w ww.e l s e v i e r . c o m / l o c a t e / b j p
Review
An
overview
of
dermatological
and
cosmeceutical
benefits
of
Diospyros
kaki
and
its
phytoconstituents
Muhammad
Kashif,
Naveed
Akhtar
∗,
Rehan
Mustafa
DepartmentofPharmacy,FacultyofPharmacyandAlternativeMedicines,TheIslamiaUniversityofBahawalpur,Punjab,Pakistan
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received30March2017 Accepted19June2017 Availableonline15August2017
Keywords: Persimmon Anti-tyrosinase Anti-wrinkle Photo-protection Cosmetics Antioxidant
a
b
s
t
r
a
c
t
DiospyroskakiL.f.belongingtofamilyEbenaceae,commonlyknownaspersimmonisusedasa medic-inalplantinChinesetraditionalmedicinesincemanyyearsfordifferentailmentsincludingcosmetics anddermatologicapplications.Traditionallythisplantisusedtotreatdifferentskinconditionsincluding pimples,skineruptionsandeczema.Presentinteresthasbeenfocusedtowarduseofnaturalbioactive compoundsinvariouscurativeandbeautifyingapplicationsindermatologicalandcosmeceutical dis-ciplines.TheobjectiveofthisarticleistopresentcumulativedataonpotentialuseofD.kakiforits possibleroleindermatologicandcosmeticapplications.Scientificdatahasrevealedanexcellent posi-tionofD.kakiinbothdermatologyandcosmeticdisciplinemakingitavaluablechoiceinrespective field.Activeprinciplesfromdifferentplantpartshaveshowntopossessanti-inflammatory, antialler-gic,photo-protective,andanti-wrinkleeffectswithappreciableactivitiesagainsttyrosinase,elastase, andcollagenaseenzymes.Promisingantioxidantactivityandskinwhiteningpotential,augmentedby reductioninsebumcontents,andreductioninsizeandnumberofskinporesmakeitasuitablechoiceas cosmeticingredient.Datahasbeensummarizedandpresentedonavailablemolecularmechanismthat cancontributetowardphytoconstituentsusageincosmeticsanddermatologymediatedbydifferent cel-lularpathways.Crudeextractsandsomeofphytochemicalobtainedfromthisplantsuchasisoquercitrin andhyperinhavebetterreportedactivitiesthanwell-knowncosmeticingredientsviz.,arbutin,kojic acidandhydroquinonewithpossibilityofhavingnosideeffects.Photoprotectionagainstdegenerative effectsofUVA,UVBandgammaradiationcanhelpskintofightwellagainstoxidativestressand reac-tiveoxygenspecies.Furtherinvestigationneedtobedirectedtowardhumansubjectsforevaluationof thesereportedactivitiesforobtainingoptimumcommercialandindustrialbenefitsfromthisvaluable plant.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Differentfactorscontributetowardchangingskinand beauty-careproductindustryincludingastrictregulatorycontrol,costand enhancedcustomerexpectationwithminimalsideeffectsofactive constituentsincosmetics.Duringthefirstdecadeof21stcentury totalexpenditureonbeautyandpersonnelcareproductsincreased from166.1billionUSDto382.3billion(ŁopaciukandŁoboda,2013) witha 25.9% saleshare ofAsiaat theendof 2007.Ingestedor appliedbioactivemoleculesinteractwithdifferenttargetsinour bodymodulatingdifferentbiologicalfunctions.Astheskinages, thesemetabolicprocessesalsochangeresultingincellulardamage
∗ Correspondingauthor.
E-mail:naveed.akhtar@iub.edu.pk(N.Akhtar).
andpoormaintenanceofskin.Cellulardamageandagingislinked withachangeincertainmetabolicenzymes,aminoacids,lipids, antioxidantsandnutrientslevels.Thenaturalbioactivemolecules presentinbotanicalextractareshowntohavepositiveregulating effectsonagingprocessandotherskinanddermatological con-ditions(Mukherjeeetal.,2011;Yeetal.,2014;Shinetal.,2015). Currentlytheresearchinfieldofskincareandother dermatologi-calconditionshavebeenshiftedconsiderablytowarduseofnatural productsandtheirbioactiveconstituentafterestablishingscientific validation,assuringsafetyandefficacy.
DiospyroskakiL.f.commonlycalledaspersimmonorJapanese persimmon,isadeciduousplantnativetoChina,KoreaandJapan, howevernowitisbeinggrowninmanyEastAsiancountriesand southernEurope.D.kakibelongstofamilyEbenaceaeandis con-sideredas oneofmostimportant speciesfromgenusDiospyros
becauseofyieldingexoticfruits(Zhuetal.,2016).Thisplantcan
http://dx.doi.org/10.1016/j.bjp.2017.06.004
Table1
ProductionstatisticsofDiospyroskakiin2014andvariousvarietiesproducedbytop 10producercountries.
Country Productionin2014 (milliontons)
Persimmoncultivarsproduced
China 3.804 Hachiya,Tamopan,Tanenashe,
Ormond,Fuyu,Imoto
Korea 0.428 Hongosi,Hachiya,DanGam(Fuyu)
Spain 0.245 Homanred
Japan 0.241 Hachiya,Tamopan,Tanenashe,
Taubata,Fuyu,Imoto,Jiro,Suruga Brazil 0.182 Sibugaki,Hachiya,Trakoukaki,
Hatemya
Azerbaijan 0.140 Gosho,Ghibrid-27235,Kiakume
Uzbekistan 0.066 Hachiya,
Italy 0.039 KakiTipo,Vaniglia,Cioccolatino, Zellonafuyu
Israel 0.037 Triumph
NewZealand 0.003 Fuyu,Jiro
Others 0.006 –
WorldTotal 5.191 –
becategorizedintotwodistinctvarietiesincludingastringent(e.g.
“Hachiya,Tamopan,Tanenashi,Triumph,HomanRed,Ormond,and
Taubata”)andnon-astringent(e.g.“Fuyu,Imoto,Izu,Jiro,Okugosho, Surugaandothers”)basedonchemicalnatureoftanninspresentin respectivevariety(Martinez-LasHerasetal.,2017).International commercialproducersofD.kakiandvariousproductvarieties pro-ducedarepresentedinTable1.AccordingtoFoodandAgriculture OrganizationStatistics(FAO-STAT)departmentofUnitedNations, 5.191milliontonsofD.kakiwasproducedgloballyin2014with 73.27%shareofChinaalonein2014(FAOSTAT,2014).Thisplant isnotendemictoBrazil,howeveritisbeingcultivatedwithgood propagationratehavingatotalgrowthof0.182milliontonsin2014
(seeTable1).InBrazilD.kakiiscultivatedinsoutheast,northeast
andcentral-westregions(Janeiro,2017).
Persimmonisenrichedwithmanynutritiousandbioactive com-ponentsincludingproteins, sugar,lipids, vitaminA,vitamin B6, vitaminB12,vitamin D,ascorbicacid(AA),vitaminE, polyphe-nols,flavonoidsandcarotenoids(KimandKim,2003).Elemental micronutrients present in persimmon fruit include potassium, sodium,iron,calciumandmanyothers.Thefruithavebeenused asakeyingredientsinsomemarketedcosmeticproductsincluding soaps,deodorizingandpurifyingbodylotion,bodywash,skintoner andbodyserum(MiraiClinical,2017).Differentreviewshavebeen publishedaboutreportedpharmacologicalactivitiesand phytocon-stituentsprofileofvariouspartsofthisplant,withverylimitedorno emphasisonitspotentialuseindermatologyandcosmetics(Piretti,
1991;Giordanietal.,2011;Xieetal.,2015).Thisreviewdescribes
availabledataaboutpotentialutilizationofdifferentpartsofD.kaki
anditsbioactivephytoconstituentsindifferentdermatologicaland cosmeceuticalapplications.
Phytochemicalsofdermatologicalandcosmeticsinterest obtainedfromDiospyroskaki
Phenolicacids
Phenolics(orphenolicacids)arewidelydistributedaromatic secondarymetabolitesinplantkingdom.Theycontainanaromatic hydrocarbonand oneormorethan onefunctionalhydroxyl(or carboxylicacid)groupattachedtoit.Theycanbecategorizedinto simplephenolsbearingonephenolunitorpolyphenolshaving mul-tiplephenolunitsinchemicalstructure.Theyperformarangeof differentfunctionsinplantsandhumanbeingincludingstructural
maintenanceandprotectionagainstoxidativestressdisorderssuch ascoronaryheartdisease,strokeandcancer(Robbins,2003). Phe-nolicacidsarepresentinfruits,vegetables,seeds,grains,leaves, rootsandstem(Robbins,2003).
In a recent report 32 low molecularweight phenolics have beendetectedfromthepulpofpersimmonandmostimportant onesinclude gallic acid (1) (itsglycoside and acyl derivatives), glycosidesofp-coumaric,vanillicandcinnamicacidsand differ-entflavonedi-C-hexosides.Catechin(5.81±0.12mg/100gofdry sample: DW), epicatechin (2) (0.61±0.023mg/100g DW), epi-gallocatechin(3)(0.28±0.02mg/100gDW) chlorogenicacid(4) (3.67±0.07mg/100gDW), caffeic acid(5)(2.83±0.07mg/100g DW),andgallicacid(19.11±0.61mg/100gDW)wereseparated fromethanolextractofMopanpersimmonandtheirantioxidant activitywasfoundtobehigherthanthatofwhiteapple,grapes, andtomato(Chenetal.,2008).Amongthesesixphenolics (con-tributingnotmorethan20%oftotalphenolic),gallicacidshowed highestantioxidantactivity.
OH OH HO
CO2H
1
O
OH HO
OH OH
OH
2 R=H
3 R=OH R
HO
HO
O HO
OH
CO2H
OH
4
CO2H
5
HO
HO
Theother polyphenolicsinvestigated frompersimmon fruits includeferulicacid,tannicacid,protocatechuicacid,vanillicacid, epicatechingallateand catechingallate(Leeetal.,2012).These andotherpolyphenoliccompoundshaveapotentialrolein pre-ventionofoxidativestressdamagebyscavengingreactiveoxygen species (ROS) (Fu et al., 2015; Zhou et al., 2016), prevention of lipid peroxidase(Toschi et al.,2000)and maybe helpfulin prevention of differentskinpathological conditions.In another studydifferentpolyphenolhavebeenseparatedfrommethanolic extract of leavesof persimmon and theirtyrosinase inhibitory effectswereelucidated.Theseparatedcompoundincludes hypero-side,isoquercitrin(6),trifolin(7),astragalin(8),chrysontemin(9), quercetin-3-O-(2′′-galloyl--d-glucopyranoside),and
kaempferol-3-O-(2′′-O-galloyl--d-glucopyranoside)(Xueetal.,2011).Among
HO
HO
HO HO
OH
OH OH
OH
OH
OH
OH HO 9
7 OH
OH
OH
OH OH
HO
HO HO OH
O
O O
O
O
O O O
O
O O
O R
6 R=H 8 R=OH
R
Flavonoids
Flavonoids,alsocalledbioflavonoidsarenaturallyoccurring sec-ondarymetabolitesofbotanicaloriginhavingageneralstructureof 15-carbonskeletoncomprisedoftwophenylringsandone hetero-cyclicring.Morethan8000phytoconstituentshavebeenidentified withthischaracteristicflavonoidstructure.Basicbenzo-␥-pyrone ringissubjectedtodifferentcombinationsofhydroxyl,methoxyl, andO-glycosylgroupsubstituentsresultinginnumerousindividual flavonoids(Benavente-GarciaandCastillo,2008).
Flavonoidsarefurtherclassifiedintotwelvedifferentsubgroups, however six of them have gained a significant dietary impor-tance,includinganthocyanidins,flavan-3-ols,flavonols,flavones, flavanones,andisoflavones(Manachetal.,2004).InD.kaki follow-ingexamplesarefoundindifferentpartsofthetreeincluding(I) anthocyanidinse.g.cyanidin,(II)flavan-3-olse.g.(+)-catechin,(− )-epicatechinand(−)-epigallocatechin,(III)flavonolse.g.kaempferol (H),quercetinandtheirglycosides.Persimmonfruitcontainshigh molecularweightcondensedproanthocyanidinsinvacuolesof tan-nincells.Theastringencyofthefruitismainlyattributedtotheir presencespeciallyduringunripestages.Catechin,gallactocatechin, gallicacid,epigallactocatechinandepigallactocatechin-3-O-gallate arethemajorsubunitsofcondensedproanthocyanidinsinthefruits
(Akagietal.,2011).
Persimmonleavescontainmanybeneficialflavonoids includ-ing quercetin and its glycoside complexes (i.e. hyperin and isoquercitrin), kaempferol and its glycoside (astragalin) along withcatechin(Ohguchietal.,2010;Sunetal.,2011,2014).Total flavonoidspresent in persimmon leavesper 100gof sample is equal to19.2gcatechin equivalent.The flavonoids present in the extracts were shown to have potent antioxidant activity, beingcapabletoscavenge superoxideanions,hydroxyl radicals withgood reducing powerand iron chelatingactivity superior than that of rutin (Sunet al.,2011). Naoxinging, a patent and authorized traditional Chinese medicine used in management of stroke and apoplexy syndrome contains flavonoids extract fromleavesofpersimmon(Beietal.,2009).Inanotherstudyfive flavonoidswereseparatedandidentifiedfromtheleavesofD.kaki
with following names kaempferol 3-O--d-galactopyranoside,
kaempferol 3-O--d-glucopyranoside, isorhamnetin 3-O--d
-glucopyranoside,quercetin3-O--d-galactopyranoside,quercetin
3-O--d-glucopyranosyl-(6→1)-a-L-rhamnopyranoside (Chen
etal.,2002b).Thepotentialuseoftheselistedflavonoidsin
der-matologyandasapossiblecosmeticingredienthasbeendescribed brieflyinBox1.
Carotenoids
Carotenoids are colored, fat soluble pigments generated as secondary metabolites in fruits, vegetables, algae, fungi, and
somemicrobes.Mostimportantcarotenoidsincludebeta-carotene, lycopene(10), lutein,and zeaxanthin (Anunciatoand da Rocha
Filho,2012).Carotenoidscanbecategorizedintotwogroupsi.e.,
“xanthophylls”whichareoxygenatedcarotenoidsand“carotenes” beingnon-oxygenated.Approximately700carotenoidshavebeen identifiedwitharound100beingconsideredfortheirdietary ben-efits(Kaulmannetal.,2014).Theyhavewiderapplicationsinfood, cosmeticsandnutritionbecauseoftheircolorproducingtendency andfreeradicalscavengingactivity(Kaulmannetal.,2014;Chang etal.,2015).Peroxylradicals,singletmolecularoxygenand super-oxideanionsarethemajorROSformedin humanskinexposed toUVirradiation,whichmayresultindegradationoflipids, pro-teinsandnucleicacids.Suchdegradationoutcomesinvariousskin pathologicalconditionssuchaserythema,pre-matureskinaging andeven dermatologicalcarcinomas.-Carotene alsoknownas “provitaminA”whichresidesintheskinimpartingagolden yel-lowcolor,havenodoubtaselectivecosmeticvalue.Luteinand zeaxanthin provide protectiontoretina against oxidative dam-agetoUV light.Lycopene canreduce erythemainduced byUV light.
10
Persimmon fruit contains different types of carotenoids includingbothxanthophyllsandcarotenes.Lutein,zeaxanthin, -cryptoxanthinand-carotenehavebeenseparatedandoptimized usingresponse surface methodologyrecently (Zaghdoudi etal.,
2015,2016).Sothepersimmoncanbeusedasasourceofimportant
polyphenolicconstituentsandcarotenoidsasasuitablecandidate forinclusionintocosmeceuticals.
Hydrolysabletannins
Anothergroupofbioactivephytoconstituentspresentin per-simmonaretannins(MW=1.12×104Da).Tanninsarecomprised ofeithergallicacidsubunits(e.g.hydrolysabletannins),flavone subunit(non-hydrolysableorcondensedtannins)or phlorogluci-nolsubunits(phloro-tannins).Tanninsfromdifferentsourceshave beenstudiedfortheirantiviral (Ueda etal.,2013),antibacterial
(Akiyamaetal.,2001),antioxidant(Guetal.,2008;Tourinoetal.,
2008), pediatric dermatoses (Fölster-Holst and Latussek, 2007), anti-inflammatory(Motaetal.,1985;Liuetal.,2015)and radiopro-tectiveeffects(Zhouetal.,2016).Tanninshavebeenusedmedically formanyyears andtheirimportanceindermatological applica-tionhavegainedsignificantimportancebecauseoftheirastringent effects,managementofsuperficialskincondition,weeping, inflam-mationanditchingwithacceptabletolerability.
Astringentfeelinguponeatingpersimmonfruitisdueto sol-uble tannins which are released from tannin vacuoles making complexwithproteininoralcavity.Whenthesetanninsare trans-formedintoinsolubleform,thefruitloses itsastringentnature considerably.Inpersimmonmajortanninpresentinclude flava-noellagitannin(moleculeofflavan-3-olattachedwithhydrolysable tanninthroughC-Clinkage),procyanidinoellagitannin (proantho-cyanidinsandellagitannins)andtheirdegradedproductssuchas gallo-catechin,catechin,catechin-gallateandgallocatechin-gallate
(Özenetal.,2004;Guetal.,2008).Inarecentstudy,tanninsfrom
persimmonhavebeenextractedbyultrasound-assistedextraction (39.56%ascatechinequivalents)andshowntopossessradio pro-tectiveeffectsagainstgammaradiationsinducedROS(Zhouetal.,
Box1:DermatologicalandcosmeticsapplicationofvariousactiveprinciplesofDiospyroskakianditsextracts
Activeprinciple/or crudedrug
Plantpart used
Pharmaceutical form
Test sub-ject/experimental condition
Pharmacologicalactionofdermatologicand/or cosmetics
Reference
•Quercetin-3-O- -d- glucopyranosyl-(1→6)- -d-glucopyranosid
Calyx Purifiedfraction
fromacetone-water (70%)extract
(10–100g/ml)
␣-MSH-stimulated B16F10mouse melanomacells
•Hypopigmentationeffects
•Inhibitmelaninsynthesis
•Inhibittyrosinaseactivity
•Reducedexpressionofmelanogenicproteins
(Jungetal.,2015)
•Chrysontemin Leaves Methanolicextract In-vitroL-DOPA
oxidation
•Antityrosinaseactivity(moderate) (Xueetal.,2011)
•Isoquercitrin (quercetin-3-O -glucoside)
•Hyperin (quercetin-3-O -galactoside)
Peel Acetoneextract B16Melanoma
cells
•InhibitsmelaninbiosynthesisinmouseB16 melanomacells(higherthankojicacidand arbutin)
(Ohguchietal.,2010)
• 2-Methoxy-4-vinylphenol
Peel Methanolicextract
anditspurified fractions
Aqueous, methanolicand acetonepurified fractions
•Antityrosinaseactivity(higherthanarbutin) (Fukai et al., 2009)
•Rotungenicacid
•
24-Hydroxyursolic acid
•Ursolicacid
•Oleanolicacid
•Spathodicacid
Leaves Methanolicextract Proteintyrosine phosphatase1B (PTP1B)
•Inhibitoryeffectsonproteintyrosine phosphatase1B
(Thuongetal.,2008)
•Crudeextract Fruit Ethanolicextract In-vitro
antityrosinase activity
Antityrosinaseactivitycomparabletothatof arbutin
(Tiechietal.,1999)
•Ethanolicextract andtheirpurified fractionsI,II&III
Leaves Purifiedfractionsof Ethanolicextract
Inhibitoryactivity againstxanthine oxidase, collagenase,and elastaseenzymes
•Antityrosinaseactivity
•Collagenaseinhibition
•Collagensynthesispromotedincultivated fibroblasts
•Xanthineoxidaseactivity
•Elastaseinhibitoryeffects
(Anetal.,2005)
Gallicacid Leavesand
fruit
Gallicaciddilutions andtopical preparation
Eiosinophil-dermal fibroblast,
SwissAlbinorats
Zebrafish,
UV-Binducedmice skinmodel
•Anti-inflammatory,
•Anti-microbial
•Inhibitshistaminerelease
•Suppressreleaseofpro-inflammatory cytokines(IL-6)andchemokines(CCL7& CXCL8)fromeosinophils-dermalfibroblast
•Suppressesthe7,12-DMBA/Crotonoilinduced two-stepskincarcinogenesisbymodulating anti-oxidantsandMMP-2/MMP-9inSwiss albinomice
•Depigmentationandskinlighteningeffect
•Anti-agingeffects(in-vivoandin-vitro)
(Tsangetal.,2016)
(Kumaretal.,2013)
•Epicatechin Fruitand
leaves
Dilutedsamples Culturedhuman skinfibroblast
•AttenuationofUVA-inducedoxidative damagetohumanskinfibroblasts
•Epigallocatechin Fruitand leaves
Topicalcream Healthyhuman
volunteers Splitfacestudy design
•Photoprotective(UV-B)
•Anti-inflammatory
•Reducesmelaninsynthesis
(Domingo et al., 2010; Jeon etal.,2010)
•Chlorogenicacid Fruitand leaves
0–500Mdilution B16melanoma cells
•Inhibitstyrosinaseactivityandsuppresses melanogenesisinB16melanomacells
•ProtectskinagainstUV-inducedoxidative damage
•Anti-inflammatoryeffects
(Lietal.,2014) (Kitagawaetal.,2011) (Tsang et al., 2016)
•-Carotene Fruitpulp
andpeel
– – •Imparting“GoldenYellow”colortoskin
•ProtectsagainstUV-skindamage
(Zaghdoudietal.,2016; Zaghdoudietal.,2015)
•Lycopene Fruit – – •Reducesskinerythemalevel
•Regulatescholesterol
(AnunciatoanddaRocha Filho,2012)
•Leutinand xeaxanthin
Fruit – – •ProtectionagainstUVdamageandROS (Kaulmann et al., 2014)
•Tanninsincluding flavanoellagitan-nin,
• Procyanidinoel-lagitannin
Fruitpulp Aqueous
methanolicextract
HEK293Tcells •Potentantioxidantproperties
•ReducingtheROSlevelsofGamma-radiation exposureinHEK293Tcells.
•Reducingcellapoptosis
(Guetal.,2008;Zhouetal., 2016)
•Coussaricacidand betulinicacid
Leaves Purifiedfractions Lipopolysaccharide-stimulatedRAW 264.7macrophages
•Anti-inflammatoryeffectswithsuppressionof NO,PGE2,TNF-␣,IL-6andIL-1
Proanthocyanidins
Proanthocyanidins (PA), secondary metabolic bioactive con-stituentsare colorless polymersresultingfromcondensation of flavan-3-olmonomericunits.They getdepositedin persimmon fruitsduringearlydevelopmentalstages.Astringentspecieof per-simmoncontainshigheramountsofPAevenafterfruitmaturation. Whileinnon-astringentfruittypesthesebioactivemoieties disap-pearonmaturationoffruits(Ikegamietal.,2007).Accordingto
Ikegamietal.(2007)PApresentinpersimmonusuallycompriseof
flavan-3-olunits,andtheyresultinproductionofbioactive com-ponentslikexavonolsandglycosylatedanthocyanidins.Multiple catechinunitsarepresentinPAobtainedfrompersimmonwith approximateMWof1.38×104Da.
Terpenoids
DifferenttriterpenoidshavebeenseparatedfromleavesofD. kaki including ursolicacid, 19-hydroxy ursolicacid and 19,24-dihydroxyursolicacid,whichdemonstratedsuppressiveactivity againststimulusinducedsuperoxidegenerationandtyrosyl phos-phorylation(Chenetal.,2002a).In2009,anotherreportindicated identification and separation of 18,19-secoursane novel triter-penoids(kakisaponinBandkakisaponinC)alongwithanursane type28-nortritepne (kakidiol)and rosamultinfromleavesofD. kaki(Chenetal.,2009).KakisaponinAwasalsopreviously iden-tifiedbythesameresearchers(Chenetal.,2007).Otherterpenoids reportedfromleavesofD.kakiincludelupeol,betulinicacid,betulic
acid(Yoshihiraetal.,1971)andpomolicacid(Thuongetal.,2008).
Coussaricacidandbetulinicacidhavebeenseparatedfromleaves ofpersimmonplant(Kimetal.,2016).
Ascorbicacid,vitaminsA,DandE
Ascorbic acid (AA) is hexuronic acid lactone micronutrient beinglipophobicinitsnature.Itcannotbesynthesizedbyhuman being and hence should be supplied externally from food. AA performsdifferentbiochemicalfunctionsinsidethebody includ-ingsynthesisandmaintenanceofcollagen(Esteban-Preteletal.,
2013;Kishimotoetal., 2013;Findiketal.,2016),
immunostim-ulant(TewaryandPatra,2008),anti-aging(Xuetal.,2012), and skinrejuvenatingagent(Zahouanietal.,2002;Crisanetal.,2015), skin whitening effects (Smith, 1999; Traikovich, 1999), neuro-modulator (Rebec and Pierce, 1994), anti-oxidant, free radical scavenger(Cathcart,1985;Erbetal.,2004)andantiviral(Jariwalla
andHarakeh, 1996).In theskinAA playsa vital roleas a
sub-strateforoxidativestressorsandhencepreventsdamagetoskin causedbyROSand otherreactiveoxidantsproducedasaresult ofUVexposure.Topicalapplication ofAAcanprovideobjective andsubjectiveimprovementsinphoto-damagedfacialskinas con-firmedbyquantitationofskinsurfacetexturechanges(Traikovich, 1999).AmountofAAinpersimmonfruitisrangedbetween180 to200mgper100goffreshweight(FW),whichissubjectedto variationduringripeningstages(DelBubbaetal.,2009).TotalAA contentsinfruitshaveameanvalueof47±39mg/100gFWwith 3.5mg/100gFWintheastringentvarietyCostatato146mg/100g FWin thenon-astringentcultivarHanaFuyu (DelBubbaet al., 2009).PersimmonleavesarealsotestedtobeenrichedwithAA contentslocalizedincytosolofpalisadeparenchymatissuecells
(Kusunokietal.,1998).
VitaminAhasbeenusedwidelyincosmeticindustryand der-matologyforitbeneficial effectsinskincareproductsincluding normalizationofkeratin,reductioninsebumproductioninacne patients,andcuringphotodamagedandagedskin(Shapiroand
Saliou,2001).VitaminDhasshowntodown-regulateepithelial
growthand facilitatesits differentiation. VitaminE being used
inexperimentalandclinicaldermatologyformorethan50years
(Thiele and Ekanayake-Mudiyanselage, 2007), is an important
membraneantioxidant,provideprotectionagainstoxidative dam-age,andwhencombinedwithAA,itcanactasaphotoprotective agent.Thesevitalvitaminsarepresentinappreciableamountin fruitsandleavesofpersimmon,strengtheningitsvalueasa cos-meticingredient.
Dermatologicalandcosmeticsbenefits
Anti-inflammatoryeffects
Inflammationisavitalimmunemechanismofinnateimmunity that protects body against various harmful factors. Inflamma-tionisusuallymediatedbydifferentexogenousandendogenous stimulithatmayactivatecellularimmunesystem,whichintern canproducesomepro-inflammatorycytokines. Cyclooxygenase-2 (COX-2) in human skin,is a main key player in UV-induced inflammation,wrinkleformation(Limetal.,2013),edema, epider-malhyperplasiaandcarcinogenesis.Lipopolysaccharide(LPS),in anexogenousbacterialendotoxinthatcanactivatemacrophages resultinginreleaseofpro-inflammatorycytokinessuchastumor necroticfactor-␣(TNF-␣),interleukin-1(IL-1),interleukin-6 (IL-6),nitricoxide(NO),andprostaglandinE2(PGE2).Inflammation is regulated by heme oxygenase-1 (HO-1) which inhibits syn-thesisofpro-inflammatory cytokinesandmediator in activated macrophages.Nuclearfactor-kB(NF-kB)hasbeenconsideredan importantfactorinvolvedinimmuneandinflammatoryresponse. Thesecytokinescancauseanexpressionofvascularanddermal adhesionmolecules,chemoattractionofinflammatorycells, and activationofotherinflammatorymediatorslikeleukotrienesand PG.
Coussaric acid (CA) and betulinic acid (BA), (triterpenoids obtained from leaves of D. kaki), has shown to possess anti-inflammatoryeffectsbyinhibitionofNF-kBpathway.Bothofthese twoacids(Kim etal.,2016)and quercetin-3-O--d-(2′′
-galloyl)-glucopyranosideandquercetin(Choetal.,2016)(separatedfrom calyxofD. kaki)can inhibitNOandPGE2along withamarked suppressionofTNF-␣,IL-6andIL-1inLPS-activatedRAW264.7 macrophages.Theywerealsofoundtosuppressprotein expres-sionofinduciblenitricoxidesynthetaseandCOX-2.BAwasalso showntohaveapositive impactonHO-1whileCAwashaving nosignificanteffects. It is commendable tonotethat in differ-entskinconditionmanifestedbyinflammation,like“inflammatory acnevulgaris”,pro-inflammatorycytokinessuchasIL-1␣,IL-1
(Chenetal.,2016;Hougeeetal.,2005;Vezzaetal.,2016;Wuetal.,
2016).
Antiallergicpropertiesandpotentialuseinpreventionof dermatitis
Skinisthelargestprotectiveorgan attheinterfacebetween hostandenvironment. Itprotectsfrompathogensasa physical barrieranddefendsourbodyagainstdifferentallergensby activat-ingimmunesystem(Skabytskaetal.,2016).Mastcellsarewidely distributedinmammaliantissuesandplayanimportantrolein regulationofallergicinflammationindifferentimmunemediated disorders.Mast cells uponactivationcan releasehistamine and otherinflammatorymediators,forexampleeicosanoids, proteogly-cans,andotherpro-inflammatorycytokinessuchasTNF-␣,IL-1, IL-6andIL-13(Kimetal.,2013).Dermatitisisacommonskin con-ditioncharacterizedbyinflamed,red,itchyskinthatmaybecome blisteredandweepy.Therearedifferenttypesofdermatitisand allofthemareprecipitatedontotheskinbyreactingwith aller-gensorirritants.Whenallergensorirritantsbecomeincontactwith skin,theymayleadtoaskinreaction,thisconditionistermedas contactdermatitis.Askindamageisusuallyseenwithanirritant whileanallergeninitiatesimmuneresponseadvancingtoallergic reaction.Atopicdermatitisoreczemaoccursdueto hypersensi-tivitytocertaintypesoffood(e.g.cow’smilk) and/orallergens. Neurodermatitisisbecauseofirritationtonerveendingsdownthe skin,leadingtoseveritchysensationandanirresistibledesireto scratchtheskinrepeatedlyresultinginthickeningandrednessof theskin.Therearesomeothertypesofdermatitisaswell includ-ing statis dermatitis, seborrheic dermatitis, perioral dermatitis anddermatitisherpetiformis.Recentadvancesinimmunological screeningofatopicdermatitishasresultedinunderstandingthat activatedmastcellsandincreasedT-helper-2lymphocytes(Th2) cellsviachemicalmediatorsandcytokinesmightplayavitalrole indevelopmentofdermatitisandIgEproduction.Topicalsteroid therapyisusefulinmanagementofthiscondition,however pro-long use of these medicinal substances is of concernto some patients.
BotanicalextractfromleavesofD.kakicontainssome antial-lergicsubstancesthancaninhibithistaminereleasefromhuman basophilic cell lines KU812. Oral administration of persimmon leavesextractandaflavonoidfractioncalledastragalin,to mod-elsofpassivecutaneousanaphylaxisand atopicdermatitismice hasresultedin suppressionofdermatitisdevelopment, scratch-ingbehavior,andserumIgElevels.Inflammatorycellinfiltration, specially degranulated mast cells, thickening of epidermis and hyperkeratosiswerereducedsignificantly.Moreover,production of IL-4 and IL-13 by spleen cells was reduced (Kotani et al., 2000).Inanotherreportpolyphenoliccompoundsfrom persim-mon leaves were shown to possess antiallergic properties and theirpotentialuseincontactdermatitiswasreported(Park,2000). Aqueous extract of D. kaki was investigated for its protective effectsonmastcellmediatedallergicreactionbyin-vivoand in-vitro mast cellbased models. The extract wasfound toinhibit therelease of histamine and -hexosaminidase fromthe mast cells by modulating cAMPand intracellular calcium levels.The releaseofpro-inflammatorycytokinessuchasTNF-␣,IL-1was alsoreducedbyinhibitionofNF-kB(Kimetal.,2013).Itwas estab-lishedthattheaqueousextractcaninhibitsystemicandcutaneous allergicreactioninasimilarwayasthatofsodiumcromoglycate. Differentphenolic compoundsincludinggallicacid, ellagicacid, hyperin,isoquercitrin,astragalin,quercetinandkaempferolfrom aherbalextracthasrevealedadosedependentinhibitoryaction againstedema induced byallergiccontact dermatitis (Fu etal.,
2015).
Anti-radiationactivity(protectionagainstphotodamage)
Electromagnetic radiation emitted from sun, is comprised of ultraviolet radiation (UVR; 200–400nm), visible light (400–780nm), and infrared (IR; 780nm to 1mm). Interna-tional commissiononillumination(CIE)divides UVRintothree categories: UVA (315–400nm), UVB (280–315nm) and UVC (100–280nm). UVC portion being most dangerous for skin, is entirelyabsorbedbytheupperatmosphericlayers.Humanbody needsaverylimitedUVAandUVBphotonsforvitaminDsynthesis
(Holick,2008; Rivaset al., 2015), longer exposuretoUVR may
lead to various skin abnormalities including photoaging and photocarcinogenesis through production of ROS, DNA damage, immunosuppression, photo-inflammation, altered remodeling of extracellular matrix (ECM) and/or angiogenesis(Bickers and
Athar, 2006; Nishigori, 2006). ROS are produced as a result
of UVR exposure to the skin(Bickers and Athar, 2006), which can activate cell surface receptors resulting in stimulation of mitogen-activated protein kinases (MAPK) (Wang et al., 2013). Cellproliferation,celldeathandcellsurvivalisregulatedby acti-vatorprotein(AP-1),andNF-kB.MoreoverNF-kBalsoregulates inflammation,oncogenesisandapoptosis(Muthusamyand Piva, 2010).UVRexposurecausesthereleaseofarachidonicacidfrom oxidizedlipidmembranes,whichisconvertedintoprostaglandin (PG)bycyclooxygenaseenzyme(COX).Thenewlyproduced PG may attractinflammatory cells. The activationof AP-1 by UVR helps promote photo-carcinogenesis and destruction of ECM. The activated AP-1 alsointerferes with collagensynthesis and blocktheeffectsoftransforminggrowthfactor-(TGF-)which is responsiblefor collagentranscription. The activationof AP-1 by UVR leads to overexpression of matrix metalloproteinases (MMP)inhumanskinandECMdestruction(CooperandBowden,
2007).
Longer exposuretoUVlight (particularly UV-A portion)can result inpremature skinaging (photo-aging)because ofhigher degreeofoxidativestressinhumanskin.Theantioxidantdefense mechanism intheskin protectsit fromharmfuleffects ofROS, however the overproduction of ROS generated from prolonged exposuretoUV-A lightcancauseanincreaseinoxidativestress damageandresultsindegradationofcertainmoleculeslike,DNA, proteins, andfattyacids.Thissituationmayleadtodestruction ofcellularandinterstitialstructurepromotingtissuenecrosisor apoptosisofskincells,andskinmaydeveloppathological condi-tionslikeskinaging,wrinklesorevencancer.UVradiationleads toamarkeddecreaseinepidermalLangerhanscells,resultinginT helper-1lymphocytes(Th1)clonalanergy.Thisresultsin immuno-suppression,anergy,andimmunologicaltolerance(Simonetal., 1991).Isomerizationofurocanicacid(UCA)fromtrans-UCAinto cis-UCAchangesUVradiationsintobioactiverecognizablesignal thatinitiatesimmunesuppression(Prateretal.,2003).
NonUVradiationssuchasvisiblelightandIRhavenotmuch beenfocusedfortheiranypossibleroleinphoto-agingasopposed byUVAandUVBradiations.Howeverrecentstudiesdemonstrate theirpossibleroleinpathogenesisofphotoaging(Sklaretal.,2013). Ionizingradiationhavebeenusedbroadlyinmedicinefor radio-diagnosticandradio-therapeuticpurposes. Theseradiationscan produceionsand causeanimbalanceinfreeradicalsinhuman. AsresultcellphospholipidsandDNAdamagemayhappen(Zhou
etal.,2016).
studies revealed a reduction in infiltration of inflammatory (degranulatedmast)cells,thickeningofepidermis,and hyperpla-sia(Choetal.,2011).UVBinducedproductionofCCL2andCCL27 isfirmlyregulatedbyactivationofNF-kB.AP-1andNF-kB, regu-latedbyintracellularredoxstate,areincreasedbyUVBirradiation. Oxidativestressandmitochondrialdysfunctionplaysamajor func-tioninapoptoticevents.FlavonoidsfromtheleavesofD.kakihave showntoreducehydrogenperoxideinducedapoptosislikeinjury toNG108-15cells(Beietal.,2005),implicatingtheirpossibleuse inreversingoxidativestresscausedbyROS.
Quercetin, kaempfetol, rutin, astragalin, hyperin and iso-quercitrinwerethoughttoplayamajorroleinimprovingtheredox status, inhibiting apoptosis, and increasing cell viability under oxidativestressin NG108-15cells (Beietal., 2005).In another studyinvolvingMC3T3-E1cells,inducedwithoxidativestressby hydrogenperoxide,flavonoidfromPLEwereshowntoprotectcells againstoxidativestressrelatedcellularinjuries.Flavonoidsfrom PLEcaninhibitapoptosisinH2O2activatedMC3T3-E1cellswith suppressionofNO,induciblenitricoxidesynthetase(iNOS),COX-2, melanonedialdehyde(MDA),indicatingthatanti-apoptosis activ-ityismediatedbysuppressionoftranslocationofNF-kB/p65into thenucleus(Sunetal.,2014).
Collagenase(amatrixmetalloproteinases;MMP)regulates pho-toaging process of skin due to ROS generated as a result of UVAexposure.Efficacyofdifferentflavonoidsincludingmyricetin, quercetin,kaempferol,luteolin,apigeninandchrysin,oncapturing ROSandinhibitionofMMPhavebeenstudiedearlierin2007by SimandLeeetal.Itwasconcludedthatflavonoidscaninhibit col-lagenaseactivityinUVAinducedhumandermalfibroblastsindose dependentmannerandcanresultinlowerexpressionofMMP.The degreeofantioxidantpropertyandinhibitionofcollagenasewas linkedwithnumbersofhydroxylgroupsinflavonoidstructure(Sim etal.,2007).Phenolicacidandtheiramidederivativescanhelp pro-tectskinagainstUV-Ainducedoxidativestressdamageandsebum peroxidation(Ley,2001).Chlorogenicacid,loadedino/w hetero-geneousemulsifiedtopicalformulation,canhelptoprotectagainst UVinducedoxidativedamage(Kitagawaetal.,2011).
Tannins extracted from persimmon were shown to possess radioprotective effectsagainst differentdoses ofgamma radia-tions(2–20kGy)exposedtoHEK293Tcells.Radiationprotection waswieldedbyanincreasedcellslifespan,reductionincell apo-ptosisandadecreaseinROSlevelsinHEK293Tcellsexposedto Gamma-radiations.Recentlyinanotherreportrestorativeeffects ofPLEagainstGammaradiationinducedoxidativestressandliver damage,wasevaluatedinirradiatedmiceandwasfoundtoreduce severityofradiationinduced liverdamageand othermetabolic parameters(Ashryetal.,2016).Sometypesofskincancersarehard totreatwithchemotherapeuticagents,andofcourse,suchagents have profound side effectprofile. Polyphenol enriched extracts havebeen evaluatedfor theirefficacytoward skincancerwith greatlypromisingoutcomes(Wangetal.,2012a)indicatingtheir potentialroleinpreventingorcuringdifferentskincancer condi-tion.
Effectsonsebumcontents,oilcontents,numberandsizeofskin pores
Excessivesebumproductionandaccumulationontheskinmay increasetheskinporesize.Aneffectiveskincleanseriscapable toreduceskinpore size byreducing production rateof sebum andpromotingitsremovalfromskin,hencereducingchancesof comedonesdevelopment.Carefulfacewashinghelpsimproveskin lesionsandpreventsacnedevelopmentbywashingaway exces-sivesebumandavoiding hairfollicularobstruction(Isodaetal., 2015).Manycosmeticingredientsusedinskincleansershavesome unwantedeffects,suchassodiumlaurylsulphatemayirritatethe
skin.Similarly,retinoidanditsderivativesareknowntobesever localskinirritants.Naturalproductsusuallyhavelessersideeffects, thatiswhycosmeticsindustryisgoingthroughashiftfrom syn-thetictonaturalcosmeticingredients.
ExtractfromD.kakifolium,Polygonumcuspidatum,andCastanea crenata(DPC)loadedtocosmeticcleanserformulationwas evalu-atedforitseffectsonskinparametersincludingnumberandsizeof skinporesandremovalofsebumfromtheskinin23healthy volun-teers.OnapplicationoftestformulationcontainingDPCextract,oil contentsdecreasedby77.3%,numberofskinporeswerereduced by24.83%andskinporesizewasreducedby71.43%ascompared tothecontrolformulation(Isodaetal.,2015).Thepreparationwas alsocapabletoremovesolidifiedsebumfromskinandcanfacilitate removalofDemodexmites(causativemicrobeforrosaceaand seb-orrheicdermatitis)fromtheskin.Furtherstudiescanbedirectedfor evaluationofdifferentformulationcontainingpersimmonextract fortheireffectsonotherskinparametersusingnon-invasivein-vivo
evaluationtechniques.
Inhibitionofmelanogenesis(skinwhiteningeffects)
Skincoloris usually determinedby fourchromophoric sub-stancesknownascarotenoids,hemoglobin,oxyhaemoglobinand melanin,thelastbeingmostabundant(Hearing,2005)relatively. Melaninisproducedbymelanosomeswhicharepresentintheskin, eyes,innerear,andhairs(Jungetal.,2015).Inhumanbeing pigmen-tationmayincreaseasaresultofUVorsolarlightexposuretothe skin,whichintern,stimulatesmelaninproductionbymelanosomes
(Coelhoetal.,2013).MelaninprovidesprotectionagainstUV
radi-ations,skinburnandcancer.Melanogenesisistheproductionof melaninfrommelanocytesinbasalepidermallayer.Every individ-ualusuallyhaveaparticularnumberofmelanocyte,howeverthe skincolorinnotdeterminedbythenumberofmelanocytes,rather itsbeingdeterminedbymelaninproducinggenes.Inmelanocytes, melanogenesisis usually regulatedby certainenzymessuchas tyrosinase-related protein-1(TRP-1),tyrosinase-related protein-2 (TRP-2) and tyrosinase (TYR)(Kameyama et al.,1995; Wang
et al.,2012b).Transcriptionof TRP-1, TRP-2,and theTYR
fam-ilygenesiscontrolledbymicrophthalmia-associatedtranscription factor (MITF), that is why it is believed that MITF is a master regulatorinmelanocyteproliferation,development,survivaland melanomaformation(Wangetal.,2012b).Recentlyitisreported thatmitogenactivatedproteinkinases(MAPK)includingp38MAPK is mainlyinvolved in MITFregulation. Activationof p38MAPK increasestranscriptionofTYR,stimulatingmelanogenesis(Galibert et al., 2001). cAMP, the second messenger derived from ATP, plays a vital role in intracellular signaltransduction. Increased cAMPconcentrationaffectsproteinkinaseA(PKA),cAMPresponse element-bindingprotein(CREB)andcAMPresponseelement(CRE). PKAhasdirect effectonmelanogenesisand itsactivation leads toMITFexpressionbyphosphorylationofCREB,whichincreases melanin synthesis (Busca and Ballotti, 2000). So, for an agent toefficientlyreduce hyperpigmentationandproduce whitening effects, it shouldact by regulatingMITF,CREB,PKA and MAPK pathways.
Many different compounds have been isolated from per-simmon and theirantityrosinase activities have been reported asoutlinedin Box 1.Quercetin-3-O--d-glucopyranosyl-(1→
6)--d-glucopyranosid (QCGG) (11) separated from the calyx of
fruitpeel,werefoundtobepotentinhibitorsofmelanin produc-tionbysuppressingtyrosinaseexpressioninmouseB16melanoma cells(Ohguchietal.,2010).2-Methoxy-4-vinylphenol(andits gly-coside)isolatedfrompeelofpersimmonhasantityrosinaseactivity higher thanthat of arbutin(Fukaiet al.,2009).Sevendifferent polyphenolsseparatedfromleavesofpersimmonhavebeentested fortheirantityrosinaseactivitiesandchrysonteminwasreportedto containmoderateantityrosinaseactivity(Xueetal.,2011).Crude ethanolicextractofpersimmonhasantityrosinaseactivity com-parabletothatofarbutinwithanti-wrinkleeffects(Tiechietal., 1999).SimilarlyamongfractionI,II,andIIIofethanolicPLE,fraction III(82%totalphenols)showedsignificantantityrosinaseactivity (higherthanthatofgreentealeaves,mushroom,garlicandblacktea extracts)alongwithinhibitionofxanthineoxidaseandcollagenase enzymes(Anetal.,2005).
O
O O
O
O
O HO
HO
HO
HO 11
OH
OH OH
OH OH
OH
OH
The other isolated phytoconstituents from persimmon have beenreportedfortheirantityrosinase/anti-melanogenicandother usefulactivitiesforcosmeticanddermatologicalinterestinclude, rotungenic acid (12), gallic acid, epicatechin, epigallocatechin, chlorogenicacid,-carotene,lycopene,lutein,zeaxanthin, cous-saric acidand betulinic acid. Gallic acid, a major polyphenolic contentfrompersimmonleavesandfruithaveshowntoreduce UVBinducedhyperpigmentationinrats(Kumaretal.,2013). Gal-licacidhasshowntosuppressmelanogenesisbydown-regulating melanogenic regulatory genes in TYR, TRP-1 and dopachrome tatamerase expression at level of transcription and translation
(Kumaretal.,2013).Moreover,gallicacidinhibitsMITF
expres-sion by reducing cAMP-mediated PKA/CREB signaling cascade. Similarlychlorogenicacidactsasasubstrateformelaninandits metabolicproductsof areshown tosuppressmelanogenesis in B16melanoma cells byinhibiting TYR activity(Li etal., 2014). Skinlightening effects ofPLE arepromising and comparableto that of hydroquinone (An et al., 2005), withoutany associated side effects. PLE beingenriched withmany valuable phytocon-stituentscanserveanefficientingredientfordifferentcosmetics formulations.
HO
HO
HO
CO
2H
H
H
12
Collagenaseandelastaseinhibition(preventionofwrinkle formation)
Collagenrepresents30%oftotal proteininman withalmost sameweightage inother animals.Collagen canexist in 27 dif-ferent types however, type I, II,and III aremost prominent in
man, comprisingapproximately80–90%of totalcollagenin the body. Somebody organsare relativelyricherin collagentype-I includingdermis,bones,tendon,andligamentwhileskin,blood vessels and intestine are enriched with type-III (Findik et al., 2016).Intheskincollagenmaybedegradedbyagingorby activ-ity ofcollagenase, producing wrinkles.Collagen is producedby maturecellscalledfibroblasts.Firstly,procollagenisproducedby fibroblasts,whichissubjectedtodifferentmodificationsincluding prolineandlysinehydroxylation.Crosslinkageoccursasaresult ofprolinehydroxylationproducingstrongcollagenfibers(Roach
etal.,1985).
Skin aging is usually estimated by wrinkles on the face. In wrinkledskin,usuallythereisdepositionofalteredelasticfibers and/ordegradedordegenerated collagenbundles inthedermis
(Antonicellietal.,2009)resultinginreducedskinelasticity(Tsuji
etal.,2001).Prolongedexposuretosunlightisconsideredtobethe mostprobablecauseforevokingskinwrinkles.Insolubleelastin is themajor partof skinelasticfiber.Elastinfibers produces a delicatedispersednetworkbetweenthecollagen(Oxlundetal., 1988).Elastinplaysavitalroleinmaintenanceandrestorationof skinelasticityanditsdegradationmayresultinwrinklesandloss ofelasticity.Higherlevelsofelastaseenzyme,diminishedelastin generationand reduced skinregeneration withincreasedaging resultsin reduced skinelasticity. Thereare two main types of elastasesintheskin;neutrophilelastase(serineproteinase)and skinfibroblastelastase(metalloproteinases).Neutrophilelastase can degrade all typesof elastin fibers while fibroblast elastase affectsoxytalanandelauninfiberswithaminimaleffectsonmature elastinfibers(Tsujietal.,2001).Overproductionofelastaseenzyme inducedbyUVirradiationaffectselastic-fibernetwork.Skin fibro-blastelastasereleasedbyfibroblastsuponUVexposure(evenat suberythmallevels) contributes at largetoward degradation of elastic fiberresulting in wrinkle formation. Topical application ofsyntheticelastaseinhibitor(N -phenethylphosphonyl-L-leucyl-L-tryptophane) inhairless mouseinduced withwrinklesbyUV irradiations,hasresultedinsignificantsuppressionofwrinkle for-mation(Tsujietal.,2001).
PLEfractionatedintothreepartshavebeenstudiedforits anti-elastaseactivityanditwasdemonstratedthatfractionhavingmore flavonoidscontents(fractionII)showedbetterinhibitionofelastase thanfractionhavingmorepolyphenoliccontents(fractionIII)(An etal.,2005).Itwasconcludedthatflavonoidsmayhaveabetter activityagainstelastaseenzyme.FlavonefromtheleavesofD.kaki
haveshowntoinhibitproliferationofadventialfibroblasts stim-ulatedbyadvancedglycationend-products(Ouyangetal.,2003), andadvancedoxidationproteinproducts(Ouyangetal.,2004).PLE separatedinto threefractions (i.e.I,II,andIII) havebeen stud-iedforitsanti-collagenaseeffects.Resultsrevealedthatfraction IIIbeingmoreenrichedwithpolyphenolsshowedhigheractivity againstcollagenaseenzyme.TheactivityofPLEagainst collagen-ase enzyme wascompared with reported activitiesof soybean trypsin inhibitor(46% at 4mg/ml) and green tea extract (100% at0.2mg/ml).PurifiedfractionofPLEindicated30%inhibitionof enzymeat20ppmconcentration,whichwas,relativelyconsidered asa higherlevel ofinhibition ofcollagenaseenzyme (Anetal., 2005).AAbeinganimportantconstituentofpersimmonleavesand fruitextract,actsasaco-factorforprolylandlysylhydroxylaseand isindispensableforbiosynthesisofcollagen.AAalsocauses provo-cationofcollagengeneexpression.Humanskinfibroblastswhen exposedtoAAforalongerdurationinvitro,showedhigherratio ofcollagentypeIandtypeIVwithincreasedprocollagensynthesis
(Kishimotoetal.,2013).SoextractofD.kakicanbeusedin
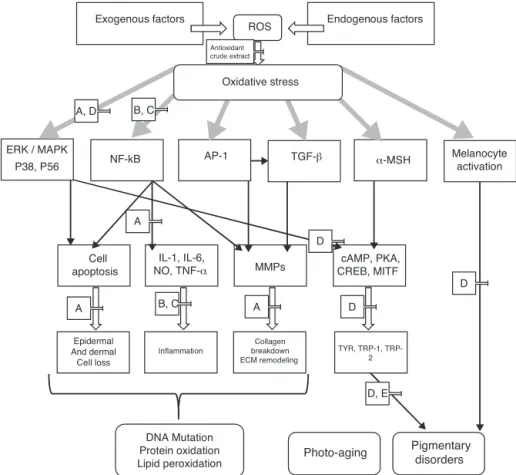
Exogenous factors Endogenous factors ROS
ERK / MAPK
P38, P56 NF-kB AP-1 TGF-β α-MSH
Melanocyte activation
Cell apoptosis
Epidermal And dermal Cell loss
IL-1, IL-6, NO, TNF-α
Inflammation
MMPs
Collagen breakdown ECM remodeling
DNA Mutation Protein oxidation
Lipid peroxidation Photo-aging cAMP, PKA, CREB, MITF
TYR, TRP-1, TRP-2
Pigmentary disorders Oxidative stress
A
B, C
Antioxidant crude extract
B, C A, D
D, E D
D
A D
A
Fig.1.PotentialusesofDiospyroskakicrudeextractanditsactiveconstituentsagainstphotoagingandpigmentarydisorders.A=phenolicacidandflavonoidsfrom persim-mon,B=coussaricacidandbetulinicacid,C=quercetin-3-O--d-(2′′-galloyl)-glucopyranosideandquercetin,D=QCGG,E=isoquercitrin,hyperin,chrysontemin,gallicacid,
rutogenicacidand2-methoxy-4-vinylphenol.
momentouslybycosmeticanddermatologicalbeneficialprofileof
D.kaki.
Potentantioxidantactivity
Skinaging beingadynamic processdependsonboth intrin-sicandextrinsicfactors,resultinginvariousskinchangesatboth estheticandfunctionallevels.Twodistinctmechanismofskinaging arechronologicalaging(determinedgenetically)andphotoaging due to repeated exposureto UV light resulting in microscopic changesinstratumcorneum(Gaoetal.,2010;Rabeetal.,2006). UVradiationsresultsin generation ofROS leading tooxidative damageandoxidativeproductswhichareindicatorsofoxidative stress(Xuetal.,2012).SkindamagecausedbyROSisthemajor factordrivingtowardphotoaging.Skin,actingasaphysical bar-rierbetweeninternalbodyandenvironment,isalsoamajortarget foroxidativestress.Itcontainsnumerousbiochemicalmolecules whicharepronetooxidativedamageinducedbyROS,including lipids,proteins,carbohydrates,andDNA.UVradiationexposureis amajorcontributoryfactorinphotoaging,sopreventivestrategies mayincludeavoidingsunlightexposureorbymaintainingcellular redoxbalancecausedbyUVradiations.Inbothcases,i.e. chrono-logicalagingorphotoaging,utilizationofdifferentantioxidantsin variousskincareproductshasproducedpromisingresult.Fig.1
showspotentialusesandmolecularmechanismofsomeselected valuablephytoconstituentsfromD.kaki.
Shieldingffectofantioxidantsagainstdifferentskinconditions has gained sufficient interest in cosmetics and dermatological practice(Parveenetal.,2014).Recently,manystudieshavebeen
conductedutilizingdifferentbotanicalextractswithproved antiox-idantactivitiesinrejuvenatingskincondition,likeskinmechanical characteristics,skinroughness,smoothness, scaliness, elasticity, andanti-agingeffects(MahmoodandAkhtar,2013;Khanetal.,
2015;Mohsinetal.,2016).Manynaturalbioactivesubstanceswith
Food,especiallyfruitsareamajorsourceofantioxidantsforthe body.Persimmonfruitsisenrichedwithmanyantioxidants includ-ingpolyphenols,phenolicacids,flavonoids,carotenoids,tannins, proanthocyanidins,catechin,vitaminsandothers(see phytochem-icalsection).Manyreportshavebeenpublishedindicatingpotent radicalscavengingactivityofcrudeextractsandtheirpurified frac-tionfromdifferentpartsofD.kaki,andtheireffectsondifferent biologicalfunctionshavewellbeenestablished(Hanetal.,2002;
Chenetal.,2008;Fukaietal.,2009;Sunetal.,2011;Leeetal.,2012).
TheantioxidantsobtainedfromD.kakihavecapabilityof scaveng-ingROS,hydroxylionradicals,superoxideradicals,peroxylradicals, singletmolecularoxygenspeciesandshowsmetalchelating
activ-ity(Fukaietal.,2009;Sunetal.,2011).Flavonoidsfromleavescan
increaselevelsofcatalase,superoxidedismutase,andglutathione peroxidaseinamannerbetterthanrutin.Totalantioxidantactivity andtotalphenoliccontentsinpersimmonweresignificantlyhigher thanthatofapple,grapesandtomato(Chenetal.,2008).Somemost importantantioxidantsthatcanserveasacandidatefordifferent cosmeticsanddermatologicpreparationsareenlistedinBox1.
Toxicologicalandsafetyaspect
Sincemorethanacentury,notoxicitycasehasbeenreportedfor persimmonleavesconsumedincrudeformasapartoftraditional medicineorbotanicalextractmadethereof.Moderntoxicological evaluationofleavesdidnotshowedanytoxiceffects.AqueousPLE wasadministeredtobothmaleandfemalemiceinanacute tox-icitytestandLD50 wasfoundtobehigherthan21.5g/kg(equal
to597.2g/kgascrudesubstance),indicatingnon-toxicnatureof extract.Micronucleustest(MNT)conductedinmousebone mar-rowusingaqueousPLE(10g/kg),hasrevealedanormaldeclinein theratioofpolychromaticerythrocytes/normochromatic erythro-cytes(PCE/NCE)ascomparedwithcyclophosphamide(20mg/kg) representingnonmutagenicnatureoftheextractinsomaticcells. ThePLEatconcentrationof10g/kgdidnotshowanysperm malfor-mationtendency(Wuetal.,2012).Inanotherstudy,ethanolicPLE wasadministeredorallyin100rats,atconcentrationof0.5,1.0,3.0, and6.4g/kgfor90days(Chenetal.,2005).Alterationsin physiolog-icalandhematologicparametersamongthecontrolandtestsubject werefoundtobeinsignificant.ItwasconcludedthatethanolicPLE atdoseof6.4g/kgdidnotproduceanymaternal,embryonicand teratogenictoxicityinstudiedsubjects.
Despite of the routine worldwide consumption of nutrient enrichedpersimmonfruits,itmaysometimepresentamoderately complicatedmedicalconditioncalleddiospyrobezoarwhichmay resultinsmallbowland/orileusobstruction(deGrootandPuylaert,
2008;de Toledoetal., 2012).Treatment modalitiesfor
diospy-robezoarresultedfromoverconsumptionofpersimmonfruits,may includeendoscopicremoval.Lasermediatedpulverization, shock-wavelithotripsy,orchemicaldissolutionbycelluloseorCoca-Cola
(DolanandThompson,1979;Chungetal.,2006;Qinetal.,2014).
Persimmonpeelextractedfractionswithvarioussolventsviz., hexane,acetone,MeOH,and70%ethanolwereevaluatedfortheir cytotoxicpotentialin two humanoral tumorcelllines (HSG-2, HSG)andonehumangingivalfibroblast(HGF)utilizing microcul-tureplatesstainedwithmethyltetrazolium(MTT)assay(Kawase et al.,2003).Twofractions of acetoneextracts showedhighest cytotoxicityin both tumorcell lines(HSG-2, HSG), and normal fibroblast(HGF)fromall23extractandfractions.AqueousPLEhas demonstratedoutstandingcytotoxicactivityagainstbrainshrimps nauplii(Artemiasalina).Thiscytotoxicityactivity(at10ppm)was comparabletothatofstandarddrugEtoposide(Nisaretal.,2015). EthylacetatePLEandvariousseparatedchemicalcompoundswere evaluatedfortheircytotoxicactivityagainstvarious cancercell linessuchasA549,HepG2andHT29.Compound2,3,and4showed
cytotoxicityagainstthesecancerouscelllineswithIC50valuesin
rangeof9.3–21.1mM(Chenetal.,2007).
Theavailableliteraturedidnotrevealedanytoxicactivitiesin PLE,whichimplicatesareliablesafetyincommonuse.However, afurtherresearchisobligatorytoevaluatemorepurifiedfractions ofvarioussolventextractstostrengthentheavailableliterature. Furthermore,variouscrudeandpurifiedextractshouldbe evalu-atedthoroughlyfortheirsuitability,safetyandtoxicitydatabefore ingestion and or topical administration. For example, Butchard patch(skinirritancy)test(Mahmoodand Akhtar,2013;Mohsin etal.,2016)canbeconductedforanytopicalformulationloaded withD.kakiphytoconstituentsintendedtobeusedbyhuman vol-unteers.
Futureperspectives
Based onliteraturesurveyit isevident thatvarious partsof
D. kakiare enrichedwithvaluable phytoconstituentsand hasa great potentialfor itsutilizationin cosmeticindustry and vari-ousskindisorders. Mostof thedatapresentedin this articleis based on either in-vitro analysis or in-vivo animal model test-ing. Thereis driving thrust toevaluatethis valuable plantand itsactive constituentsincosmetics and dermatological applica-tionsafterestablishingscientificvalidation,safetyandefficacyby usingdifferentnon-invasivein-vivoevaluationtechniques.Because oflowerriskprofileinvolved inusinghumanvolunteersin der-matologic/andorcosmeticevaluation,topicalformulationcanbe formulatedandsubjectedtoin-vivostudiesindiseasedorhealthy humanvolunteerstoestablishclinicalrelevancy.Further purifica-tion,identificationandstandardizationofactiveprinciplesfrom fruits,leavesandstemcanbecarriedoutforobtainingoptimum benefitsfromthisvaluableGodgiftedplant.Loadingtheactive prin-ciplesseparatedfromD.kakiintodifferenttopicalformulationsviz., emulsions,gels,emulgels,creamsandotherbeautycareproducts canbeofvaluetoestablishrelevancybetweenin-vitrodataand in-vivooutcomefortheirbeneficialeffectsonhumanskinandesthetic parameters.Topical formulationloadedwithcrude andpurified extract can besubjected toin-vivo evaluation and theireffects canbeevaluatedonvariousskinparameterssuchastopographic changes,wrinkleassessment,skinhydrationlevels,numberand sizeofskinpores.Despiteofitsutilizationasavaluabletraditional medicinesindifferentAsiancountriesformorethan100years,it cansafelybeforecastedthatstilloptimumtherapeuticbenefitsof
D.kakihavenotfullybeenexplored.Specifically,theactive princi-plesfromroot,barks,stem,flowerandaerialpartsofthetreeare stilltobeevaluatedfortheirpossibleinvolvementincosmeticand dermatologicbenefits.Cosmeceuticalandcommercialinterestis gainingmorepopularityforusingthisplantasasourceofvaluable ingredientsforcurativeandbeautifyingpurposesinvariousskin disorders.
Conclusion
improveskinconditionbyreversingsignsofphoto-agedskin, pro-ducingskinlighteningeffectsbyreducingmelaninlevelsandmay helptoreducesebumproduction.
Authorscontributions
Alltheauthorshavecontributedequallytowardcompletionand contentsofthisreviewarticlearewellunderstoodbythem.
Conflictsofinterest
This statement is to certify that all Authors have seen and approved the manuscript. We warrant that the article is the Authors’originalworkanddeclarenoconflictofinterest.
References
Akagi,T.,Katayama-Ikegami,A.,Yonemori,K.,2011.Proanthocyanidin biosynthe-sisofpersimmon(DiospyroskakiThunb.)fruit.Sci.Hortic.Amsterdam130, 373–380.
Akiyama,H.,Fujii,K.,Yamasaki,O.,Oono,T.,Iwatsuki,K.,2001.Antibacterialaction ofseveraltanninsagainstStaphylococcusaureus.J.Antimicrob.Chemother.48, 487–491.
An,B.J.,Kwak,J.H.,Park,J.M.,Lee,J.Y.,Park,T.S.,Lee,J.T.,Son,J.H.,Jo,C.,Byun,M.W., 2005.Inhibitionofenzymeactivitiesandtheantiwrinkleeffectof polyphe-nolisolatedfromthepersimmonleaf(Diospyroskakifolium)onhumanskin. Dermatol.Surg.31,848–854.
Antonicelli,F.,Bellon,G.,Lorimier,S.,Hornebeck,W.,2009.Roleoftheelastin receptorcomplex(S-Gal/Cath-A/Neu-1)inskinrepairandregeneration.Wound RepairRegen.17,631–638.
Anunciato,T.P.,daRochaFilho,P.A.,2012.Carotenoidsandpolyphenolsin nutricos-metics,nutraceuticals,andcosmeceuticals.J.Cosmet.Dermatol.11,51–54. Ashry,O.M.,Hussein,E.M.,AbdEl-Azime,A.S.,2016.Restorativeroleofpersimmon
leaf(Diospyroskaki)togammairradiation-inducedoxidativestressandtissue injuryinrats.Int.J.Radiat.Biol.,1–6.
Basal,E.,Jain,A.,Kaushal,G.P.,2004.Antibodyresponsetocrudecelllysateof Pro-pionibacteriumacnesandinductionofpro-inflammatorycytokinesinpatients withacneandnormalhealthysubjects.J.Microbiol.42,117–125.
Bei,W.,Peng,W.,Ma,Y.,Xu,A.,2005.FlavonoidsfromtheleavesofDiospyros kakireducehydrogenperoxide-inducedinjuryofNG108-15cells.LifeSci.76, 1975–1988.
Bei,W.,Zang,L.,Guo,J.,Peng,W.,Xu,A.,Good,D.A.,Hu,Y.,Wu,W.,Hu,D.,Zhu,X., Wei,M.,Li,C.,2009.Neuroprotectiveeffectsofastandardizedflavonoidextract fromDiospyroskakileaves.J.Ethnopharmacol.126,134–142.
Benavente-Garcia,O.,Castillo,J.,2008.Updateonusesandpropertiesofcitrus flavonoids:newfindingsinanticancer,cardiovascular,andanti-inflammatory activity.J.Agric.FoodChem.56,6185–6205.
Bergler-Czop, B., Brzezinska-Wcislo, L., 2014. Pro-inflammatory cytokines in patientswithvariouskindsofacnetreatedwithisotretinoin.Postep.Dermatol. Alergol.31,21–28.
Bickers,D.R.,Athar,M.,2006.Oxidativestressinthepathogenesisofskindisease.J. Invest.Dermatol.126,2565–2575.
Busca,R.,Ballotti,R.,2000.CyclicAMPakeymessengerintheregulationofskin pigmentation.Pigment.CellRes.13,60–69.
Cathcart3rd,R.F.,1985.VitaminC:thenontoxic,nonrate-limited,antioxidantfree radicalscavenger.Med.Hypotheses18,61–77.
Chang,H.P.,Sheen,L.Y.,Lei,Y.P.,2015.Theprotectiveroleofcarotenoidsand polyphenolsinpatientswithheadandneckcancer.J.Chin.Med.Assoc.78, 89–95.
Chen,B.F.,Huang,J.M.,Bei,W.J.,Huang,J.K.,Bin,T.J.,2005.Studyonthesubchronic toxicityandteratogenesisofpersimmonleavesethanolextractfor90day. Tox-icology19,326–327.
Chen,G.,Lu,H.,Wang,C.,Yamashita,K.,Manabe,M.,Meng,Z.,Xu,S.,Kodama,H., 2002a.EffectoffiveflavonoidcompoundsisolatedfromleavesofDiospyros kakionstimulus-inducedsuperoxidegenerationandtyrosylphosphorylation ofproteinsinhumanneutrophils.Clin.Chim.Acta326,169–175.
Chen,G.,Lu,H.,Wang,C.,Yamashita,K.,Manabe,M.,Xu,S.,Kodama,H.,2002b. EffectoffivetriterpenoidcompoundsisolatedfromleavesofDiospyroskakion stimulus-inducedsuperoxidegenerationandtyrosylphosphorylationinhuman polymorphonuclearleukocytes.Clin.Chim.Acta320,11–16.
Chen,G.,Wang,Z.Q.,Jia,J.M.,2009.Threeminornoveltriterpenoidsfromtheleaves ofDiospyroskaki.Chem.Pharm.Bull.57,532–535.
Chen,G.,Xue,J.,Xu,S.X.,Zhang,R.Q.,2007.Chemicalconstituentsoftheleavesof Diospyroskakiandtheircytotoxiceffects.J.AsianNat.Prod.Res.9,347–353. Chen,H.,Pu,J.,Liu,D.,Yu,W.,Shao,Y.,Yang,G.,Xiang,Z.,He,N.,2016.
Anti-Inflammatoryandantinociceptivepropertiesofflavonoidsfromthefruitsof blackmulberry(MorusnigraL.).PLOSONE11,e0153080.
Chen,X.N.,Fan,J.F.,Yue,X.,Wu,X.R.,Li,L.T.,2008.Radicalscavengingactivityand phenoliccompoundsinpersimmon(DiospyroskakiL.cv.Mopan).J.FoodSci.73, C24–C28.
Cho,J.K.,Park,J.M.,Jeon,I.H.,Kim,H.S.,Jang,S.I.,2011.Effectofpersimmonleaf extractonutravioletB-inducedinflammationinHaCaTkeratinocytesandmice. J.KoreanSoc.Appl.Biol.Chem.54,583–590.
Cho,Y.H.,Kim,N.H.,Khan,I.,Yu,J.M.,Jung,H.G.,Kim,H.H.,Jang,J.Y.,Kim,H.J.,Kim,D.I., Kwak,J.H.,Kang,S.C.,An,B.J.,2016.Anti-inflammatoryPotentialof Quercetin-3-O-beta-d-(2′′-galloyl)-glucopyranosideandquercetinisolatedfromDiospyros kakicalyxviasuppressionofMAPsignalingmoleculesinLPS-inducedRAW264.7 macrophages.J.FoodSci.81,C2447–C2456.
Chung,Y.,Han,D.,Park,Y.,Son,B.,Paik,C.,Jeon,Y.,Sohn,J.,2006.Hugegastric diospyrobezoarssuccessfullytreatedbyoralintakeandendoscopicinjectionof Coca-Cola.Dig.LiverDis.38,515–517.
Coelho,S.G.,Zmudzka,B.Z.,Yin,L.,Miller,S.A.,Yamaguchi,Y.,Tadokoro,T.,Hearing, V.J.,Beer,J.Z.,2013.Non-invasivediffusereflectancemeasurementsof cuta-neousmelanincontentcanpredicthumansensitivitytoultravioletradiation. Exp.Dermatol.22,266–271.
Cooper,S.J.,Bowden,G.T.,2007.UltravioletBregulationoftranscriptionfactor fam-ilies:rolesofnuclearfactor-kappaB(NF-kappaB)andactivatorprotein-1(AP-1) inUVB-inducedskincarcinogenesis.Curr.CancerDrugTargets7,325–334. Crisan,D.,Roman,I.,Crisan,M.,Scharffetter-Kochanek,K.,Badea,R.,2015.Therole
ofvitaminCinpushingbacktheboundariesofskinaging:anultrasonographic approach.Clin.Cosmet.Invest.Dermatol.8,463–470.
deGroot,B.,Puylaert,J.B.,2008.Diospyrobezoar:anuncommoncauseofobstructive ileus.Int.J.Emerg.Med.1,333–334.
deToledo,A.P.,Rodrigues,F.H.,Rodrigues,M.R.,Sato,D.T.,Nonose,R.,Nascimento, E.F.,Martinez,C.A.,2012.Diospyrobezoarasacauseofsmallbowelobstruction. CaseRep.Gastroenterol.6,596–603.
DelBubba,M.,Giordani,E.,Pippucci,L.,Cincinelli,A.,Checchini,L.,Galvan,P.,2009. Changesintannins,ascorbicacidandsugarcontentinastringentpersimmons duringon-treegrowthandripeningandinresponsetodifferentpostharvest treatments.J.FoodCompos.Anal.22,668–677.
Dolan,P.A.,Thompson,B.W.,1979.Managementofpersimmonbezoars (diospy-robezoars).South.Med.J.72,1527–1528,1531.
Domingo,D.S.,Camouse,M.M.,Hsia,A.H.,Matsui,M.,Maes,D.,Ward,N.L.,Cooper, K.D.,Baron,E.D.,2010.Anti-angiogeniceffectsofepigallocatechin-3-gallatein humanskin.Int.J.Clin.Exp.Pathol.3,705–709.
Erb,C.,Nau-Staudt,K.,Flammer,J.,Nau,W.,2004.Ascorbicacidasafreeradical scavengerinporcineandbovineaqueoushumour.OphthalmicRes.36,38–42. Esteban-Pretel,G.,Marin,M.P.,Renau-Piqueras,J.,Sado,Y.,Barber,T.,Timoneda, J.,2013.VitaminAdeficiencydisturbscollagenIVandlaminincomposition anddecreases matrixmetalloproteinaseconcentrationsin ratlung.Partial reversibilitybyretinoicacid.J.Nutr.Biochem.24,137–145.
FAOSTAT,2014.http://www.fao.org/faostat/en/.
Findik,R.B.,Ilkaya,F.,Guresci,S.,Guzel,H.,Karabulut,S.,Karakaya,J.,2016.Effect ofvitaminConcollagenstructureofcardinalanduterosacralligamentsduring pregnancy.Eur.J.Obstet.Gynecol.Reprod.Biol.201,31–35.
Fölster-Holst,R.,Latussek,E.,2007.Synthetictanninsindermatology—atherapeutic optioninavarietyofpediatricdermatoses.Pediatr.Dermatol.24,296–301. Fu,R.,Zhang,Y.,Peng,T.,Guo,Y.,Chen,F.,2015.Phenoliccompositionandeffects
onallergiccontactdermatitisofphenolicextractsSapiumsebiferum(L.)Roxb. leaves.J.Ethnopharmacol.162,176–180.
Fukai,S.,Tanimoto,S.,Maeda,A.,Fukuda,H.,Okada,Y.,Nomura,M.,2009. Pharma-cologicalactivityofcompoundsextractedfrompersimmonpeel(Diospyroskaki Thunb.).J.OleoSci.58,213–219.
Galibert,M.D.,Carreira,S.,Goding,C.R.,2001.TheUsf-1transcriptionfactorisanovel targetforthestress-responsivep38kinaseandmediatesUV-inducedTyrosinase expression.EMBOJ.20,5022–5031.
Gao,Y.Y.,Luo,D.,Zhou,B.R.,Li,W.,Min,W.,Lin,B.J.,2010.Mechanismoftelomere shorteninginphotoagingmodelinducedby8-methoxypsoralenandultraviolet A.ZhonghuaYiXueZaShi90,1698–1702.
Giordani,E.,Doumett,S.,Nin,S.,DelBubba,M.,2011.Selectedprimaryandsecondary metabolitesinfreshpersimmon(DiospyroskakiThunb.):areviewofanalytical methodsandcurrentknowledgeoffruitcompositionandhealthbenefits.Food Res.Int.44,1752–1767.
Gu,H.-F.,Li,C.-M.,Xu,Y.-j.,Hu,W.-f.,Chen,M.-h.,Wan,Q.-h.,2008.Structural fea-turesandantioxidantactivityoftanninfrompersimmonpulp.FoodRes.Int.41, 208–217.
Han,J.,Kang,S.,Choue,R.,Kim,H.,Leem,K.,Chung,S.,Kim,C.,Chung,J.,2002. FreeradicalscavengingeffectofDiospyroskaki,LaminariajaponicaandUndaria pinnatifida.Fitoterapia73,710–712.
Hearing,V.J.,2005.Biogenesisofpigmentgranules:asensitivewaytoregulate melanocytefunction.J.Dermatol.Sci.37,3–14.
Holick,M.F.,2008.Sunlight,UV-radiation,vitaminDandskincancer:howmuch sunlightdoweneed?Adv.Exp.Med.Biol.624,1–15.
Hougee,S.,Sanders,A.,Faber,J.,Graus,Y.M.,vandenBerg,W.B.,Garssen,J.,Smit,H.F., Hoijer,M.A.,2005.Decreasedpro-inflammatorycytokineproductionby LPS-stimulatedPBMCuponinvitroincubationwiththeflavonoidsapigenin,luteolin orchrysin,duetoselectiveeliminationofmonocytes/macrophages.Biochem. Pharmacol.69,241–248.
Humbert,P.,Binda,D.,Robin,S.,Krutmann,J.,2010.Beautyfrominside: nutrition-basedstrategiesincosmeticdermatology.In:Nutr.HealthSkin.Springer,pp. 189–196.
Ikegami,A.,Eguchi,S.,Kitajima,A.,Inoue,K.,Yonemori,K.,2007.Identificationof genesinvolvedinproanthocyanidinbiosynthesisofpersimmon(Diospyroskaki) fruit.PlantSci.172,1037–1047.
mois-turizersforthecareofmildacnepatientswithsensitiveskin.J.Dermatol.42, 181–188.
Janeiro, J.B.d.R.d., 2017. Ebenaceae in Flora do Brasil 2020 em construc¸ão, http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB603406 (accessed 01.06.17).
Jariwalla,R.J.,Harakeh,S.,1996.Antiviralandimmunomodulatoryactivitiesof ascorbicacid.Sub-CellBiochem.25,213–231.
Jeon,H.Y.,Kim,J.K.,Seo,D.B.,Cho,S.Y.,Lee,S.J.,2010.Beneficialeffectofdietary epigallocatechin-3-gallateonskinviaenhancementofantioxidantcapacityin bothbloodandskin.SkinPharmacol.Phys.23,283–289.
Jung,H.G.,Kim,H.H.,Paul,S.,Jang,J.Y.,Cho,Y.H.,Kim,H.J.,Yu,J.M.,Lee,E.S.,An, B.J.,Kang,S.C.,Bang,B.H.,2015.Quercetin-3-O--d-glucopyranosyl-(1→ 6)--d-glucopyranosidesuppressesmelaninsynthesisbyaugmentingp38MAPKand CREBsignalingpathwaysandsubsequentcAMPdown-regulationinmurine melanomacells.SaudiJ.Biol.Sci.22,706–713.
Kameyama,K.,Sakai,C.,Kuge,S.,Nishiyama,S.,Tomita,Y.,Ito,S.,Wakamatsu,K., Hearing,V.J.,1995.Theexpressionoftyrosinase,tyrosinase-relatedproteins 1and2(TRP1andTRP2),thesilverprotein,andamelanogenicinhibitorin humanmelanomacellsofdifferingmelanogenicactivities.Pigment.CellRes.8, 97–104.
Kaulmann,A.,Jonville,M.C.,Schneider,Y.J.,Hoffmann,L.,Bohn,T.,2014.Carotenoids, polyphenolsandmicronutrientprofilesofBrassicaoleraceaeandplumvarieties andtheircontributiontomeasuresoftotalantioxidantcapacity.FoodChem. 155,240–250.
Kawase,M.,Motohashi,N.,Satoh,K.,Sakagami,H.,Nakashima,H.,Tani,S.,Shirataki, Y.,Kurihara,T.,Spengler,G.,Wolfard,K.,Molnar,J.,2003.Biologicalactivityof persimmon(Diospyroskaki)peelextracts.Phytother.Res.17,495–500. Khan,B.A.,Akhtar,N.,Menaa,A.,Menaa,F.,2015.AnovelCassiafistula(L.)-based
emulsionelicitsskinanti-agingbenefitsinhumans.Cosmetics2,368–383. Kim,H.H.,Kim,D.S.,Kim,S.W.,Lim,S.H.,Kim,D.K.,Shin,T.Y.,Kim,S.H.,2013.
InhibitoryeffectsofDiospyroskakiinamodelofallergicinflammation:roleof cAMP,calciumandnuclearfactor-kappaB.Int.J.Mol.Med.32,945–951. Kim,H.J.,Kim,M.K.,2003.AnticancereffectofpersimmonleafextractsonKorean
gastriccancercell.KoreanJ.Nutr.36,133–146.
Kim,K.S.,Lee,D.S.,Kim,D.C.,Yoon,C.S.,Ko,W.,Oh,H.,Kim,Y.C.,2016. Anti-inflammatoryeffectsandmechanisms ofactionofcoussaric andbetulinic acidsIsolatedfromDiospyroskakiinlipopolysaccharide-stimulatedRAW264.7 macrophages.Molecules21,http://dx.doi.org/10.3390/molecules21091206. Kishimoto,Y.,Saito,N.,Kurita,K.,Shimokado,K.,Maruyama,N.,Ishigami,A.,2013.
Ascorbicacidenhancestheexpressionoftype 1and type4collagenand SVCT2inculturedhumanskinfibroblasts.Biochem.Biophys.Res.Commun.430, 579–584.
Kitagawa,S.,Yoshii,K.,Morita,S.Y.,Teraoka,R.,2011.Efficienttopicaldeliveryof chlorogenicacidbyanoil-in-watermicroemulsiontoprotectskinagainst UV-induceddamage.Chem.Pharm.Bull.59,793–796.
Kotani,M.,Matsumoto,M.,Fujita,A.,Higa,S.,Wang,W.,Suemura,M.,Kishimoto, T.,Tanaka,T.,2000.Persimmonleafextractandastragalininhibitdevelopment ofdermatitisandIgEelevationinNC/Ngamice.J.AllergyClin.Immunol.106, 159–166.
Kumar,K.J.,Vani,M.G.,Wang,S.Y.,Liao,J.W.,Hsu,L.S.,Yang,H.L.,Hseu,Y.C.,2013. Invitroandinvivostudiesdisclosedthedepigmentingeffectsofgallicacid:a novelskinlighteningagentforhyperpigmentaryskindiseases.Biofactors39, 259–270.
Kusunoki,K.,Hara,T.,Fujita,M.,Minari,Y.,Tadokoro,T.,Innami,S.,Maekawa,A., 1998.Histochemicalobservationandcellulardistributionofascorbicacidin persimmonleaves.J.Nutr.Sci.Vitaminol.44(1),11–23.
Lee,J.H.,Lee,Y.B.,Seo,W.D.,Kang,S.T.,Lim,J.W.,Cho,K.M.,2012.Comparative studiesofantioxidantactivitiesandnutritionalconstituentsofpersimmonjuice (DiospyroskakiL.cv.Gapjubaekmok).Prev.Nutr.FoodSci.17(2),141–151. Ley,J.P.,2001.PhenolicacidamidesofphenolicbenzylaminesagainstUVA-induced
oxidativestressinskin.Int.J.Cosmet.Sci.23(1),35–48.
Li,H.R.,Habasi,M.,Xie,L.Z.,Aisa,H.A.,2014.Effectofchlorogenicacidon melano-genesisofB16melanomacells.Molecules19(9),12940–12948.
Li,S.,Zhang,Z.,Cain,A.,Wang,B.,Long,M.,Taylor,J.,2005.Antifungalactivityof camptothecin,trifolin,andhyperosideisolatedfromCamptothecaacuminata.J. Agric.FoodChem.53(1),32–37.
Lim,T.G.,Kim,J.E.,Jung,S.K.,Li,Y.,Bode,A.M.,Park,J.S.,Yeom,M.H.,Dong,Z.,Lee, K.W.,2013.MLK3isadirecttargetofbiochaninA,whichplaysaroleinsolar UV-inducedCOX-2expressioninhumankeratinocytes.Biochem.Pharmacol.86 (7),896–903.
Liu,J.B.,Ding,Y.S.,Zhang,Y.,Chen,J.B.,Cui,B.S.,Bai,J.Y.,Lin,M.B.,Hou,Q.,Zhang,P.C., Li,S.,2015.Anti-inflammatoryhydrolyzabletanninsfromMyricariabracteata.J. Nat.Prod.78(5),1015–1025.
Łopaciuk,A.,Łoboda,M.,2013.Globalbeautyindustrytrendsinthe21stcentury. In:ManagKnowlLearnInternConf,pp.19–21.
Mahmood,T.,Akhtar,N.,2013.Combinedtopicalapplicationoflotusandgreentea improvesfacialskinsurfaceparameters.RejuvenationRes.16(2),91–97. Manach,C.,Scalbert,A.,Morand,C.,Remesy,C.,Jimenez,L.,2004.Polyphenols:food
sourcesandbioavailability.Am.J.Clin.Nutr.79(5),727–747.
Martinez-LasHeras,R.,Pinazo,A.,Heredia,A.,Andres,A.,2017.Evaluationstudiesof persimmonplant(Diospyroskaki)forphysiologicalbenefitsandbioaccessibility ofantioxidantsbyinvitrosimulatedgastrointestinaldigestion.FoodChem.214, 478–485.
MiraiClinical,2017.www.miraiclinical.com/all-products(accessed12.02.17). Miyachi,Y.,1995.Photoagingfromanoxidativestandpoint.J.Dermatol.Sci.9(2),
79–86.
Mohsin,S.,Akhtar,N.,Mahmood,T.,Khan,H.,Mustafa,R.,2016.Formulationand stabilityoftopicalwaterinoilemulsioncontainingcornsilkextract.Trop.J. Pharm.Res.15(6),1115–1121.
Mota,M.L.,Thomas,G.,BarbosaFilho,J.M.,1985.Anti-inflammatoryactionsof tan-ninsisolatedfromthebarkofAnacardiumoccidentaleL.J.Ethnopharmacol.13 (3),289–300.
Mukherjee,P.K.,Maity,N.,Nema,N.K.,Sarkar,B.K.,2011.Bioactivecompoundsfrom naturalresourcesagainstskinaging.Phytomedicine19(1),64–73.
Muthusamy,V.,Piva,T.J.,2010.TheUVresponseoftheskin:areviewoftheMAPK, NFkappaBandTNFalphasignaltransductionpathways.Arch.Dermatol.Res.302 (1),5–17.
Nisar,M.,Shah,S.M.,Khan,I.,Sheema,Sadiq,A.,Khan,S.,Shah,S.M.,2015.Larvicidal, insecticidal,brineshrimpcytotoxicityandanti-oxidantactivitiesofDiospyros kaki(L.)reportedfromPakistan.Pak.J.Pharm.Sci.28(4),1239–1243. Nishigori,C.,2006.Cellularaspectsofphotocarcinogenesis.Photochem.Photobiol.
Sci.5(2),208–214.
Ohguchi,K.,Nakajima,C.,Oyama,M.,Iinuma,M.,Itoh,T.,Akao,Y.,Nozawa,Y.,Ito,M., 2010.InhibitoryeffectsofflavonoidglycosidesisolatedfromthepeelofJapanese persimmon(Diospyroskaki‘Fuyu’)onmelaninbiosynthesis.Biol.Pharm.Bull. 33(1),122–124.
Ouyang,P.,Bei,W.J.,Lai,W.Y.,Xu,D.L.,Peng,W.L.,2003.Effectsofflavonefromleaves ofDiospyroskakionadventitialfibroblastproliferationinducedbyadvanced glycationend-productsinvitro.Di1junyidaxuexuebao23(12),1260–1262. Ouyang,P.,Liu,S.,Bei,W.,Lai,W.,Hou,F.,Xu,A.,2004.Effectsofflavonefromleaves ofDiospyroskakionadventitialfibroblastsproliferationbyadvancedoxidation proteinproductsinvitro.ZhongyaocaiZhongyaocai27(3),186–188. Oxlund,H.,Manschot,J.,Viidik,A.,1988.Theroleofelastininthemechanical
prop-ertiesofskin.J.Biomech.21(3),213–218.
Özen,A.,Colak,A.,Dincer,B.,Güner,S.,2004.Adiphenolasefrompersimmonfruits (DiospyroskakiL.,Ebenaceae).FoodChem.85(3),431–437.
Park,M.-H.,2000.Effectofpolyphenolcompoundsfrompersimmonleaves( Diospy-roskakifolium)onallergiccontactdermatitis.J.KoreanSoc.FoodSci.Nutr. Parveen,R.,Akhtar,N.,Mahmood,T.,2014.TopicalmicroemulsioncontainingPunica
granatumextract:itscontroloverskinerythemaandmelanininhealthyAsian subjects.Adv.Dermatol.Allergol.31(6),351.
Piretti,M.,1991.PolyphenolconstituentsoftheDiospyroskakifruit.Areview. Fitoterapia62,3–13.
Prater,M.R., Blaylock,B.L.,Holladay,S.D., 2003.Molecularmechanismsof cis-urocanic acidandpermethrin-induced alterations incutaneousimmunity. Photodermatol.Photoimmunol.Photomed.19(6),287–294.
Qin,B.,Wan,X.L.,Guo,X.Y.,Dong,L.,2014.Successfulendoscopictreatmentofan intestinaldiospyrobezoarmigratedfromthestomach.BMJCaseRep.2014. Rabe,J.H.,Mamelak,A.J.,McElgunn,P.J.,Morison,W.L.,Sauder,D.N.,2006.
Photoag-ing:mechanismsandrepair.J.Am.Acad.Dermatol.55(1),1–19.
Rebec,G.V.,Pierce,R.C.,1994.Avitaminasneuromodulator:ascorbatereleaseinto theextracellularfluidofthebrainregulatesdopaminergicandglutamatergic transmission.Prog.Neurobiol.43(6),537–565.
Rivas,M.,Rojas,E.,Araya,M.C.,Calaf,G.M.,2015.Ultravioletlightexposure,skin cancerriskandvitaminDproduction.Oncol.Lett.10(4),2259–2264. Roach,H.I.,Hillier,K.,Shearer,J.R.,1985.Ascorbicacidrequirementsforcollagen
synthesis(prolinehydroxylation)duringlong-termcultureofembryonicchick femurs.Biochim.Biophys.Acta842(2–3),139–145.
Robbins,R.J.,2003.Phenolicacidsinfoods:anoverviewofanalyticalmethodology. J.Agric.FoodChem.51(10),2866–2887.
Rogerio, A.P., Kanashiro,A., Fontanari, C.,daSilva, E.V.,Lucisano-Valim, Y.M., Soares,E.G.,Faccioli,L.H.,2007.Anti-inflammatoryactivityofquercetinand isoquercitrininexperimentalmurineallergicasthma.Inflamm.Res.56(10), 402–408.
Shapiro,S.S., Saliou,C.,2001.Roleofvitaminsinskincare.Nutrition17(10), 839–844.
Shin,S.,Son,D.,Kim,M.,Lee,S.,Roh,K.-B.,Ryu,D.,Lee,J.,Jung,E.,Park,D.,2015. AmelioratingeffectofAkebiaquinatafruitextractsonskinaginginducedby advancedglycationendproducts.Nutrients7(11),9337–9352.
Sim,G.S.,Lee,B.C.,Cho,H.S.,Lee,J.W.,Kim,J.H.,Lee,D.H.,Kim,J.H.,Pyo,H.B.,Moon, D.C.,Oh,K.W.,Yun,Y.P.,Hong,J.T.,2007.Structureactivityrelationshipof antiox-idativepropertyofflavonoidsandinhibitoryeffectonmatrixmetalloproteinase activityinUVA-irradiatedhumandermalfibroblast.Arch.Pharmacol.Res.30 (3),290–298.
Simon,J.C.,Edelbaum,D.,Bergstresser,P.R.,CruzJr.,P.D.,1991.Distorted antigen-presentingfunctionofLangerhanscellsinducedbytumornecrosisfactoralpha viaamechanismthatappearsdifferentfromthatinducedbyultravioletB radi-ation.Photodermatol.Photoimmunol.Photomed.8(5),190–194.
Skabytska,Y.,Kaesler,S.,Volz,T.,Biedermann,T.,2016.Howtheinnateimmune systemtrainsimmunity:lessonsfromstudyingatopicdermatitisandcutaneous bacteria.J.Deutsch.Dermatol.Ges.14(2),153–156.
Sklar,L.R.,Almutawa,F.,Lim,H.W.,Hamzavi,I.,2013.Effectsofultravioletradiation, visiblelight,andinfraredradiationonerythemaandpigmentation:areview. Photochem.Photobiol.Sci.12(1),54–64.
Smith,W.P.,1999.Theeffectsoftopicall(+)lacticacidandascorbicacidonskin whitening.Int.J.Cosmet.sci.21(1),33–40.
Sun,L.,Zhang,J.,Fang,K.,Ding,Y.,Zhang,L.,Zhang,Y.,2014.Flavonoidsfrom persimmon(Diospyroskaki)leaves(FPL)attenuateH2O2-inducedapoptosisin MC3T3-E1cellsviatheNF-kappaBpathway.FoodFunct.5(3),471–479. Sun,L.,Zhang,J.,Lu,X.,Zhang,L.,Zhang,Y.,2011.Evaluationtotheantioxidant