w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Acute
genotoxicity
analysis
in
vivo
of
the
aqueous
extract
of
Maytenus
guyanensis
Amazonian
chichuá
Dionatas
Ulises
de
Oliveira
Meneguetti
a,b,c,∗,
Renato
Abreu
Lima
c,d,
Francisco
Carlos
da
Silva
e,f,
Guilherme
Matos
Passarini
g,
João
Bezerra
Facundo
g,
Rubiani
de
Cassia
Pagotto
g,
Júlio
Sancho
Linhares
Teixeira
Militão
c,h,
Valdir
Alves
Facundo
a,c,d,haProgramadePós-graduac¸ãoemBiologiaExperimental,UniversidadeFederaldeRondônia,PortoVelho,RO,Brazil bColégiodeAplicac¸ão,UniversidadeFederaldoAcre,RioBranco,AC,Brazil
cLaboratóriodeQuímicadeProdutosNaturais,UniversidadeFederaldeRondônia,PortoVelho,RO,Brazil
dProgramadePós-graduac¸ãoemBiodiversidadeeBiotecnologiadaAmazôniaLegal(RedeBionorte),Manaus,AM,Brazil eProgramadePós-graduac¸ãoemBiologiaCelulareMolecularAplicadaàSaúde,UniversidadeLuteranadoBrasil,Canoas,RS,Brazil fCentroUniversitárioLuteranodeJi-Paraná,Ji-Paraná,RO,Brazil
gDepartamentodeCiênciasBiológica,UniversidadeFederaldeRondônia,PortoVelho,RO,Brazil hDepartamentodeQuímica,UniversidadeFederaldeRondônia,PortoVelho,RO,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received21January2015 Accepted9March2015 Availableonline30March2015
Keywords:
Mutagenicity
Maytenusguyanensis
WesternAmazon
a
b
s
t
r
a
c
t
ThespeciesMaytenusguyanensisKlotzschexReissek,Celastraceae,presentawidevarietyofpossible
pharmacologicalactivitiesanditsrootsandstemsareusedbypopularmedicineinthewesternAmazon
rainforest.Fewstudieshavedemonstratedthegenotoxicsafetyofthepopularuseofthisspecies,and
owingtothis,thepresentstudyaimedtoperformananalysisoftheacutegenotoxicityinvivoofthe
aqueousextractofM.guyanensis.MaleandfemalemicefromMusmusculusspecies,ofweightsranging
from20to40g,organizedineightgroupswithdifferenttreatmentswereused.Theaqueousextractsofthe
barkofM.guyanensiswereadministeredorallybygavagewith0.1mlofthetestsubstanceper10gofthe
animal,followedbyperformanceofcometassayinperipheralblood,PCE/NCEcorrelationandoccurrence
ofmicronucleiinthebonemarrow.ItwasfoundthattheaqueousextractofM.guyanensis,withten
timeshigherconcentrationthanthoseusedinethnopharmacology,didnotpresentgenotoxiceffectand,
moreover,ithasantigenotoxicactioninmicetreatedacutely.Furtherstudiesregardingbioaccumulation
andchroniceffectsofthisspeciesaresuggested,inordertoimprovetheunderstandingofitsmechanism
ofaction,ensuringtheefficacyandsafetyofitsutilizationanddevelopingphytotherapicsanddrugs.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Brazilpossessesalmost19%oftheworld’sflora,ofwhichthe AmazonForestisoneofthemostrichanddiversifiedareaonthe planet;however,roughly99%ofthemedicinalplantsdonothave itsefficacyandpharmacologicalsafetyproven(Giuliettietal.,2005; Fãoetal.,2012;Meneguettietal.,2014),makingnecessarya phy-tochemicalandpharmacologicalapproach(Andradeetal.,2007), whichmayrepresentagreat economicpotentialtobeexplored by the pharmaceutical industry (Cechinel-Filho and Rosendo, 1998).
∗ Correspondingauthor.
E-mail:dionatas@icbusp.org(D.U.d.O.Meneguetti).
Thepopularmedicinalspecies MaytenusguyanensisKlotzsch exReissekbelongstoCelastraceaebotanicalfamilyandisasmall endemictree,popularlyknownas“chichuá”,“xixuá”,“chuchauasi”, “chuchuhuashu”, “chuchuasi”, “chuchasa” or “tonipulmon” (Revilla, 2001; Prata, 2007; Prata and Mendonca, 2009). The speciespresentawidevarietyofpossiblepharmacological activi-ties,whereinitsrootsandstemsareusedpopularlyasanalgesic, musclerelaxant,woundhealing,insecticide,immunosuppressive, anti-inflammatory, anti-ulcerogenic, anti-rheumatism, anti-diarrheal,antibacterial,antifungal,anti-helminthic,antiprotozoal, antitumor,aphrodisiacandgynecologicallyactive(Revilla,2002; Borrás,2003;Macarietal.,2006;Fonsecaetal.,2007).
PhytochemicalstudiesofspeciesfromthegenusMaytenushave demonstratedpresenceofseveralchemicalgroups,inwhichwe can contrast thequinone-methide triterpenoids that presented several biological activities, such as tripanocide (Duarte et al.,
http://dx.doi.org/10.1016/j.bjp.2015.03.003
2002), anti-helminthic(Mena-Rejónet al., 2007), cytotoxic and antitumor(Moritaetal.,2008).Othermetabolitesidentifiedwithin thisgenuswere:tannins,flavonoidsandalkaloids(Santos-Oliveira etal.,2009;Gonzalezetal.,1996;Chavezetal.,1998).
Phytochemical studiesof leaves, stembarks and roots ofM. guyanensisleadtoisolationand identificationoftenterpenoids, whereinfive arefriedelane: friedeline, friedelol,16-friedeline, 29-hydroxifriedelineand 3,7-friedelodione;oneof themis a -amerineoleanane;oneofthemisa␣-amerineursane,andthreeare friedo-nor-oleanane(quinine-methides):tingenone, 22-hydroxy-tingenoneand22-hidroxi-pristimerine.Inaddition,twosteroids: -sitosteroland sitostenone,one sesquiterpene alkaloid named
N,N-dimethylserine(Sousaetal.,1986;Facundoetal.,2015)and one flavonoid: 4-methyl-epigalocatequine (Macari et al., 2004) werealsoidentified;however,fewstudieshavedemonstratedthe genotoxicsafetyofthepopularuseofthisspecies,andowingto it,thepresent studyaimedtoperformananalysisoftheacute genotoxicityinvivooftheaqueousextractofM.guyanensis.
Materialsandmethods
Collectionandidentificationofbotanicalmaterial
ThebarksofMaytenusguyanensisKlotzschexReissek, Celas-traceae,werecollectedinFebruary2008attheAdolphoDucke’s Forest Reserve, located at the km 26, AM-010 road (Manaus-Itacoatiara–Lat02◦53′S,Long59◦58′W).Theidentificationofthe
specieswasdoneattheHerbáriodoInstitutoNacionaldePesquisa daAmazônia(INPA),Exsiccaten◦188.485.
Extractpreparation
BarksfromM.guyanensisweregrindedtoimprovesolvent con-tactarea.Theextractwaspreparedbyinfusionwithdistilledwater for10minat80◦C,atthefollowing concentrations:3.85mg/ml,
value normally used by populations and ten, twenty and fifty timesmoreconcentrated(Camparotoetal.,2002;Meneguettietal., 2014).
Animals’treatment
ThetestswereperformedfromJanuarytoMarch2014,at Lab-oratory deGeneticeToxicology Applied fromCentro academic LutherandeJi-Paraná(CEULJI/ULBRA),inJi-Paranácity,Rondônia, Brazil.AlloftheexperimentswereapprovedbyEthics Commit-teeonAnimalUse(CEUA)fromOswaldoCruzFoundation– RO (Fiocruz-RO),protocolnumber2013/12.
Musmusculus’smaleandfemalemicewereused,acquiredfrom CEULJI/ULBRAvivarium,withweightrangingfrom20to40g.The aqueousextractsfrombarksofM.guyanensiswereadministered
viaoralgavage,twoamountsat48and24h,respectively,before thetestbegan.Theanimalswereweighedbeforethedosageand thevolumeadministeredwascalculatedas0.1mlofthetesting substanceper10goftheanimal.
Theanimalsweredividedintoeightgroups,eachone contain-ingeightanimals,fourweremaleandfourfemale,andtheywere maintainedinenvironmentswithcontrolledtemperature(25◦C),
withcyclesof12hoflightand12hofdarkness,inpolyethylene cages,withaccesstowaterandfood.
Thefollowinggroupswereorganizedasfollows:
G1–negativecontrol(CN),whereonlyH2Owasadministered;
G2to G5 –treated withaqueousextracts of M.guyanensis in concentrations3.85mg/ml,38.5mg/ml,77mg/mland192mg/ml, respectively;
G6 and G7 – treated, respectively, with 3.85mg/ml and 38.5mg/ml,withadditionof2.0mgcyclophosphamideper100g ofanimalweight,treatedviaintraperitonealinjection,24hbefore theapplicationofthefirstdoseoftheaqueousextractofM. guya-nensis.Theseconcentrationswerechosen,basedonastudycarried outbyMeneguettietal.(2014),inwhichthesesame concentra-tionspresentedanticytotoxicandanti-mutagenicactionininvitro
studies;
G8–positivecontrol(CP),just2.0mgofcyclophosphamidewas administeredper100gofmiceweight(Magalhãesetal.,2010),via
intraperitonealinjection,24hbeforetheapplicationofthefirst doseoftheaqueousextractofM.guyanensis,beingalsotreated with0.1mlofH2Oper10goftheanimal,viagavage,atthesame
periodsastoomuchgroups.
Cometassay
Thegenotoxicityandacuteantigenotoxicityanalysisweredone bycometassay,fromthemethoddescribedbySinghetal.(1988)
andreviewedbyTiceetal.(2000).Theexperimentwasperformed inbloodcellsfromtheanimalssubmittedtothetreatmentwiththe aqueousextractofM.guyanensis.Theperipheralbloodofanimals wascollectedafterthedecapitationwithaguillotine,whereintwo slidesperanimalwereprepared.
Thesamplesincellularsuspensionweremixedwithathinlayer ofagar“lowmelting”0.75%andlaiduponlayerspre-coveredwith normalagaroseat1.5%.Theseslideswereplungedinalysissolution (2.5MNaCl,100mMEDTAand10mMTris,pH10withadditionof 1%TritonX-100and10%DMSOatthetimeofuse),for96hat4◦C.
ThelysisenablesthemigrationoftheDNAfragmentsthatwere broughtaboutafterincubation oftheslides inalkaline tampon (300mMNaOHand1mMEDTA,pH>13)for20minand, subse-quently,anelectriccurrent of300mAand25V(0.90V/cm)was appliedfor15mininaDNAelectrophoresisvat.Theslideswere neutralizedafterelectrophoresiswiththreetamponsat0.4M,pH 7.5andcoloredwithsilvernitrate.
The analysisof thecells was performedrandomlyby visual evaluation, totaling 100 cells/slide, wherein two parameters wereconsidered:DamageIndex(0–400)andDamageFrequency (0–100%).
CorrelationPCE/NCEandoccurrenceofmicronucleiinbone marrow
Phosphatetampon
ThesolutionsAandBwerepreparedseparately.Forthe solu-tionA, 27.6g ofanhydride potassium phosphate (KH2PO4)was
addedperliterofdistilledwater.InthesolutionBwasadded35.6g ofsodiumdibasicphosphate(NA2PO4·7H2O)perliterofdistilled
water.After,16.5mlofthesolutionAwasaddedto46mlofthe solutionBand37.5mlofdistilledwaterwasadded,fillingwith 100mlwiththephosphatetamponwithpH=5.8(Silvaetal.,2011).
Bonemedullagatheringandpreparationofthemicroscopicslides
Aftertheanimalsweresacrificedbyguillotinedecapitation,the femurswerewithdrawn,cleanedandthetwoendsremovedwitha surgicalscissor.Thebonemarrowwasextractedwithahistological needledirectlyupontheslidewith10loffetalbovineserum,and withacurvedhistologicalneedlethemarrowwashomogenized withserum,whereinasmearofeachfemurwasmade,andtwo slidesperanimalwereprepared.
Theslidesweredriedinanincubatorat37◦C,coloredwitha
100
80
60
40
20 5.9
M
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
6.1 5.4
mg/ml
M
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
mg/ml
Damage inde
x
9.4 18.0
29.2 57.2***
***
*
***
### ###
24.6
3.2 5.4 4.1 6.7
12.6 20.9 34.6***
***
*** ***
### ###
18.6
3.6 5.1 4.9 9.2
12.5 23.1 41.4***
***
*
***
### ###
22.9
6.4 5.9
11.6 8.4
20.6 31.9 70.4***
***
**
***
### ###
30.7
6.1 6.0
10.5 6.9
19.3 30.6 63.8***
***
***
***
### ###
27.7
3.4 5.2
38.0
4.5 8.0
***
***
*** ***
### ###
12.6
22.0 22.7 0
100
80
60
40
20
F
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
mg/ml
Damage inde
x
0
100
80
60
40
20
Damage inde
x
0
100
80
60
40
20
F
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
mg/ml
T
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
mg/ml
T
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
mg/ml
0 100
80
60
40
20
Damage frequency
Damage frequency
100
80
60
40
20
0
Damage frequency
0
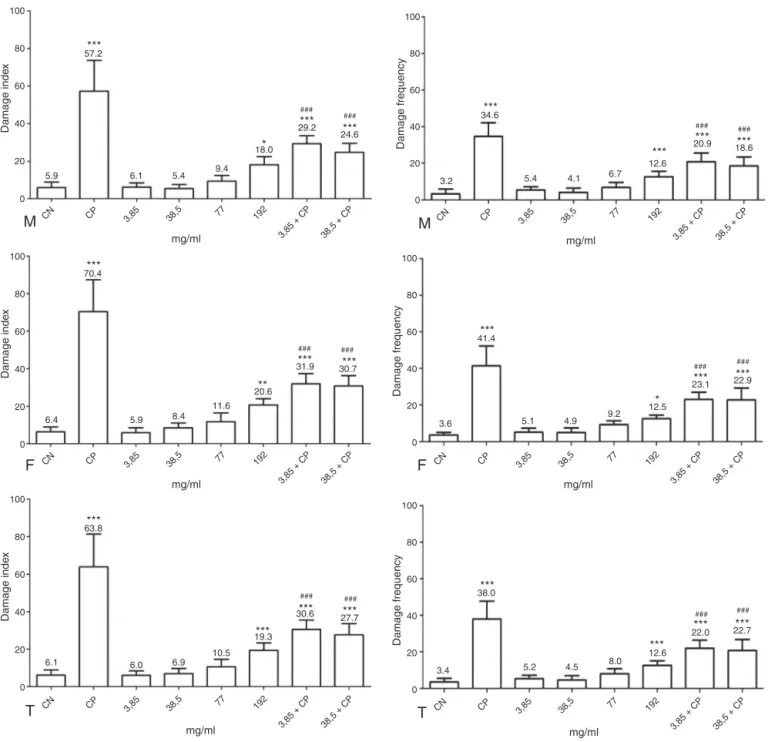
Fig.1.Genotoxicandantigenotoxicacuteeffectsevaluatedindamageindexanddamagefrequencybythecomettestinperipheralbloodofmice,whichunderwentthe treatmentwithaqueousextractofM.guyanensis.M:Males;F:Females;T:Total–MaleandFemale.Statisticallysignificantforgenotoxicity*(p<0.05),**(p<0.01)and ***(p<0.001)whencomparedtoCN.Statisticallysignificantforantigenotoxicity#(p<0.05),##(p<0.01)and###(p<0.001)whencomparedtoCP.
differentiatelightbluepolychromaticerythrocytes(PCE)andred normochromaticerythrocytes(NCE)(Ribeiroetal.,2003).
Analysesofslides
Thezig-zagmodelwasusedtodeterminesimultaneouslythe amount of micronuclei of each 1000 PCEand the relationship betweenPCEandNCEin1000erythroidcellsperslide.
Thisrelationship was established to avoid determination of false-negatives,demonstratingwhethertherewascytotoxicityor cellulardepression(Shahrimetal.,2006).Afterwards,thecountof onlymicronucleiinPCEwascontinueduntilthecountof1000cells. ThecountofmicronucleioccurredonlyinPCE,duetoitsindication fororganismswithacuteexpositionanalysis(Villelaetal.,2003), thesameasusedinthepresentstudy.
Statisticalanalysis
The variance analysis was done by the ANOVA test, using Turkey’stest asacounterproof,affectingthecomparisonofthe meansofdifferenttreatmentswiththecontrol groups.For this purpose,thesoftwareGraphpadPrism5.0wasused.
Results
Cometassay
Intheanalysisoftheantigenotoxicpotential,3.85mg/mland 38.5mg/ml concentrationsdemonstrated significantdecreasein boththedamageindexandthedamagefrequencyrelativetothe CPgroup.
CorrelationPCE/NCE
ThedatacorrespondingtothecorrelationPCE/NCEinfluenced bytheaqueousextractofM.guyanensisareorganizedinTable1. ThecorrelationPCE/NCEofthe3.85mg/mland18.5mg/ml concen-trationsdidnotpresentsignificantdifferencerelativetoCN.This resultwasnotobservedinthe77mg/mland192mg/ml concentra-tions,thatdecreasedthecorrelation,demonstratingtheoccurrence of cytotoxicity(Shahrim et al.,2006; Silva et al.,2011), that is normallyconfirmedduetothedose-responseeffect(Krishnaand Hyashi,2000)andthelikelyexplanationisthatthefrequencyofcell depression(Nesslanyetal.,2004)caninducetoapoptosis(Ouanes etal.,2003),duetoitbeingsubjectedtobalanceregulationbetween theactivationandsuppressionofcelldeathincertainsituations (KaufmannandHengartner,2001).
The 3.85mg/ml and 38.5mg/ml concentrations that did not present cytotoxity, also demonstrated anticytotoxic action, increasing the PCE/NCE correlation, significantly reversing the damagecausedbycyclophosphamide.
Micronucleiinbonemarrow
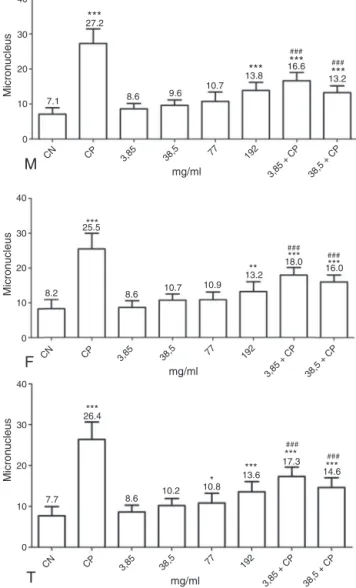
Themeanofmicronucleiforeach1000cellsofmicebone mar-rowsubjectedtotreatmentwithdifferentconcentrationsofthe aqueousextractofM.guyanensis,canbevisualizedinFig.2.
The3.85mg/ml,38.5mg/mland77mg/mlconcentrationsdid notpresentmutageniceffectsinmaleandfemalemice,however, whenthetotalmean wasassessed,the77mg/mlconcentration showedtobemutagenicrelativetoCN,whichwasalsoobserved inthe192mg/mlconcentrationinmale,femaleandtotalmean.
The3.85mg/mland38.5mg/mlconcentrationsdemonstrated antigenotoxic effects again,acting asantimutagenic, decreasing significantlythenumberofmicronucleirelativetoCP.
Discussion
Thegenotoxicsafetyandantigenotoxicactionof3.85mg/mland 38.5mg/mlconcentrationsoftheaqueousextractofM.guyanensis
wasalreadyseeninapreviousstudyusingAlliumcepa,wherethese sameconcentrations demonstrated anticytotoxic and antimuta-genic actionrelative tomeristemsgermination,mitotic indexes andmicronuclei formation(Meneguettiet al.,2014),beingalso inagreementwithHurtado(2013),inwhichfractionsofM. guya-nensisshowedanti-proliferativeactivitywithinawideperiodof time,notpresentingclastogenicoraneugenicactions,wherethis latterwasalsoobservedinastudycarriedoutbyMendesetal. (2012),demonstratingthattheethanolicextractofM.rigidadoes notinducechromosomalabnormalities.
The antimutagenic action was also observed by Salmonela/
Microsometest(AmesTest)inwhichthehydroalcoholicextractof thebarkofM.krukoviipresentedexhibitsinhibitoryactivityagainst theT98andT100lines(Brunietal.,2006).
Previousstudieshavedemonstratedthatthequinone-methide triterpenesareoneofthemaincausesoftheantigenotoxicaction foundinsomespeciesofthegenusMaytenus,actinginvitroagainst tumorcellsandagainstexperimentaltumors,inwhichthespecies
Maytenusilicifoliapresentedinhibitoryactivitiesagainstdifferent sarcomasandneoplasticcells(Santanaetal.,1971;Santos-Oliveira etal.,2009),strengtheningtheresultsfoundinthe3.85mg/mland 38.5mg/mlconcentrationsoftheaqueousextractofM.guyanensis, thatprobablyoccurredduetothepresenceofquinone-methide
40
30
20
10 7.1
8.2 8.6
25.5
10.7 10.9 13.2
18.0 16.0
M
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP
8.6 9.6
mg/ml
F
CN CP 3,85 38,5 77 1923,85 + CP 38,5 + CP mg/ml
T
CN CP 3,85 38,577 192
3,85 + CP 38,5 + CP mg/ml
Micron
ucleus
10.7
13.8 16.6 27.2***
***
*** ***
###
*** *** ***
26.4
8.6 10.2 10.8
13.6 17.3
7.7
14.6
***
*** ***
* **
### ###
###
*** ### ###
13.2
0
40
30
20
10
Micron
ucleus
0
40
30
20
10
Micron
ucleus
0
Fig.2.Mutageniceffectevaluatedbythemeanofthenumberofmicronucleiin micebonemarrowcells,subjectedtotreatmentwithaqueousextractofM. guya-nensis.M:Males;F:Females;T:Total–MaleandFemale.Statisticallysignificantfor mutagenicity*(p<0.05),**(p<0.01)and***(p<0.001)whencomparedtoCN. Sta-tisticallysignificantforantigenotoxicity#(p<0.05),##(p<0.01)and###(p<0.001)
whencomparedtoCP.
triterpenes identified previously inthis species (Facundo etal., 2015).Similarresultswereobservedwiththetingenone-quinone methide(maitenine)invitroteststhatdemonstratedinhibitionof Leuk-P138, CA9KBandV79neoplasticcelllines(Kupchanand Karim,1976).
Studiesperformedinhumanswithothertriterpenesisolated fromMaytenusssp.demonstratedthedecreaseofwoundsonthe baseofthetongueandpharynxbyapproximately50%,causedby epidermoidcarcinomasand40%ofthelymphoepitheliomawith invasiontotheorbit,besidespositiveresultsin2ofthe7patients withuterineepidermoidcarcinoma(Santanaetal.,1971).
Inanotherclinicalseries,thetriterpenemaitenine“tingenone analog” (Moritaet al., 2008; Gullo et al.,2012; Facundo et al., 2015),alsoisolatedfromthegenusMaytenus,potentiatedother anti-cancerogenicsubstancesofnaturalsourcesinelevenpatients withadvanced basal-cellcarcinoma,inwhichallofthepatients presentedclinicalrecovery,withreductionofwoundsizebymore than50%(Meloetal.,1974;Corsinoetal.,2000).
Table1
Mean±standarddeviationofthenumberofPolychromaticErythrocytes(PCE),NormochromaticErythrocytes(NCE)andofthePCE/NCEratioinmicebonemarrowcells, whichunderwentthetreatmentwithaqueousextractofMaytenusguyanensis.
Treatment(mg/ml) Gender N (PCE) (NCE) Correlation(PCE/NCE)
CN Male 4 2571±8.2 2429±8.2 1.06±0.07
Female 4 2616±5.8 2384±5.8 1.10±0.05
Total 8 2594±7.5 2406±7.5 1.08±0.06
CP Male 4 1835±13.6 3165±13.6 0.58±0.07***M
Female 4 1737±4.7 3262±4.7 0.53±0.02***F
Total 8 1786±11.3 3214±11.3 0.56±0.06***T
3.85 Male 4 2622±8.1 2377±8.1 1.10±0.07
Female 4 2581±6.7 2419±6.7 1.07±0.06
Total 8 2602±7.8 2398±7.8 1.08±0.07
38.5 Male 4 2526±5.8 2474±5.8 1.02±0.05
Female 4 2541±7.3 2459±7.3 1.03±0.06
Total 8 2534±6.6 2466±6.6 1.03±0.05
77 Male 4 2362±13.2 2637±13.2 0.90±0.09**M
Female 4 2381±11.3 2619±11.3 0.91±0.08***F
Total 8 2372±12.3 2628±12.3 0.90±0.09***T
192 Male 4 2161±11.2 2839±11.2 0.76±0.07***M
Female 4 2165±9.4 2835±9.4 0.77±0.06***F
Total 8 2163±10.4 2837±10.4 0.76±0.07***T
3.85+CP Male 4 2155±6.6 2845±6.6 0.76±0.04***M###M
Female 4 2194±7.4 2806±7.4 0.78±0.05***F###F
Total 8 2174±7.3 2826±7.3 0.77±0.04***T###T
38.5+CP Male 4 2212±8.6 2787±8.6 0.79±0.05***M###M
Female 4 2221±7.9 2779±7.9 0.80±0.05***F###F
Total 8 2217±8.2 2783±8.2 0.80±0.05***T###T
Statisticallysignificantforgenotoxicity:*M(p<0.05),**M(p<0.01)and***M(p<0.001)whencomparedtomaleCN.*F(p<0.05),**F(p<0.01)and***F(p<0.001)when com-paredtofemaleCN.*T(p<0.05),**T(p<0.01)and***T(p<0.001)whencomparedtototalCN.Statisticallysignificantforantigenotoxicity:#M(p<0.05),##M(p<0.01)and ###M(p<0.001)whencomparedtomaleCP.#F(p<0.05),##F(p<0.01)and###F(p<0.001)whencomparedtofemaleCP.#T(p<0.05),##T(p<0.01)and###T(p<0.001)when comparedtototalCP.
wayonthewoundofthedigestivetract,inhibitingthedamage ofmucosacellsbyfreeradicalsgenerated bytheverydigestive process.Thiseffectisalsorelatedtoananti-mutagenicaction, pro-tectingagainstgenotoxicagents thatmayinducethemalignant transformationoftheintestinalmucosacells(Kruletal.,2001).
TheantioxidantactionwasalreadyreportedforthespeciesM. guyanensis(Macarietal.,2006),forM.procumbens(Momtazetal., 2013)andforilicifolia(Macarietal.,2006;Negrietal.,2009)in whichthelipid peroxidationisinhibited (Santos-Oliveiraet al., 2009)andshowschelantactivityofheavymetals,besidesacting ondifferentfreeradicals(Hoetal.,1992;Meloetal.,2001).
In this study,theconcentrationof 77mg/mlof theaqueous extractofM.guyanensisdidnotpresentgenotoxicactionforthe indexandfrequencyofdamageevaluatedbythecometassay,or fortheformationofmicronucleionthebonemarrowofmaleand femalemice.Ontheotherhand,itpresentedgenotoxiceffectsin thetotalmeanofthemicronucleitestandalsointheratioPCE/NCE. Thenegativeresultsfortheconcentrationof77mg/mlinthecomet assaymighthaveoccurredduetoanalysesofonlytheacuteeffects, inwhichfurtherstudiesaresuggestedforabettercomprehension ofthechroniceffectscausedbytheingestionofinfusionsof aque-ousextractsofM.guyanensis;accordingtoBodeandDong(2014)
severalnaturalcompoundsfoundinvariousplantsconsumedby traditionalpopulationarepotentialcarcinogensortumor promo-tersanditsuseforlongperiodsoftimeshouldbeavoided.
Inthe192mg/mlconcentrationthedatademonstrated geno-toxiceffectsinallofthetestsperformed,beinginagreementwith astudydonebyMeneguettietal.(2014),inwhichthe77mg/ml and192mg/mlconcentrationspresentedcytotoxicandmutagenic actions.
It is important to highlight that 77mg/ml and 192mg/ml concentrationsare,respectively,twenty-andfifty-foldmore con-centratedthantheconcentrationnormallyusedbythepopulation: 3.85mg/ml(Camparotoetal.,2002),bringingacertaintranquility relativetotheuseofthisspeciesintheethnopharmacology.
This genotoxicity found in high concentrations was not evidencedinotherstudiesusingplantsofthesamegenus,inwhich
thechronictoxicologyoftheinfusionofM.ilicifoliawastestedin ratsandmicewithdosesrangingfrom20to40foldhigherthan thatcommonlyusedbyhumans,notcausingchangesinweight, behavior,temperatureandinthebiochemicalparametersofthe serumandhematologicalparameters(CarliniandFrochtengarten, 1988).
Inothertoxicologicstudies,thistimeacute,performedinmice andratsbyinfusionsandlyophilizedextractofM.ilicifolia,andtoxic effectswerenotevidencedindosesuntil1600-foldhigherthanthe dosesusedbyhumans(Santos-Oliveiraetal.,2009).
Inhumanbeings,astudyofclinicaltoxicology(PhaseItrial), administeredduring14days,thedoubleofthedosageofM. ilici-foliausedinethnopharmacology,didnotobserveabnormalresults thatcouldbeattributedtotheplantuse.Onlysymptomslikedry mouth,nausea,andgastralgiainafewvolunteerswererecorded, withrecoveryduringthestudy,demonstratingthusthatM. ilicifo-liaisnottoxicforhumanswhenusedinthesamewayaspopular medicine(CarliniandFrochtengarten,1988).
It was found that aqueous extract of M. guyanensis in con-centrationsuptotenfoldhigherthan theconcentrationusedin ethnopharmacologydoesnotpresentgenotoxiceffectsand, more-over,ithasantigenotoxicactionsinmicetreatedacutely.Further studiesofbioaccumulationandchroniceffectsofthisspeciesare suggested in order to improve the understandingof its action mechanisms,ensuringtheefficacyandsafetyofitsutilizationand developmentofphytotherapicsanddrugs.
Authors’contribution
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Andrade,S.F.,Cardoso,L.G.,Bastos,J.K.,2007.Anti-inflammatoryandantinociceptive activitiesofextract,fractionsandpopulnoicacidfrombarkwoodof
Austrop-lenckiapopulnea.J.Ethnopharmacol.109,464–471.
Bode,A.M.,Dong,Z.,2014.Toxicphytochemicalsandtheirpotentialrisksforhuman cancer.CancerPrev.Res.8,1–8.
Borrás,M.R.L.,2003.PlantasdaAmazônia:medicinaisoumágicas-Plantas com-ercializadasnoMercadoMunicipalAdolphoLisboa.EditoraValer/Governodo EstadodoAmazonas,Manaus.
Bruni,R.,Rossi,D.,Muzzoli,M.,Romagnoli,C.,Paganetto,G.,Besco,E.,etal.,2006.
Antimutagenic,antioxidantandantimicrobialpropertiesofMaytenuskrukovii bark.Fitoterapia77,538–545.
Camparoto,M.L.,Teixeira,R.O.,Mantovani,M.S.,Vicentini,V.E.P.,2002.Effectsof MaytenusilicifoliaMart.andBauhiniacandicansBenthinfusionsononionroot-tip andratbone-marrowcells.Genet.Mol.Biol.25,85–89.
Carlini,E.A.,Frochtengarten,M.L.,BrasíliaDistritoFederal1988.Toxicologiaclínica (FaseI)daespinheira-santa(Maytenusilicifolia).
Cechinel-Filho,V.,Rosendo,A.Y.,1998.Estratégiasparaaobtenc¸ãode compos-tosfarmacologicamenteativosapartirdeplantasmedicinais.Conceitossobre modificac¸ãoestruturalparaotimizac¸ãodaatividade.Quím.Nova21,99–105.
Chavez,H.,Valdivia,E.,Estevez-Braun,A.,Ravelo,A.G.,1998.Structureofnew bioactive triterpenes related to 22-B-hidroxy-tingenone. Tetrahedron 54, 13579–13590.
Corsino,J.,DeCarvalho,P.R.,Kato,M.J.,Latorre,L.R.,Oliveira,O.M.M.F.,Araujo,A.R., etal.,2000.Biosynthesisoffriedelaneandquinonemethidetriterpenoidsis com-partmentalizedinMaytenusaquifoliumandSalaciacampestris.Phytochemistry 55,741–748.
Duarte,L.P.,VieiraFilho,S.A.,Silva, G.D.F.,Sousa,J.R.,Pinto, A.S.,2002. Anti-trypanosomalactivityofpentacyclictriterpenesisolatedfromAustroplenckia populnea(Celastraceae).Rev.Inst.Med.Trop.SãoPaulo44,109–112.
Facundo,V.A.,Meneguetti,D.U.O.,Militão,J.S.L.T.,Lima,R.A.,Hurtado,F.B.,Casseb, A.A.,etal.,2015.ChemicalconstituentsfromMaytenusguianensisKlotzschex Reissek(Celastraceae)AmazonRainforest.Biochem.Syst.Ecol.58,270–273.
Fão,F.,Zan,R.A.,Brondani,F.M.M.,Ramos,L.J.,Meneguetti,D.U.O.,2012.Análisedo potencialmutagênicodaseivadacascadeCrotonlechleri(Müll.Arg),noestado deRondônia,Amazôniaocidental.SaBios:Rev.SaúdeBiol.7,91–98.
Fonseca,A.P.N.D.,Silva,G.D.F.,Carvalho,J.J.,Salazar,G.D.C.M.,Silva,R.P.,Jorge,R.M., etal.,2007.EstudofitoquímicododecoctodasfolhasdeMaytenustruncata Reissekeavaliac¸ãodasatividadesantinociceptiva,antiedematogênicae antiul-cerogênicadeextratosdodecocto.Quim.Nova30,842–847.
Giulietti,A.M.,Harley,R.M.,Queiroz,L.P.,Wanderley,M.G.L.,Berg,C.V.D.,2005.
Biodiversidadeeconservac¸ãodasplantasnoBrasil.Rev.Megadiver.1,54–61.
Gonzalez,A.G.,Alvarenga,N.L.,Estevez-Braun,A.,Ravelo,A.G.,Bazzocchi,I.L., Mou-jir,L.,1996.Structureandabsoluteconfigurationoftriterpenedimersfrom
Maytenusscutioides.Tetrahedron52,9597–9608.
Gullo,F.P.,Sardi,J.C.O.,Santos,V.A.F.F.M.,Sangalli-Leite,F.,Pitangui,N.S.,Rossi, S.A.,etal.,2012.Antifungalactivityofmayteninandpristimerin.Evid.Based Complement.Altern.Med.340787,1–6.
Ho,C.T.,Chen,Q.,Shi,H.,1992.Antioxidativeeffectsofpolyphenolextractprepared fromvariousChineseherbs.Prev.Med.2,520–525.
Hurtado,F.B.,(Tese)DoutoradoemBiologiaExperimental2013.Contribuic¸ãoao estudofitoquímicoebiológicodaentrecascadaespécieMaytenusguyanensis klotzshexReissek.Fundac¸ãoUniversidadeFederaldeRondônia(UNIR),Porto Velho,Rondônia.
Kaufmann,S.H.,Hengartner,M.O.,2001.Programmedcelldeath:aliveandwellin thenewmillennium.TrendsCellBiol.11,526–534.
Krishna,G.,Hyashi,M.,2000.Invivorodentmicronucleusassay:protocol,conduct anddatainterpretation.Mutat.Res.455,155–166.
Krul,C.,Luiten-Schuite,A.,Tenfelde,A.,Ommem,B.,Verhagen,H.,Havenaar,R.,2001.
Antimutagenicactivityofgreenteaandblackteaextractsstudiedinadynamic invitrogastrointestinalmodel.Mutat.Res.474,71–85.
Kupchan,S.M.,Karim,A.,1976.Tumorinhibitors.114.Aloeemodin:antilukemic principleisolatedfromRhamnusfrangulaL.Lloydia39,223–224.
Macari,P.A.T.,Portela,C.N., Celani,F.B.,Pohlit,A.M., 2004.Isolamento deum flavonóidedacascadeMaytenusguyanensis.XXVIReuniãoAnualsobreEvoluc¸ão, SistemáticaeEcologiaMicromoleculares.InstitutodeQuímica,Universidade FederalFluminense.
Macari,P.A.T.,Portela,C.N.,Pohlit,A.M.,2006.Antioxidant,cytotoxicand UVB-absorbingactivityofMaytenusguyanensisKlotzch(Celastraceae)barkextracts. ActaAmaz.36,513–518.
Magalhães,E.A.,Silva,J.G.J.,De-Campos,T.A.,Silva,L.P.,Silva,R.M.G.,2010.Avaliac¸ão dopotencialgenotóxicodoextratobrutodePyrostegiavenusta(KerGawl.)Miers, Bignoneaceae,emmedulaósseadecamundongos.Rev.Bras.Farmacogn.20, 65–69.
Melo,A.M.,D’Albuquerque,I.L.,Lacet,Y.,1974.Primeirasobservac¸õesdousotópico deprimina,plumbaginaemaiteninaempacientescomcâncerdepele.Rev.Inst. Antibiot.14,9–16.
Melo,S.F.,Soares,S.F.,Costa,R.F.,Da-Silva,C.R.,Oliveira,M.B.N.,Bezerra,R.J.A.C., etal.,2001.EffectoftheCymbopogoncitratus,Maytenusilicifoliaand
Baccha-risgenistelloidesextractsagainstthestannouschlorideoxidativedamagein
Escherichiacoli.Mutat.Res.496,33–38.
Mena-Rejón,G.J.,Pérez-Espadas,A.R.,Moo-Puc,R.E.,Cedillo-Rivera,R.,Bazzocchi, I.L.,Jiménez-Diaz,I.A.,Quijano,L.,2007.Antigiardialactivityoftriterpenoids fromrootbarkofHippocrateaexcelsa.J.Nat.Prod.70,863–865.
Mendes,S.S.,Andrade,J.A.,Xavier,M.A.,Secundo,J.J.A.,Pantaleão,S.M.,Estevam, C.S.,etal.,2012.GenotoxicitytestofMaytenusrigidaandAristolochiabirostris intheradicularmeristemoftheonion,Alliumcepa.Rev.Bras.Farmacogn.22, 76–81.
Meneguetti,D.U.O.,Lima,R.A.,Silva,J.B.,Silva,R.P.,Pagotto,R.C.,Facundo,V.A., 2014.AnálisecitotóxicaemutagênicadoextratoaquosodeMaytenus guyanen-sisKlotzschExReissek(Celastraceae)chichuá(xixuá)amazônico.Ciên.Nat.36, 301–309.
Momtaz,S.,Hussein,A.A.,Ostad,S.N.,Abdollahi,M.,Lall,N.,2013.Growth inhibi-tionandinductionofapoptosisinhumancancerousHeLacellsbyMaytenus
procumbens.FoodChem.Toxicol.51,38–45.
Morita,H.,Hirasawa,Y.,Muto,A.,Yoshida,T.,Sekita,S.,Shirota,O.,2008.Antimitotic quinoidtriterpenesfromMaytenuschuchuhuasca.Bioorg.Med.Chem.Lett.18, 1050–1052.
Negri,M.L.S.,Possamai,J.C.,Nakashima,T.,2009.Atividadeantioxidantedas fol-hasdeespinheira-santa-MaytenusilicifoliaMart.exReiss.,secasemdiferentes temperaturas.Rev.Bras.Farmacogn.19,553–556.
Nesslany,F.,Brugier,S.,Mouries,M.A.,Curieux,F.,Marzin,D.,2004.Invitroandinvivo chromosomalaberrationsinducedbymegazol.Mutat.Res.560,147–158.
Ouanes,Z.,Abid,S.,Ayed,I.,Anane,M.T.,Creppy,E.E.,Bacha,H.,2003.Inductionof micronucleibyZearalenoneinVeromonkeykidneycellsandinbonemarrow cellsofmice:protectiveeffectofVitaminE.Mutat.Res.538,63–70.
Prata,R.R.,Dissertac¸ão(mestrado)2007.Aspectosanatômicose etnofarmacológi-cosdocauleeraizdeMaytenusguyanensisKlotzschexReissek(Celastraceae). INPA/UFAM,Manaus.
Prata,R.R.,Mendonca,M.S.,2009.Estudoanatômicodoxilemasecundáriodaraize docauledeMaytenusguyanensisKlotzschexReissek(Celastraceae).ActaAmaz. 39,261–266.
Revilla,J.,2002.Apontamentosparaacosméticaamazônica.SEBRAE–AM/INPA, Manaus.
Revilla,J.,2001.PlantasdaAmazônia–oportunidadeseconômicasesustentáveis. SEBRAE–AM/INPA,Manaus.
Ribeiro,L.R.,Salvadori,D.M.F.,Marques,E.K.,2003.MutagêneseAmbiental.EdUlbra. Canoas.
Santana,C.F.,Asfora,J.J.,Cotias,C.T.,1971.Primeirasobservac¸õessobreoemprego damaiteninaempacientescancerosos.Rev.Inst.Antibiot.11,37–49.
Santos-Oliveira,R.,Coulaud-Cunha,S.,Colac¸o,W.,2009.RevisãodaMaytenus ili-cifoliaMart.exReissek,Celastraceae.Contribuic¸ãoaoestudodaspropriedades farmacológicas.Rev.Bras.Farmacogn.19,650–659.
Shahrim,Z.,Baharuddin,P.J.N.M.,Yahya,A.,muhammad,H.,Bakar,R.A.,Ismail,Z., 2006.TheinvivorodentmicronucleusassayofKacipFatimah(Labisiapumila) extract.Trop.Biomed.23,214–219.
Silva,F.C.,Barros,M.A.B.,Viana,R.R.,Romão,N.F.,Oliveira,M.S.,Meneguetti,D.U.O., 2011.Avaliac¸ãodemutagêneseprovocadaporsulfatodeferroatravésdoteste micronúcleoemcélulasdamedulaósseadecamundongos.Rev.Cie.Fac.Edu. Mei.Amb.2,13–21.
Singh,N.P.,Mccoy,M.T.,Tice,R.R.,Scheneider,A.,1988.Asimpletechniquefor quan-tificationoflowlevelsofDNAdamageinindividualcells.Exp.CellRes.175, 184–191.
Sousa,J.R.,Pinheiro,J.A.,Ribeiro,E.F.,Souza,E.,Maia,J.G.S.,1986.Asesquiterpene evoninoatealkaloidfromMaytenusguianensis.Phytochemistry25,1776–1778.
Tice,R.R.,Agurell,E.,Anerson,B.,Burlinson,B.,Hartmann,A.,Kobayashi,H.,etal., 2000.Singlecellgel/cometassay:guidelinesforinvitroandinvivogenetic toxicologytesting.Environ.Mol.Mutagen.35,206–210.