfantuminfection ofdogsfromanurbanareaofBrazilasidentifiedbymolecular meth-ods.PLoSNegl.Trop.Dis.5,e1291.
Coura-Vital,W.,Reis,A.B.,Fausto,M.A.,Leal,G.G.A.,Marques,M.J.,Veloso,
V.M.,Carneiro,M.,2013.RiskfactorsforseroconversionbyLeishmania
infantuminacohortofdogsfromanendemicareaofBrazil.PLOSONE 8,e71833.
daCosta-Val,A.P.,Cavalcanti,R.R.,deFigueiredoGontijo,N.,Michalick,
M.S.,Alexander,B.,Williams,P.,Melo,M.N.,2007.Caninevisceral
leishmaniasis:relationshipsbetweenclinicalstatus,humoralimmune response,haematologyandLutzomyia(Lutzomyia)longipalpis infectiv-ity.Vet.J.174,636–643.
deFreitas,J.C.,Lopes-Neto,B.E.,deAbreu,C.R.,Coura-Vital,W.,Braga,S.L.,
Reis,A.B.,Nunes-Pinheiro,D.C.,2012.Profileofanti-Leishmania
anti-bodiesrelatedtoclinicalpictureincaninevisceralleishmaniasis.Res. Vet.Sci.93,705–709.
De Luna, R.,Ferrante, M., Severino, L.,Ambrosio, R.,Piantedosi, D.,
Gradoni,L.,Lucisano,A.,Persechino,A.,2000.Decreasedlipidfluidity
oftheerythrocytemembraneindogswithleishmaniasis-associated anaemia.J.Comp.Pathol.122,213–216.
Degrave,W.,Fernandes,O.,Campbell,D.,Bozza,M.,Lopes,U.,1994.Useof
molecularprobesandPCRfordetectionandtypingofLeishmania—a mini-review.Mem.Inst.OswaldoCruz89,463–469.
Fraga,D.B.,Solca,M.S.,Silva,V.M.,Borja,L.S.,Nascimento,E.G.,Oliveira,
G.G.,Pontes-de-Carvalho,L.C.,Veras,P.S.,Dos-Santos,W.L., 2012.
Temporal distribution of positive results of tests for detecting Leishmaniainfectioninstraydogsofanendemicareaofvisceral leish-maniasisintheBraziliantropics:a13yearssurveyandassociation withhumandisease.Vet.Parasitol.190,591–594.
Giunchetti,R.C.,Correa-Oliveira,R.,Martins-Filho,O.A.,Teixeira-Carvalho,
A.,Roatt,B.M.,deOliveiraAguiar-Soares,R.D.,deSouza,J.V.,dasDores
Moreira,N.,Malaquias,L.C.,MotaeCastro,L.L.,deLana,M.,Reis,A.B.,
2007.ImmunogenicityofakilledLeishmaniavaccinewithsaponin
adjuvantindogs.Vaccine25,7674–7686.
Giunchetti,R.C.,Mayrink,W.,Genaro,O.,Carneiro,C.M.,Correa-Oliveira,
R.,Martins-Filho,O.A.,Marques,M.J.,Tafuri,W.L.,Reis,A.B.,2006.
Rela-tionshipbetweencaninevisceralleishmaniosisandtheLeishmania (Leishmania)chagasiburdenindermalinflammatoryfoci.J.Comp. Pathol.135,100–107.
GrimaldiJr.,G.,Teva,A.,Santos,C.B.,Ferreira,A.L.,Falqueto,A.,2012.
Theeffectofremovingpotentiallyinfectiousdogsonthenumbersof canineLeishmaniainfantuminfectionsinanendemicareawithhigh transmissionrates.Am.J.Trop.Med.Hyg.86,966–971.
Guerra,L.L.,Teixeira-Carvalho,A.,Giunchetti,R.C.,Martins-Filho,O.A.,
Reis,A.B.,Correa-Oliveira,R.,2009.Evaluationoftheinfluenceof
tissueparasitedensityonhematologicaland phenotypiccellular parametersofcirculatingleukocytesandsplenocytesduringongoing caninevisceralleishmaniasis.Parasitol.Res.104,611–622.
Lage,R.S.,Oliveira,G.C.,Busek,S.U.,Guerra,L.L.,Giunchetti,R.C.,
Correa-Oliveira, R., Reis, A.B.,2007. Analysis of the cytokine profile in
spleencellsfromdogsnaturallyinfectedbyLeishmaniachagasi.Vet. Immunol.Immunopathol.115,135–145.
Lowry, O.H., Rosebrough, N.J., Farr, A.L.,Randall, R.J., 1951. Protein
measurementwith theFolinphenolreagent. J. Biol.Chem.193, 265–275.
McFarlane,E.,Perez,C.,Charmoy,M.,Allenbach,C.,Carter,K.C.,Alexander,
J.,Tacchini-Cottier,F.,2008.Neutrophilscontributetodevelopment
ofaprotectiveimmuneresponseduringonsetofinfectionwith Leish-maniadonovani.Infect.Immun.76,532–541.
Menezes-Souza,D.,Correa-Oliveira,R.,Guerra-Sa,R.,Giunchetti,R.C.,
Teixeira-Carvalho,A.,Martins-Filho,O.A.,Oliveira,G.C.,Reis,A.B.,
2011.Cytokineandtranscriptionfactorprofilesintheskinofdogs
naturallyinfectedbyLeishmania(Leishmania)chagasipresenting dis-tinctcutaneousparasitedensityandclinicalstatus.Vet.Parasitol.177, 39–49.
Moreno,J.,Alvar,J.,2002.Canineleishmaniasis:epidemiologicalriskand
theexperimentalmodel.TrendsParasitol.18,399–405.
Moreno,J.,Nieto,J.,Chamizo,C.,Gonzalez,F.,Blanco,F.,Barker,D.C.,
Alva,J.,1999.TheimmuneresponseandPBMCsubsetsincanine
vis-ceralleishmaniasisbefore,andafter,chemotherapy.Vet.Immunol. Immunopathol.71,181–195.
Passos,V.M.,Fernandes,O.,Lacerda,P.A.,Volpini,A.C.,Gontijo,C.M.,
Degrave,W.,Romanha,A.J.,1999.Leishmania(Viannia)braziliensisis
thepredominantspeciesinfectingpatientswithAmericancutaneous leishmaniasisintheStateofMinasGerais,SoutheastBrazil.ActaTrop. 72,251–258.
Peruhype-Magalhaes,V.,Martins-Filho,O.A.,Prata,A.,Silva,L.A.,Rabello,
A.,Teixeira-Carvalho,A.,Figueiredo,R.M.,Guimaraes-Carvalho,S.F.,
Ferrari,T.C.,Correa-Oliveira,R.,2005.Immuneresponseinhuman
visceralleishmaniasis:analysisofthecorrelationbetweeninnate immunitycytokineprofileanddiseaseoutcome.Scand.J.Immunol. 62,487–495.
Peters,N.C., Sacks, D.L., 2009. Theimpact of vector-mediated
neu-trophilrecruitmentoncutaneousleishmaniasis.CellMicrobiol.11, 1290–1296.
Pinelli,E.,Gonzalo,R.M.,Boog,C.J.,Rutten,V.P.,Gebhard,D.,delReal,
G.,Ruitenberg,E.J.,1995.Leishmaniainfantum-specificTcelllines
derivedfromasymptomaticdogsthatlyseinfectedmacrophagesina majorhistocompatibilitycomplex-restrictedmanner.Eur.J.Immunol. 25,1594–1600.
Pinelli,E., Killick-Kendrick,R.,Wagenaar,J.,Bernadina,W.,del Real,
G.,Ruitenberg,J.,1994.Cellularandhumoralimmuneresponsesin
dogsexperimentallyandnaturallyinfectedwithLeishmaniainfantum. Infect.Immun.62,229–235.
Pinelli, E.,van derKaaij, S.Y., Broeren,C.P., Ruitenberg,E.J.,Rutten,
V.P.,1999a.Measurementofdogcytokinesbyreverse
transcription-quantitativecompetitivepolymerasechainreaction.Immunogenetics 49,696–699.
Pinelli,E.,VanderKaaij,S.Y.,Slappendel,R.,Fragio,C.,Ruitenberg,E.J.,
Bernadina,W.,Rutten,V.P.,1999b.Detectionofcaninecytokinegene
expressionbyreversetranscription-polymerasechainreaction.Vet. Immunol.Immunopathol.69,121–126.
Quinnell,R.J.,Courtenay,O.,Shaw,M.A.,Day,M.J.,Garcez,L.M.,Dye,C.,
Kaye,P.M.,2001.Tissuecytokineresponsesincaninevisceral
leish-maniasis.J.Infect.Dis.183,1421–1424.
Reis,A.B.,Giunchetti,R.C.,Carrillo,E.,Martins-Filho,O.A.,Moreno,J.,2010.
ImmunitytoLeishmaniaandtherationalsearchforvaccinesagainst canineleishmaniasis.TrendsParasitol.26,341–349.
Reis, A.B., Martins-Filho, O.A., Teixeira-Carvalho, A., Carvalho, M.G.,
Mayrink,W.,Franca-Silva,J.C.,Giunchetti,R.C.,Genaro,O.,
Correa-Oliveira, R., 2006a. Parasite density and impaired
biochemi-cal/hematologicalstatusareassociatedwithsevereclinicalaspects ofcaninevisceralleishmaniasis.Res.Vet.Sci.81,68–75.
Reis, A.B.,Martins-Filho, O.A.,Teixeira-Carvalho, A., Giunchetti,R.C.,
Carneiro,C.M.,Mayrink,W.,Tafuri,W.L.,Correa-Oliveira,R.,2009.
Sys-temicandcompartmentalizedimmuneresponseincaninevisceral leishmaniasis.Vet.Immunol.Immunopathol.128,87–95.
Reis,A.B.,Teixeira-Carvalho,A.,Giunchetti,R.C.,Guerra,L.L.,Carvalho,
M.G.,Mayrink,W.,Genaro,O.,Correa-Oliveira,R.,Martins-Filho,O.A.,
2006b.Phenotypicfeaturesofcirculatingleucocytesas
immunolog-icalmarkersforclinicalstatusandbonemarrowparasitedensityin dogsnaturallyinfectedbyLeishmaniachagasi.Clin.Exp.Immunol.146, 303–311.
Reis,A.B.,Teixeira-Carvalho,A.,Vale,A.M.,Marques,M.J.,Giunchetti,R.C.,
Mayrink,W.,Guerra,L.L.,Andrade,R.A.,Correa-Oliveira,R.,
Martins-Filho,O.A.,2006c.Isotypepatternsofimmunoglobulins:hallmarks
forclinicalstatusandtissueparasitedensityinBraziliandogs nat-urallyinfectedbyLeishmania(Leishmania)chagasi.Vet.Immunol. Immunopathol.112,102–116.
Ritter,U.,Frischknecht,F.,vanZandbergen,G.,2009.Areneutrophils
importanthostcellsforLeishmaniaparasites?TrendsParasitol.25, 505–510.
Rodriguez-Cortes,A.,Fernandez-Bellon,H.,Ramis,A.,Ferrer,L.,Alberola,
J.,Solano-Gallego,L.,2007.Leishmania-specificisotypelevelsand
theirrelationship with specific cell-mediated immunity parame-ters in canineleishmaniasis. Vet.Immunol. Immunopathol. 116, 190–198.
Rousseau,D.,Demartino,S.,Ferrua,B.,Michiels,J.F.,Anjuere, F.,
polymorphonuclearneutrophilsin Leishmaniainfantum infection. BMCMicrobiol.1,17.
Strauss-Ayali,D.,Baneth,G.,Jaffe,C.L.,2007.Splenicimmuneresponses
duringcaninevisceralleishmaniasis.Vet.Res.38,547–564.
Tropia deAbreu, R.,Carvalho, M.G., Carneiro, C.M., Giunchetti,R.C.,
Teixeira-Carvalho,A.,Martins-Filho,O.A.,Coura-Vital,W.,
Correa-Oliveira,R.,Reis,A.B.,2011.Influenceofclinicalstatusandparasite
loadonerythropoiesisandleucopoiesisindogsnaturallyinfected withLeishmania(Leishmania)chagasi.PLoSONE6,e18873.
Tsagozis,P.,Karagouni,E.,Dotsika,E.,2003.CD8(+)Tcellswith
parasite-specificcytotoxicactivityandaTc1profileofcytokineandchemokine
secretiondevelopinexperimentalvisceralleishmaniasis. Parasite Immunol.25,569–579.
Volpini,A.C.,Passos,V.M.,Oliveira,G.C.,Romanha,A.J.,2004.PCR-RFLP
toidentifyLeishmania(Viannia)braziliensisandL.(Leishmania) ama-zonensiscausingAmericancutaneousleishmaniasis.ActaTrop.90, 31–37.
WorldHealthOrganization,2010.WorkingtoOvercometheGlobalImpact