rev bras hematol hemoter. 2017;39(3):274–277
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Case
Report
Flow
cytometry
to
identify
bone-marrow
relapse
in
blastic
plasmacytoid
dendritic
cell
neoplasm:
a
case
report
Mariela
Granero
Farias
a,∗,
Fabiane
Spagnol
Pedrazzani
a,
Luis
Carlos
Zanandrea
Contin
a,
Ana
Paula
Alegretti
a,
Lisandra
Della
Costa
Rigoni
a,
Liane
Esteves
Daudt
a,baHospitaldeClínicasdePortoAlegre(HCPA),PortoAlegre,RS,Brazil
bUniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29November2016 Accepted17April2017 Availableonline16May2017
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rareacuteleukemiasubtypecharacterizedbyclonal expan-sion of dendritic-lineage cells.1 These cells are identified immunophenotypically by weak CD45 expression and co-expressionoftheCD4andCD56antigensintheabsenceof otherlineage-specificmarkers.2,3Previouslyknownasblastic naturalkiller(NK)-celllymphomaorCD4+/CD56+ hematoder-micneoplasm,itiscurrentlyclassifiedbytheWorldHealth Organization (WHO) as a distinct entity, under the acute myeloid leukemia (AML) and related precursor neoplasm group.4
Few studies have assessed the incidence of BPDCN in the general population. The limited existing data suggest an extremely low overall incidence, representing 0.44% of all hematological malignancies3,5 and 0.7% of cutaneous
∗ Correspondingauthorat:Servic¸odePatologiaClínica,UnidadedeDiagnósticoPersonalizado,HospitaldeClínicasdePortoAlegre,Rua
RamiroBarcelos2350,90035-903PortoAlegre,RS,Brazil. E-mailaddress:mgfarias@hcpa.edu.br(M.G.Farias).
lymphomas.6 BPDCN mostlyaffects men, witha 3:1 male-to-female ratio, and usually occurs in patients aged60–70 years7,8 althoughcaseshavebeen reportedinchildrenand youngadults.9
BPDCNisparticularlydifficulttodiagnosebecauseofits clinicalandbiologicalheterogeneitythatoverlapswithother clinical malignancies, and frequent lack of chromosomal abnormalities.5,10Onlaboratorytesting,thecellmorphology can be misleading, with a pseudolymphocytic appearance, abundant cytoplasm with a low nuclear-cytoplasmic ratio, strongbasophilia,andnogranulation.Microvacuolesareoften visible,possiblyrepresentingglycogencompoundsinclinedto forma“pearlnecklace”onthenuclearmembrane,aswellas cytoplasmicextensionssimilartopseudopodia.11,12 Thecell lineagemustbeevaluatedbyflowcytometry immunopheno-typing,whichidentifiesdendriticcellsintheirthreestagesof differentiation.10
http://dx.doi.org/10.1016/j.bjhh.2017.04.005
revbrashematolhemoter.2017;39(3):274–277
275
Arecentstudyshowedthat,accordingtotheirCD34and CD117 expressions, dendritic cells can be categorized into threematurationalstages:(1) inBPDCN, CD34isexpressed insomeoftheimmatureblasts;(2)intermediatecellsare par-tiallyCD117-positivewhentheexpressionofCD34isabsent inblast cells;and (3) maturecells donot expressCD34or CD117.These stagesofmaturation explainthe variationin theclinicalpresentationofBPDCN,aswellasitslaboratory characteristics.10
The most differentiated stage is characterized by cells withweakexpressionof CD45,no expressionofCD34, co-expression of CD4/CD56, presence of HLADR/CD123, and absenceofspecificmarkersofmyeloid, BandT lymphoid, andNKcells.13,14Co-expressionofCD2,cytoplasmicCD3,CD5, CD7,CD33,nTdT,CD79a,and/orCD117canbecommon.13,15,16 CD123isthe␣chainoftheinterleukin-3receptor,andisa
sensitiveandspecificmarkerofplasmacytoiddendriticcells (pDC).However,althoughCD123iscurrentlythemost impor-tantmarkerinthe diagnosis ofBPDCN,it isnotunique to the plasmacytoiddendritic cell lineage. CD4and CD56are expressedinotherhematologicalmalignancies,andarenot enoughtoestablishdiagnosis.17,18
Clinically,thisdiseasemanifestswithisolatedcutaneous involvementinthe formofsingleormultiplelesions,and, despiteinitialindolentbehavior,itischaracterizedby aggres-sive,rapidsystemicdissemination.19,20 Manypatients have cytopenia, particularly thrombocytopenia, as a result of extremely variable rates ofdendritic-cell infiltration ofthe bonemarrow.Althoughthereisaninitialresponsetosystemic chemotherapy,thediseaserelapsesasamatterofcourse,and survivalrangesfrom12to14months.19
Currently,thereisnoconsensusastotheidealtreatment for BPDCN.1,21 Retrospective studies have evaluated differ-enttreatmentstrategies,includingmulti-agentchemotherapy according to established protocols for acute lymphoblastic leukemia(ALL)oracutemyeloidleukemia(AML),whileafew caseshavebeensubmittedtoallogeneichematopoieticstem celltransplantation (HSCT).1,21 Theneoplastic cellsare ini-tially sensitivetochemotherapyagentswhichare typically activeagainstlymphoblasts,suchassteroids,vincristine,and asparaginase.Therefore,itisrecommendedthatALLprotocols befollowed,withsubsequentHSCTwhenappropriate.1,20Itis importanttohighlightthattheeffectivenessofthisprocedure stillneedstobeinvestigatedbyclinicalstudies,andtherole oftransplantationhasyettobedefined.1TheMartín-Martín etal.studydemonstratedthatthisstrategy(ALLtherapyplus HSCT)wasassociatedwiththebestprognosis.10
Within this context, the aim of this paper is to report acaseofthis rare,difficult-to-diagnose neoplasminwhich flowcytometryimmunophenotypingcontributedtothe cor-rectidentificationofleukemiccelllineage.
Case
report
A 51-year-old previously healthy female presented with a 3-month history of progressively disseminating cutaneous lesions,withnodefineddiagnosis andnoclinical response to topical treatments. Immunohistochemistry skin biopsy was performedand revealed blast-appearing cells,positive
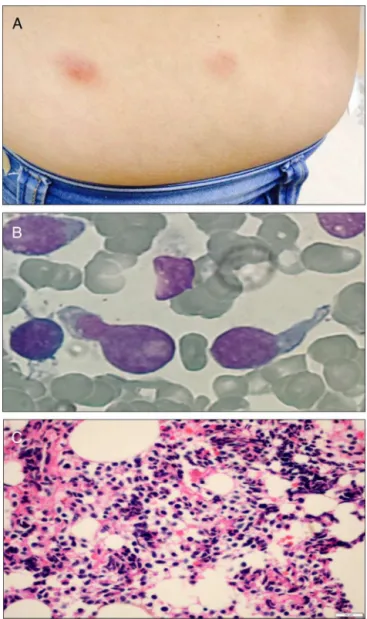
Figure1–Physicalandmorphologicfeaturesofblastic plasmacytoiddendriticcellneoplasm.Cutaneouslesions onthepatient’sabdomen(A);morphologyofleukemic blastsinthebone-marrowaspiratesmear(B);
anatomopathologicalstudyshowinginfiltrationbyblast cells(C).
276
revbrashematolhemoter.2017;39(3):274–2770
1 1
1 1
1E1 1E1
1E2 1E2
1E3 1E3
1E4 1E4
1 1E1 1E2 1E3 1E4
1E1 1E2 1E3 1E4 1E1 1E2 1E3 1E4 1 1E1 1E2 1E3 1E4
SSC-A
Exp-SSC Low
SSC-A
Exp-SSC Low
SSC-A
Exp-SSC Low
200 900
500
300
200
100
0
0 0
100 100
200 200
300 300
500 500
900 900
400
FSC-A linear
CD 123 APC-A:APC-A LOGA CD 123 APC-A:APC-A LOG CD56 PE-A:PE-A LOGARITHM
CD38 APC-A:APC-A
LOGARITHM
CD4 PE-A:PE-A
LOGARITHM
HLADR FITC-A:FITC-A
LOG
CD45 APC-A:APC-A LOGA CD34 PerCP-A:PerCP-A LOG
600 800 1000
1 1E1 1E2 1E3 1E4 1 1E1 1E2 1E3 1E4
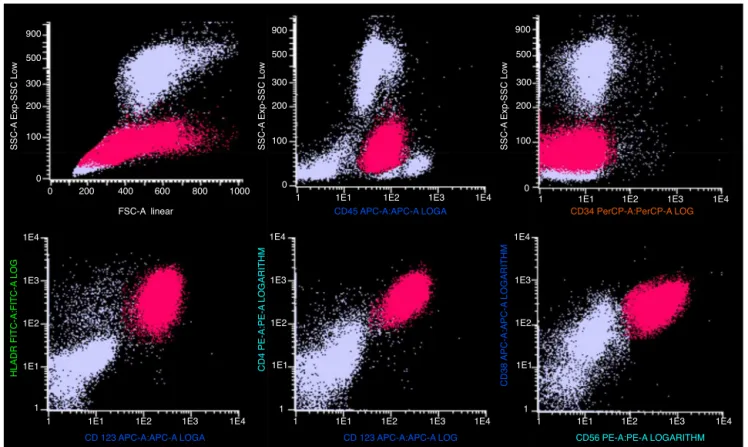
Figure2–Immunophenotypingbyflowcytometryofabone-marrowaspiratesample,showingthemainmarkersthat identifyandcharacterizeplasmacytoiddendriticblastcells.
strongandhomogeneousCD123andweakCD45expression. TherewasnoexpressionofCD34ormarkersoflymphoidB (cCD79a,CD19),lymphoidT(CD7,CD3,CD5,CD2),NK(CD11b, CD16),ormyeloidcells(cMPO,CD13,CD15,CD64,CD65) sug-gesting BPDCL. The patient began a HyperCVAD regimen (cyclophosphamide,vincristine,doxorubicinand dexametha-sone) andunderwent allogeneic HSCT.Thepost-transplant course was complicated by septic shock and hemorrhagic alveolitis,andthepatientdied18daysafterHSCT.
Discussion
BPDCNisrare,aggressive,anddifficulttodiagnose. Knowl-edgeaboutthisconditionhasbeenimprovinginrecentyears becauseoftheincreasingnumberofreportedcasesanda bet-tercomprehensionofitsbiologicalcharacteristics.22Giventhe clinicalandbiologicalheterogeneityofthisneoplasm,correct identificationandclassificationrequiretheexperienceofthe clinicianandhistopathologist,aswellasappropriate diagnos-tictests.
DiagnosisofBPDCNbyflowcytometrymaybechallenging insomecasesbecauseofthedifferent maturationalstages or because cells do not exhibit the distinctive profile due toeithertheabsenceofthe mainmarkersorthe presence ofadditional markersindicative ofother lineages.10,13 The advantageof flow cytometryin the diagnosis of BPDCN is thatitisaquantitativeandqualitativemethod,inadditionto beingmoresensitiveandspecific,asitnotonlydemonstrates
antigenco-expressionbutalsoshowsthedensityofeach anti-gen quantitatively.Thistechniquealsohasbetterabilityto detectnumerousantigenssimultaneously,includingsomenot routinelytestedbyimmunohistochemistry.12,23
ExpressionofCD123istypicalofBPDCN,butthismarker canbefoundinbasophilsandinmanycasesofacuteleukemia ofotherlineages.24Intensityofexpressionofthisantigencan varyduringdendriticcellmaturation,althoughstrong, homo-geneousexpressionisanimportantindicatorofdifferentiated plasmacytoiddendriticorigin;therefore,itmustbeevaluated togetherwithothermarkers.13
ItisimportanttodistinguishBPDCNfromother malignan-ciesthatexpressCD4andCD56,suchasaggressiveNK-cell leukemia/lymphoma, nasal NK-cell lymphoma, and AML, whichcanfeatureaberrantexpressionsofthesemarkersand cutaneousinvolvement.25
WereportedthecaseofBPDCNinafemalepatientwithan indolentclinicalpresentation,cutaneousandCNSinfiltration, noinitialbonemarrowinvolvement,andnoresponsetoAML treatment.Duringrelapse,ahighpercentageofplasmacytoid dendritic-lineagecellswasidentifiedinthebonemarrowby flowcytometry.Thepatientdied18daysafterHSCT.
revbrashematolhemoter.2017;39(3):274–277
277
Funding
HCPAResearchandEventPromotionFund(FIPE-HCPA).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. PaganoL,ValentiniCG,PulsoniA,FisogniS,CarluccioP, MannelliF,etal.Blasticplasmacytoiddendriticcellneoplasm withleukemicpresentation:anItalianmulticenterstudy. Haematologica.2013;98(2):239–46.
2. JacobMC,ChaperotL,MossuzP,FeuillardJ,ValensiF,Leroux D,etal.CD4+CD56+lineagenegativemalignancies:anew entitydevelopedfrommalignantearlyplasmacytoid dendriticcells.Haematologica.2003;88(8):941–55. 3. BuenoC,AlmeidaJ,LucioP,MarcoJ.Incidenceand
characteristicsofCD4+/HLADRhidendriticcellmalignancies. Haematologica.2004;89(1):58–69.
4. FacchettiF,JonesDM,PetrellaT.Blasticplasmacytoid dendriticcellsneoplasm.In:SwerdlowSH,CampoE,Harris NL,JaffeES,PileriSA,SteinH,ThieleJ,VardimanJW,editors. WHOclassificationoftumoursofhaematopoieticand lymphoidtissues.4thed.Lyon,France:IARCPress;2008.p. 146–7.WorldHealthOrganizationClassificationofTumours, vol.2.
5. RiazW,ZhangL,HornaP,SokolL.Blasticplasmacytoid dendriticcellneoplasm:updateonmolecularbiology, diagnosis,andtherapy.CancerControl.2014;21(4):279–89. 6. NgAP,LadeS,RutherfordT,McCormackC,PrinceHM,
WestermanDA.PrimarycutaneousCD4+/CD56+ hematodermicneoplasm(blasticNK-celllymphoma):a reportoffivecases.Haematologica.2006;91(1):143–4. 7. Garnache-OttouF,FeuillardJ,SaasP.Plasmacytoiddendritic
cellleukaemia/lymphoma:towardsawelldefinedentity?BrJ Haematol.2007;136(4):539–48.
8. FacchettiF,UngariM,MarocoloD,LonardiS,VermiW.Blastic plasmacytoiddendriticcellneoplasm.HaematolMeetRep. 2009;3(3):1–3.
9. FeuillardJ,JacobMC,ValensiF,MaynadiéM,GressinR, ChaperotL,etal.Clinicalandbiologicfeaturesof CD4(+)CD56(+)malignancies.Blood.2002;99(5):1556–63. 10.Martín-MartínL,LópezA,VidrialesB,CaballeroMD,
RodriguesAS,FerreiraSI,etal.Classificationandclinical behaviorofblasticplasmacytoiddendriticcellneoplasms accordingtotheirmaturationassociatedimmunophenotypic profile.Oncotarget.2015;6(22):19204–16.
11.JegalianAG,BuxbaumNP,FacchettiF,RaffeldM,PittalugaS, WayneAS,etal.Blasticplasmacytoiddendriticcellneoplasm inchildren:diagnosticfeaturesandclinicalimplications. Haematologica.2010;95(11):1873–9.
12.TsagarakisNJ,KentrouNA,PapadimitriouKA,PagoniM, KokkiniG,PapadakiH,etal.Acutelymphoplasmacytoid dendriticcell(DC2)leukemia:resultsfromtheHellenic DendriticCellLeukemiaStudyGroup.LeukRes. 2010;34(4):438–46.
13.Garnache-OttouF,FeuillardJ,FerrandC,BiichleS.Extended diagnosticcriteriaforplasmacytoiddendriticcellleukaemia. BrJHaematol.2009;145(5):624–36.
14.SaadehD,KurbanM,AbbasO.Plasmacytoiddendriticcell roleincutaneousmalignancies.JDermatolSci.2016;83(1):3–9. 15.PaganoL,ValentiniCG,GrammaticoS,PulsoniA.Blastic
plasmacytoiddendriticcellneoplasm:diagnosticcriteriaand therapeuticalapproaches.BrJHaematol.2016;174(2):188–202. 16.FachettiF,CigognettiM,FisogniS,RossiG,LonardiS,Vermi
W.Neoplasmderivedfromplasmacytoiddendriticcells.Mod Pathol.2016;29(2):98–111.
17.MarafiotiT,PatersonJC,BallabioE,ReichardKK,TedoldiS, HollowoodK,etal.Novelmarkersofnormalandneoplastic humanplasmacytoiddendriticscells.Blood.
2008;111(7):3778–92.
18.WangW,LiW,JiaJJ,ZhengY,WangH,GaoXM,etal.Blastic plasmacytoidcellneoplasm:acasereport.OncolLett. 2015;9(3):1388–92.
19.ChenJ,ZhouJ,QinD,XuS,YanX.Blasticplasmacytoid dendriticcellneoplasm.JClinOncol.2011;29(2):e27–9. 20.LaribiK,DenizonN,Besanc¸onA,FarhiJ,LemaireP,SandriniJ,
etal.Blasticplasmocytoiddendriticcellneoplasm:from originofthecelltotargetedtherapies.BiolBloodMarrow Transplant.2016;22(8):1357–67.
21.HeinickeT,HüttenH,KalinskiT,FrankeI,BonnekohB,Fischer T.Sustainedremissionofblasticplasmacytoiddendriticcell neoplasmafterunrelatedallogeneicstemcelltransplantation –asinglecenterexperience.AnnHematol.2015;94(2):283–7. 22.BrodyJP,AllenS,SchulmanP,SunT,ChanWC,FriedmanHD,
etal.AcuteagranularCD4-positivenaturalkillercell leukemia:comprehensiveclinicopathologicstudiesincluding virologicandinvitroculturewithinducingagents.Cancer. 1995;75(10):2474–83.
23.ShiY,WangE.Blasticplasmacytoiddendriticcellneoplasm:a clinicopathologicreview.ArchPatholLabMed.
2014;138(4):564–9.
24.TobaK,KoikeT,ShibataA,HashimotoS,TakahashiM, MasukoM,etal.Noveltechniqueforthedirectflow
cytofluorometricanalysisofhumanbasophilsinunseparated bloodandbonemarrow,andthecharacterizationof
phenotypeandperoxidaseofhumanbasophils.Cytometry. 1999;35(3):249–59.