ABSTRACT
I N TROD UCTI ON
A st u d y m od el is a p r ocess t h at sim u lat es som e r eal- w or ld ph en om en on of in t er est , t h u s allow in g t h e r esear ch er t o d er iv e in f or m at ion about t his phenom enon73. Despit e a plet hora of
in sit u st udies and clinical t r ials, in vit r o m odels
ar e t h e m ost com m on ly em ploy ed m et h ods in
p H- cy clin g m od els f or in v it r o ev alu at ion of
the eficacy of luoridated dentifrices for caries
cont r ol: st r engt hs and lim it at ions
Marília Afonso Rabelo BUZALAF1, Angélica Reis HANNAS2, Ana Carolina MAGALHÃES3, Daniela RIOS4, Heitor Marques HONÓRIO5, Alberto Carlos Botazzo DELBEM6
1- DDS, MS, PhD, Full Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil. 2- DDS, MS, PhD, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil.
3- DDS, MS, PhD, Assistant Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil.
4- DDS, MS, PhD, Assistant Professor, Department of Pediatric Dentistry, Orthodontics and Community Health, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil.
5- DDS, MS, PhD, Associate Professor, Department of Pediatric Dentistry, Alfenas Federal University, Alfenas, MG, Brazil.
6- DDS, MS, PhD, Associate Professor, Department of Pediatric Dentistry, Araçatuba Dental School, Paulista State University, Araçatuba, SP, Brazil.
Corresponding address: Profa. Dra. Marília Afonso Rabelo Buzalaf - Faculdade de Odontologia de Bauru - USP - Departamento de Ciências Biológicas - Al. Octávio Pinheiro Brisolla, 9-75 Bauru-SP, 17012-901 Brazil - Phone + 55 14 32358346 - Fax + 55 14 32271486 - e-mail: mbuzalaf@fob.usp.br
Received: June 25, 2009 - Modiication: March 17, 2010 - Accepted: March 30, 2010
D
espite a plethora of in situ studies and clinical trials evaluating the eficacy of luoridated dentifrices on caries control, in vitro pH cycling models are still broadly used because they mimic the dynamics of mineral loss and gain involved in caries formation. This paper critically reviews the current literature on existing pH-cycling models for the in vitro evaluation of the eficacy of luoridated dentifrices for caries control, focusing on their strengths and limitations. A search was undertaken in the MEDLINE electronic journal database using the keywords “pH-cycling”, “demineralization”, “remineralization”, “invitro”, “luoride”, “dentifrice”. The primary outcome was the decrease of demineralization or the increase
of remineralization as measured by different methods (e.g.: transverse microradiography) or tooth luoride
uptake. Inclusion of studies, data extraction and quality assessment were undertaken independently and in duplicate by two members of the review team. Disagreements were solved by discussion and consensus or by a third party. One hundred and sixteen studies were included, of which 42 addressed
speciically the comparison of dentifrices using different pH-cycling models. The other studies included meta-analysis or reviews, data about the effect of different luoride sources on de-remineralization,
different methods for analysis de-remineralization and chemical variables and characteristics of dental
hard tissues that might have inluence on de-remineralization processes. Generally, the studies presented
ability to detect known results established by clinical trials, to demonstrate dose-related responses in
the luoride content of the dentifrices, and to provide repeatability and reproducibility between tests. In
order to accomplish these features satisfactorily, it is mandatory to take into account the type of substrate
and baseline artiicial lesion, as well as the adequate response variables and statistical approaches
to be used. This critical review of literature showed that the currently available pH-cycling models are
appropriate to detect dose-response and pH-response of luoride dentifrices, and to evaluate the impact of new active principles on the effect of luoridated dentifrices, as well as their association with other
anti-caries treatments.
Key words: Dental caries. Dentifrices. Eficacy. Fluorides. In vitro.
the high level of scientiic control and the resulting low er var iabilit y int r insic t o in vit r o m odels, as w ell as t he sm aller sam ple size r equir ed. Addit ionally, t he r esponse var iables t hat can be em ployed in pH- cycling m odels ar e m or e sensit ive t han t hose available for use in t he clinical sit uat ion. Due t o t hese k ey advant ages, pH- cy cling m odels hav e helped im pr oving t he under st anding of t he car ies pr ocess and t he possible m echanism s by w hich luoride exerts its anti-caries effect. Furthermore, they are broadly used in proile studies for rapid and inexpensive t est ing of developing and r ecent ly m ar k et ed pr oduct s1 0 6 , 1 1 6. The r ole of pH- cy cling m odels is t her efor e t o facilit at e t he generat ion of suficient quantitative data to give investigators the conidence to appropriately design clinical trials18.
How ev er, p H- cy clin g m od els as all in v it r o p r ot ocols h av e im p or t an t lim it at ion s:
(1)
t h ey ar e unable t o com plet ely sim ulat e t he com plex int raoral condit ions leading t o car ies developm ent , even when “artiicial mouth” systems, bacterial bioilms and saliva are employed. This is particularly relevant for testing luoridated dentifrices with monoluorophosphate (MFP), since the enzyme syst em s r equir ed for MFP hydr olysis ar e pr esent in saliva and plaque in vivo, but ar e absent in m ostin vit r o t est m et hods; ( 2) t hey cannot m im ic solid
surface area/solution ratios or the saliva/plaque luid com posit ion encount er ed in vivo107, since differ ent oral sur faces ar e bat hed in differ ent volum es and source com binat ions of saliva, ( 3) t here are art ifact s associat ed w it h t he choice of subst rat e and t est condit ions, par t icular ly t he t im e per iods of de- and r em ineralizat ion, w hich ar e m uch fast er t han t hose expect ed t o occur in in vivo condit ions106; and ( 4) t hey ar e not able t o adequat ely sim ulat e t opical use and clearance of pr oduct s fr om t he oral cavit y. While dent ifr ices ar e t ypically slur r ied t o sim ulat e dilut ion dur ing br ushing, t he upt ake and r eact ivit y of luoride are consistently lower in vivo t han in vit r o, w hich m ay r esult in inaccurat e assessm ent s
of t he ant i- car ies pot ent ial of for m ulat ions dir ect ed toward enhancement of luoride delivery107. All t hese lim it at ions m ust be kept in m ind w hen dat a fr om pH- cycling st udies ar e int ended t o be ext rapolat ed for t he clinical sit uat ions.
This paper crit ically reviews t he current lit erat ure on ex ist in g p H- cy clin g m od els f or t h e in v it r o evaluation of the eficacy of luoridated dentifrices f or car ies con t r ol, f ocu sin g on t h eir st r en g t h s an d lim it at ion s. Addit ion ally, t h e im pact of t h e characteristics of previous artiicial caries lesions on subsequent de- r em ineralizat ion, t he r esponse var iables usually chosen, and t he Am er ican Dent al Associat ion ( ADA) gu idelin es1 , 2 f or t h is k in d of t est s ar e con sider ed. Fin ally, st u dies inv olv in g d i f f er en t p H- cy cl i n g p r o t o co l s ar e co m p ar ed , considering separately those focusing on luoride
dose- r esponse or pH- r esponse of t he dent ifr ices, type of luoride compound present in the dentifrice, t he im pact of new act ive pr inciples on t he effect of luoridated dentifrices, as well as their association w it h ot her ant i- car ies t r eat m ent s. The validit y of t he pH- cycling pr ot ocols is discussed in t he light of dat a fr om clinical st udies, w henever possible. Based on t he cur r ent ly available lit erat ur e, fut ur e p er sp ect i v es i n p H- cy cl i n g p r o t o co l s ar e al so included in t he discussion.
M ETH OD S
A sear ch w as u n d er t ak en i n t h e MED LI NE elect ronic j ournal dat abase using t he keywords “ pH-cycling”, “ dem ineralizat ion”, “ r em ineralizat ion”, “ in
vit r o”, “luoride”, “dentifrice”. The primary outcome
was t he decrease of dem ineralizat ion or t he increase o f r em i n er al i zat i o n as m easu r ed b y d i f f er en t m et hods ( e.g.: m icr ohar dness, m icr oradiography, polarized light microscopy) or tooth luoride uptake. One hundred and sixt een papers referring t o t he follow ing issues w er e r et r ieved: 1) t he com par ison of dent ifr ices using differ ent pH- cycling m odels; 2) m et a- analy sis/ r ev iew s about m et hods or t he effect of luoride on dental de-remineralization; 3) the effect of different luoride sources on de-r em in ede-r alizat ion in v it de-r o; 4 ) dif f ede-r en t m et h ods t o analysis de- r em ineralizat ion and; 5) chem ical var iables and charact er ist ics of dent al har d t issues that might have inluence on de-remineralization pr ocess.
I nclusion of st udies, dat a ext ract ion and qualit y assessm ent w er e under t aken independent ly and in duplicat e by t w o m em ber s of t he r eview t eam . Disagr eem ent s w er e r esolved by discussion and consensus or by a t hir d par t y.
RESULTS AN D LI TERATURE REV I EW
The genesis of m oder n pH- cycling m odels was pr oduced by t en Cat e and Duij st er s87 ( 1982) . I n typical pH-cycling studies for testing the eficacy of luoridated dentifrices, a dental substrate (enamel or dent in, fr om per m anent or pr im ar y t eet h, fr om hum an or bovine or igin) is sequent ially exposed t o dem ineralizing and r em ineralizing solut ions w it h int er m ediar y t r eat m ent s w it h t he dent ifr ices.
I n v i t r o p H- cy cl i n g m o d e l s ca n g e n e r a l l y
fr om t he r em ineralizing solut ion. Dem ineralizing models can also employ substrates with artiicial car ies lesion t o m easu r e t h e ex t en t of f u r t h er dem ineralizat ion. I n r em ineralizing m odels, dent al substrates with artiicial caries lesions are used an d t h e r esp o n se v ar i ab l es w i l l m easu r e t h e m ineral gain in t he lesions as a consequence of t he t r eat m ent w it h t he dent ifr ices.
Som e im por t ant m et hodological aspect s have been found in t he lit erat ur e r eview. They w ill be pr esent ed and discussed below in or der t o m ake easier t h e ch oice f or appr opr iat e pr ot ocols f or t h e in com in g st u d ies as w ell as t h e ad eq u at e int er pr et at ion of t he r esult s of exist ing publicat ions b y t h e r ead er s. Fin ally, t h e d at a f r om st u d ies ab ou t t h e ef f ect of f lu or id e d en t if r ices on d e-r em inee-ralizat ion w ill be pe-r esent ed in t ables and discussed in t he t ext . The fut ur e per spect ives for st udies using pH- cycling as an in vit r o m odel w ill also be addr essed.
Ty pe of de n t a l su bst r a t e s t h a t ca n be u se d in pH - cy clin g m ode ls
Excellent r eview s t o guide t he choice of dent al subst rat es for in v it r o and in sit u st udies hav e been pr esent ed by Mellber g60 ( 1992) and Ogaar d an d Rolla6 8 ( 1 9 9 2 ) . Regar din g t h e or igin of t h e subst rat es, hum an t eet h can be r egar ded as t he m ost appr opr iat e sour ce fr om t he per spect ive of clinical r elevance. How ever, t heir com posit ion is variable, due to genetic inluences, environmental conditions (diet, luoride exposure, previous caries challenge) and age ( post - er upt ive m at urat ion and dent in scler osis) . These differ ences lead t o lar ge variat ions in t heir response under acidic challenges.
Am o n g t h e d i f f e r e n t t y p e s o f h u m a n t e e t h , perm anent m olars and prem olars are t he m ost oft en em ployed t eet h. Prim ary t eet h are only occasionally used because it is dificult to obtain them and they have a sm all surface for experim ent al m anipulat ion. When pr im ar y t eet h ar e used, it m ust be t aken int o account t hat t he pr ogr ession of lesions in vit r o is fast er t han w ould occur for per m anent t eet h114. Regar ding t he age, im pact ed or par t ially er upt ed t eet h ar e m or e por ous t han t eet h t hat have been exposed t o t he oral cavit y for a longer t im e and car ies pr ogr ession should be expect ed t o occur in a fast er rat e10. I n addit ion, dent in scler osis, w hich occurs wit h age, can alt er t he dent in suscept ibilit y t o caries developm ent since a gradual m ineralizat ion of t he per it ubular dent in occur s, event ually r esult ing in com plet e oblit erat ion of t he t ubules79.
On t h e ot h er h an d , b ov in e t eet h ar e m or e r e a d i l y a v a i l a b l e a n d h a v e a m o r e u n i f o r m com p osit ion w h en com p ar ed t o h u m an t eet h , t hus pr ov iding a less var iable r esponse t o bot h car iogenic challenge and ant i- car ies t r eat m ent s, such as luoridated dentifrices60. Addit ionally, bovine t eet h h av e a bigger su r face ar ea w h ich m ak es easier ex per im ent al m anipulat ion. Fur t her m or e, bovine t eet h pr esent higher por osit y, w hich allow s a f ast er dif f u sion of ion s t o t h e dem in er alized ar ea2 6 , 2 8 , 5 5. Th u s, lon g er ex p er im en t al p er iod s m ight com pr om ise t he abilit y of t he m odel t o show luoride dose-response. Although bovine enamel is m or e por ous t han hum an enam el, w hich leads t o fast er dem ineralizat ion and r em ineralizat ion, t hese differ ences r esult in quant it at iv e and not qualit at iv e differ ences in behav ior. Addit ionally, the artiicial caries lesions produced from bovine
t eet h have a m ineral dist ribut ion and st ruct ure t hat r esem bles lesions pr oduced fr om hum an t eet h, bot h for enam el and dent in20,28,37,60. Thus, bovine t oot h can be consider ed an accept able alt er nat ive t o hum an t oot h in Car iology r esear ch and m ay offer advant ages t o hum an t oot h by decr easing t he r esponse t im e and var iabilit y of t he har d t issue subst rat e in t he m odel60.
Consider ing t he differ ent t ypes of m ineralized d en t al t i ssu es, en am el an d d en t i n h av e v er y d i f f er en t st r u ct u r es a n d co m p o si t i o n s, w h i ch int er fer e in t heir suscept ibilit ies t o dent al car ies. Basi cal l y, p er m an en t en am el i s co m p o sed b y m ineral ( 85% volum e) in t he for m of hydr oxy- or luorapatite crystals organized in prisms. Upon a cariogenic challenge ( 4.5< pH< 5.5) , hydroxyapat it e cr yst als ar e dissolved fr om t he subsur face, w hile luorapatite crystals are deposited at the surface, or iginat ing a subsur face lesion. The dissolut ion pr ocess is m er ely a chem ical event .
Per m an en t d en t i n , h ow ev er, con t ai n s 4 7 % apat it e, 33% or ganic com ponent s ( 90% collagen and 10% non- collagenous prot eins) and 20% wat er by volum e. The m ineral phase is hydr oxyapat it e, sim ilar t o enam el, but t he cr yst allit es have m uch sm a l l e r d i m e n si o n s. Th e h e x a g o n a l d e n t i n a l cr yst allit es ar e 3- 30 nm in cr oss- sect ion and about 50 nm in lengt h. This r esult s in a m uch lar ger surface area t o cryst allit e volum e rat io and t herefore a m or e r eact ive m ineral phase. The or ganic m at r ix is m ainly com posed of collagen. I t is pr esent as a ver y st r uct ur ed t r iple helix of t hr ee int er t w ined poly pept ide chains. I n addit ion, t her e ar e m any non- collagenous ( phosphopr ot eins, phospholipids and pr ot eogly cans) com ponent s t hat det er m ine t he m at r ix pr oper t ies. These com pounds play a r ole in t he nucleat ion and r egulat ion of m ineral for m at ion dur ing odont ogenesis90. Then t hey m ay int erfere wit h dem ineralizat ion and rem ineralizat ion pr ocesses by a sim ilar m echanism of act ion. Ther e is a syner gism bet w een m at r ix and apat it e. The m ineral phase can only be part ially dissolved during an acid at t ack, w hile t he m at r ix cannot be digest ed by enzym at ic act ion w hile it s sur face is pr ot ect ed by apat it e. Dent in car ies is t her efor e a biochem ical pr ocess charact er ized init ially by t he dissolut ion of t he m ineral par t t hus exposing t he or ganic m at r ix t o br eak dow n50,66,79 by bact er ia- der ived enzy m es an d h ost - der iv ed en zy m es, su ch as t h e m at r ix m et al l o p r o t ei n ases ( MMPs) p r esen t i n d en t i n and saliva15,100. The dent in dem ineralizat ion rat e decreases when t he am ount of degradable collagen incr eases, w her eby t he dem ineralized m at r ix is at t r ibut ed t o ham per ionic diffusion int o and out of t he dem ineralizing ar ea49,50. How ever, it should be r em em ber ed t hat dur ing car ies lesion for m at ion in
vivo, t eet h w it h a vit al pulpo- dent inal or gan w ill
r espond t o m ost exogenous st im uli t hr ough t he
apposit ion of m inerals along and wit hin t he dent inal t u bu les3 2. Th is ph en om en on , t oget h er w it h t h e outward low of dentinal luid from the pulp, may
be expected to signiicantly reduce the rate of in
vivo lesion pr ogr ession in dent in com par ed t o in vit r o sit uat ion80.
I t has been shown t hat MMPs get act ivat ed when t he pH drops in t he presence of acids from cariogenic ch al l en g es. Th e su b seq u en t n eu t r al i zat i on b y salivar y buffer sy st em s enhances t he degrading act iv it y of t h e or g an ic m at r ix1 0 0 ( Fig u r e 2 ) . I t is n ot ew or t h y t h at d en t in m at r ix d eg r ad at ion occu r s w h en bact er ial collagen ase is added t o r em ineralizing solut ion but not t o dem ineralizing solut ion in pH- cycling m odels47, i.e. t he act ivit y of bact er ial collagenase added t o dem ineralizat ion solut ion is lost , w hile t he act ivit y of host MMPs is enhanced at low pH, em phasizing t he im por t ance of host collagenases100. Thus, pH- cycling m odels w it hout t he addit ion of collagenases or gelat inases can only sim ulat e t he chem ical event s involved in r oot dent in car ies, since t hey do not t ake int o considerat ion t he biochem ical role of salivary MMPs in t he degradat ion of t he dem ineralized or ganic m at r ix. Addit ionally, w hen sim ulat ing dent in car ies
in vit r o, it is also im por t ant t o use fr eshly ext ract ed
t eet h and st or e t hese t eet h pr oper ly ( 0.02% NaN3, 0.9% NaCl solut ion, at 4oC)70 in order t o assure t hat t he act ivit y of dent in MMPs is pr eser ved.
for dent in lesions m ineral upt ake is pr edom inant at t he sur face and m ineral loss at t he lesion fr ont86.
I t m u st be also poin t ed ou t t h at t h e den t al subst rat es have usually t o be polished befor e t he beginning of t he exper im ent in or der t o pr oduce m or e u n if or m an d h om ogen eou s su r f aces t h at can be m or e accurat ely st andar dized, r esult ing in abraded sur faces. This pr ocedur e is essent ial for som e r esponse var iables such as sur face har dness analysis. The removal of the outermost luoride-rich enam el layer will render a fast er dem ineralizat ion of t he subsurface during t he subsequent pH- cycles5,96.
Characteristics of Artiicial Caries Lesions t h a t M a y Affe ct D e - a n d Re m in e r a liz a t ion in pH - Cy clin g M ode ls
In some studies, artiicial caries lesions are init ially pr oduced by im m er sion of t he subst rat es in buffer ed lact at e or acet at e gels110 or solut ions,
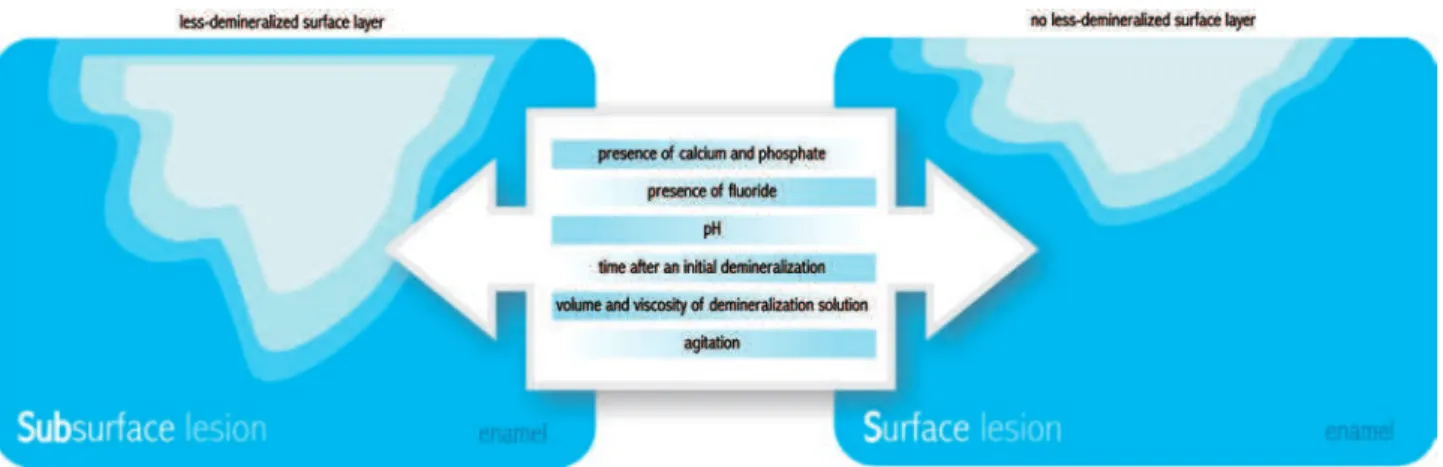
under sat urat ed in r espect t o apat it e, w it h a pH ranging bet w een 4.4 and 5.0, for a t im e ranging bet w een 16 h and 28 days ( Figur es 3, 4, 5 and 6) . These dist inct pr ot ocols w ill lead t o differ ent t ypes of lesions for m ed ( sur face soft ened lesions, also know n as er osion- like lesions, or subsur face lesions, also called car ies- like lesions) ( Figur e 7) . The distinct integrated mineral loss (∆Z) and depth at baseline54- 56 in t hese lesions can have a profound im pact on t he subsequent de- and r em ineralizat ion rat es t hus affect ing t he per for m ance of t he t est ed pr oduct s.
I t is required t hat t he dem ineralizing procedures induce car ies- like ( subsur face lesion w it h a less-dem ineralized sur face layer ) rat her t han er osion-like lesions. Many fact or s ar e im por t ant for t he p r eser v at ion of t h e su r f ace lay er, su ch as t h e pr esence of calcium and phosphat e19 and luoride99 in t h e solu t ion , it s pH9 7 an d t h e t im e af t er an
Figure 2- Schematic illustration of the process of dentin dissolution by bacterial acids. Under resting conditions (pH 7.0)
PROTOCOL DENTAL TISSUE R E S P O N S E VARIABLE
RESULTS
pH-cycling Human enamel CSH x TMR [Ten Cate, et al.92 (1985)]
The NaF dentifrice was found to be extremely effective in reducing the progression of caries in enamel Re:14d, 37ºC, on the weekends and before De
De: for 6h/day in 40 ml of acid buffer containing 2.0 mM Ca, 2.0 mM PO4, 0.075 M acetate, pH 4.3
Treatment: with slurry 1:4 in water for 5 min/Re for 17 h in 20 mL of a mineralizing solution containing 1.5 mM Ca, 0.9 mM PO4,
0.15 M KCl and 20 mM cacodylate buffer, pH 7.0 [as described by Featherstone, et al. 29 (1986)]
Ref: White and Featherstone113(1987)
Method 1 H u m a n s o u n d a n d b o v i n e carious enamel
Method 1: F dentifrice is very effective in inhibiting lesion formation in initially sound enamel as well as in inhibiting lesion progression .
pH cycling sound human enamel: CSH The effect of F dentifrice on prevention of demineralization and increase of remineralization depends on the type of lesion
De: 6h/day (75mM acetic acid, 2mM Ca(NO3)2, 2mM KH2PO4, pH 4.3, 20 mL/sample)
Method 2:
Re: 17 h/day (20 mM cacodylate buffer at pH 7.0, 130 mM KCl, 1.5 mM Ca(NO3)2, 0.9 mM KH2PO4, 20 mL/ sample). Total:15 d
(remineralizing solution, 37oC, on the weekend)
F analysis after acid etch biopsy (samples) a n d C a l o s s a n d uptake (solutions) by AAS
F treatment: slurry 1:3 water, 5 mL/ 5 min, under agitation before or after De.
Artiicial caries before Method 2
(7 d, 10 mL, 37oC): calcium-phosphate-luoride-acetate system
(2.2 mM Ca(NO3)2, 2.2 mM KH2PO4, 0.5 mM F, 50 mM acetate, pH
4.5) or 0.2 mM MHDP in 100 mM lactate buffer (pH 4.5)
Method 2
pH cycling carious bovine enamel:
De: 4 weeks –3 h/day (50 mM acetic acid, 1.5 mM Ca(NO3)2, 0.9
mM KH2PO4, pH 4.5-4.75)
Re: 21 h/day (20 mM cacodylate buffer at pH 7.0, 130 mM KCl, 1.5 mM Ca(NO3)2, 0.9 mM KH2PO4, 20 mL/ sample)
F treatment: as described above Ref: Ten Cate, et al.93 (1988)
Test 1: 5 min test solution (2 mL), 1 min water (2 mL) Bovine enamel w i t h s a l i v a r y pellicle
Calcium analysis by AAS (De-Re solutions)
Residual salivary [F] by water luoridation or toothpaste may give some protection to enamel demineralization
De: 1 h acid treatment (50 mM acetic acid, 1.5 mM KH2PO4, pH 5) (5-10mm 2)
Re: 1 h remineralization (20 mM cacodylic acid, 1.5 mM KH2PO4, pH 7).
Total: 8 cycles (18 h)
Test 2: Re solution (1 h/overnight), water rinse (1 min) and acid solution (1 h) during 3 days
Ref: Page69 (1991)
Artiicial caries: 8% methylcelulose gel, 0.1 M lactic acid (pH 4.6/7 d –Enamel and pH4.8/5 d–dentin)
Bovine Calcium uptake and loss by AAS (De and Re solutions)/ TMR and loosely and irmly bound F (samples)
Low F levels - less effective to inhibit caries lesion in dentin than in enamel
pH cycling: enamel and F dentifrice has a more pronounced effect on dentin than on enamel
De:(3 mL, 1.5 mM CaCl2, 0.9 mM KH2PO4 and 50 mM acetic acid,
pH 5.0, 6x0.5 h/day)
dentin
Re: (3 mL, 1.5 mM CaCl2, 0.9 mM KH2PO4, 130 mM KCl and 20
mM Hepes, pH 7.0, 6x 2.5 h/day, overnight and weekend)
(22 mm2)
3 days without treatment/ 7 days with treatment
Treatment: dentifrice slurry (1:3 in water, 5 min) x 3 µM F in de-remineralizing solutions x deionized water (5 min)
Ref: Ten Cate, et al.86 (1995)
Caries lesion: 96 h De solution Primary enamel The 10-day pH cycling model is inappropriate for primary teeth de/remineralization analysis.
pH-cycling: (1-mm) Positive regarding the treatment
De: 2.2 mM CaCl2, 2.2 mM NaH2PO4, 0.05 M acetic acid, pH
4.4 . 2x3 h/day
Re:1.5 mM CaCl2, 0.9 mM NaH2PO4, 0.15 M KCl ,pH 7.0, 2 h
between De, according to Ten Cate and Duijsters87 (1982)
Treatment: slurry (30 mL deionized water) for 1min before 1st De
and before and after 2nd De
TMR and PLM
Model I: 10-day pH-cycling Model II: 7-day pH-cycling
Ref: Thaveesangpanich, et al.95 (2005)
Caries lesion: 96 h De solution Primary enamel Positive results regarding the treatment.
pH-cycling: (1-mm window) Both 10-day (containing 0.25ppm F) and 7-day (without F) pH-cycling models were suitable for studying caries lesion progression in primary teeth
De: 2.2 mM CaCl2, 2.2 mM NaH2PO4, 0.05M acetic acid, pH 4.4
. 2x3 h/day
TMR and PLM
Re:1.5 mM CaCl2, 0.9 mM NaH2PO4, 0.15 M KCl, pH 7.0, 2 h
between DE, according to Ten Cate and Duijsters87 (1982)
Treatment: slurry (30 ml deionized water) for 1 min before 1st De
and before and after 2nd De
Model I: as mentioned above, 7-day pH-cycling
Model II: 0.25 ppm F added to de- and re- solutions, pH of de solution adjusted to 4.5, 10-day pH-cycling
Ref: Thaveesangpanich, et al.94 (2005)
Artiicial caries: 8 % methyl cellulose gel, 0.1 M lactate buffer, pH 4.6, 7 d and 28 d, for shallow (50 µm) and deep (200 µm) lesions, respectively
Bovine enamel (22mm2)
Ca loss and uptake (de-re solutions) , TMR (samples)
Dose-response was shown for Ca loss but not for Ca uptake. Signiicant difference was found for F response between shallow and deep lesions
pH cycling: (6x3 h/day):
Re: (2.0 or 2.5 h and overnight, weekend, 1.5 mM CaCl2, 0.9 mM KH2PO4, 130 mM KCl, 20 mM Hepes, pH 7.0, 3 mL), rinse,
De: (0.5 or 1h, 1.5 mM CaCl2, 0.9 mM KH2PO4, 50 mM acetic acid,
pH 4.6-4.8, 3 ml) and rinse (1.5 mM CaCl2, 0.9 mM KH2PO4, 130
mM KCl, pH 7.0 unbuffered). The solutions were changed daily. pH cycling without treatment: 3 days
Treatment: once/ day (moderate challenges) or twice/day (severe challenges) – 5 mL slurry (1:3 in water, 1x5 min or 2x/2 min) A robot was used for pH-cycling
Ref: Ten Cate, et al.88 (2006)
pH-cycling (7 d at 37°C): Bovine enamel (4x4 mm)
S M H a n d C S H (samples) and Ca, P and F analysis (pH-cycling solutions)
The 550 ppm F acidified dentifrice had the same anticariogenic action as the 1,100 ppm F neutral formulation.
De: (2 mM Ca, 2 mM P, 0.04 ppm F, 75 mM acetate buffer, pH 4.7, 2.2 mL/mm2) for 6 h
Re: (1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.05 ppm F, 0.1 M cacodylate buffer, pH 7.0, 1.1 mL/mm2) for 18 h
Treatment: 1-min soak in slurries (1:3 water) between solution changes (twice a day). Last 2 days only in Re. According to Vieira, et al.103 (2005)
Ref: Brighenti, et al.11 (2006)
pH-cycling (7 d at 37°C): Bovine enamel (4x4mm)
S M H , C S H , a n d analysis of F, Ca and P in enamel (microdrill biopsy technique)
The acidic dentifrices with 412 and 550 ppm F had the same eficacy as the neutral 1,100 ppm F dentifrice and commercial 1,100 ppm F dentifrice.
De: (2 mM Ca, 2 mM P, 0.04 ppm F, 75 mM acetate buffer, pH 4.7, 2.2 mL/mm2) for 6 h
Re: (1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.05 ppm F, 0.1 M cacodylate buffer, pH 7.0, 1.1 mL/mm2) for 18 h
Treatment: 1-min soak in slurries (1:3 water) between solution changes (twice a day). Last 2 days only in Re. According to Vieira et al.103 (2005)
Ref: Alves, et al.3 (2007)
Artificial caries (for re only): 0.05 M acetate buffer, pH 5.0 containing 1.28 mM Ca, 0.74 mM P, 0.03 ppm F, 2 mL/mm2 for 32 h
Bovine enamel (4x4x3 mm)
SMH, CSH, PLM The low-F dentifrice presented anticaries potential, but it was not equivalent to the dentifrices containing 1,100 ppm F
De pH-cycling (8 d, 37°C): Both de and remineralizing models seem to be adequate to evaluate the anticaries potential of low-F dentifrice De: 0.05 M acetate buffer, pH 5.0 containing 1.28 mM Ca, 0.74
mM P, 0.03 ppm F- 4 h/day, 6.25 mL/mm2
Re: 1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.05 ppm F in 0.1 M Tris, pH 7.0 20 h/day, 3.12 mL/mm2
Treatment: (before and after immersion in de): F solutions (0, 70, 140, 280 ppm F, NaF) or slurries (1:3) of dentifrices containing 0, 500 ppm F, 1,100 ppm F or Crest (1,100 ppm F – Gold standard) all NaF, for 5 min under agitation
After the 8th cycle remained in Refor 24 h
Re pH-cycling: same as De, but 2 h in De and 22 h in Re, 3 treatments of 1 min/day
Ref: Queiroz, et al.75 (2008)
Figure 3- pH-cycling studies evaluating the dose-response or pH-response of luoridated dentifrices for caries prevention
PROTOCOL DENTAL TISSUE R E S P O N S E VARIABLE
CONCLUSION
pH-cycling (6d + weekend only in Re solution, 37o C) Human enamel R e f l e c t e d l i g h t microscopy, CSH, F/ Ca content by probe ( s a m p l e s ) a n d F a n a l y s i s i n d e - r e solutions
NaF dentifrice and mouthrinse lead to a higher uptake of F into the lesion compared to MFP dentifrice, but the mineral content proile did not differ among the groups
De: 17h/day (40mL, 2 M Ca, 2mM PO4, 0.075M acetate, pH 4.3) ( 4 w i n d o w s 1x3mm, buccal and lingual) Re: 6h/day (20mL, 1.5mM Ca, 0.9mM PO4, 0.15mM KCl, 20mM
cacodylate buffer, pH 7.0)
Treatment :2x/day, dentifrice slurry 1:3 water or mouthrinse (1 min, under agitation)
Ref: Nelson, et al.64 (1992)
pH-cycling (7 or 14d at 21°C): Human dentin Fluoride analysis in samples (microdrill technique), TMR
The model provided reproducible results, demonstrated signiicant dose-related differences in the effects of both NaF and NaMFP-containing dentifrices on dentin F uptake and De, and detected a F-induced reduction in dentin caries, relative to a non-F control, similar to results established in a clinical trial
De: (0.1M lactic acid, 0.2% Carbopol 907, 50% saturated with hydroxyapatite, pH 5.0) for 4h/day [White110 (1987)]
(1mm in diameter window) Re: pooled human stimulated saliva for 20h
Treatment: slurry (1:3 in water or human saliva) for 1min (4x1min/ day, 2 before and 2 after De)
Initially pellicle formation for 0.5h in saliva Ref: Dunipace, et al.25 (1994)
Artiicial caries: demineralizing solution for 96h, 10mL/sample Human enamel TMR and PLM Chinese and Indian dentifrices failed to show “healing” eficacy even though they claimed to contain varying levels of F.
pH cycling (10d): (1mm window of molars) De: (2.2mM CaCl2, 2.2mM NaH2PO4, 0.05M acetic acid, pH 4.4 adjusted with 1M KOH, 2x3h/day)
Re: (1.5mM CaCl2, 0.9mM NaH2PO4, 0.15M KCl, pH 7, 2h between DE/day, overnight). According to Ten Cate and Duijsters87 (1982)
Treatment: slurry 1:3 in water 1min, 5mL/section, 3 times/day (before 1st De and before and after 2nd De).
The solutions were replaced in each challenge Ref: Itthagarun, et al.43 (2000)
Artiicial caries: demineralizing solution for 96h, 10mL/sample Primary enamel TMR and PLM Colgate Pokemon remineralized initial carious lesions, but Perioe children’s toothpaste did not.
pH cycling (7d): (1-mm window) De: (2.2mM CaCl2, 2.2mM NaH2PO4, 0.05M acetic acid, pH 4.4
adjusted with 1M KOH, 2x3h/day)
Re: (1.5mM CaCl2, 0.9mM NaH2PO4, 0.15M KCl, pH 7, 2h between De/day, overnight).
According to Ten Cate and Duijsters87 (1982)
Treatment: slurry 1:3 in water 1min, 5mL/section, 3 times/day (before 1st De and before and after 2nd De).
The solutions were replaced in each challenge Ref: Itthagarun, et al.42 (2007)
Artiicial caries: 0.1M lactic acid and 0.2% Carbopol C907, 50% saturated with hydroxyapatite, pH 5.0, according to White110 (1987)
Human enamel SMH and F uptake ( m i c r o d r i l l b i o p s y t e c h n i q u e a n d F electrode)
AmF and MFP only dentifrices were less effective in enamel remineralization than NaF only and NaF+MFP formulations. The inclusion of human saliva as product diluents is a critically important aspect to consider for any
in vitro study and most importantly when testing either
AmF or MFP containing formulations.
pH-cycling (5d): (3mm diameter)
natural saliva (1min)/ slurry (1:3 natural saliva) (1min)/natural saliva (1h)/ slurry 1min/ natural saliva (1h)/De solution (3h)/saliva (1h)/slurry (1min)/saliva (1h)/ slurry (1min)/saliva overnight Ref: Casals, et al.14 (2007)
pH cycling (14 days, 37oC): Human enamel CSH AmF and NaF dentifrices had the same effect. However, NaMFP, without hydrolysis, had nearly no effect. De: 6h/day (40mL, 2mM Ca, 2mM PO4,0.075M acetate, pH 4.3) (4x2mm)
Treatment :(dentifrice slurry 1:3 in water and solution, 1min, under agitation)
Re: 17h/day and on the weekend (20mL, 1.5mM Ca, 0.9mM PO4, 0.15mM KCl, 20mM cacodylate buffer, pH 7.0) according to Ten Cate and Duijsters87 (1982)
Ref: Toda and Featherstone101 (2008)
PROTOCOL D E N T A L TISSUE
R E S P O N S E VARIABLE
CONCLUSION
pH-cycling (14 d, 37ºC): Human enamel CSH Inclusion of pyrophosphate in NaF dentifrice did not affect the net outcome of the cycling De/Re
De: 6 h/day (2 mM Ca, 2 mM PO4, 0.075 mM acetate, pH 4.3,
40 mL/sample)
Treatment: 5 min dentifrice slurry 1:3 water (4 mL/sample, under agitation), water rinse
Re: 17 h (1.5 mM Ca, 0.9 mM PO4, 150 mM KCl, cacodylate
buffer pH 7, 20 mL/sample). Solutions were changed each 7d. On the weekends, there was only remineralization. According to Featherstone et al.29 (1986)
Ref: Featherstone, et al.30 (1988)
pH-cycling (6x3 h/day): Bovine enamel Ca uptake and loss (solutions)
The addition of triclosan and zinc citrate does not affect the caries-preventing property of F dentifrice
Re: (2.5 h and overnight, weekend, 1.5 mM CaCl2, 0.9 mM KH2PO4,
130 mM KCl, 20 mM cacodylate buffer, pH 7.0, 3 mL)
De: (0.5 h, 1.5 mM CaCl2, 0.9 mM KH2PO4, 50 mM acetic acid,
pH 5, 3 mL), according to Ten Cate and Duijsters87 (1982) with
slight modiications.
Treatment: 1 min daily in slurry (1:3 in water) followed by water rinse A pH-cycling robot was used to change the solutions
pH cycling without treatment: 3 days pH cycling with treatment: 14 days Ref: Ten Cate84 (1993)
pH-cycling: dentifrices were applied to sound enamel windows for 3 min at 8-h intervals for 14 d.
Human enamel PLM (lesion depth) The addition of ACaPO4 to a luoride dentifrice resulted in a trend toward further reductions in lesion depth following
in vitro lesion formation and progression over those
obtained with a luoride dentifrice. Dentifrices were removed, enamel rinsed for 3 min with deionized
water and placed in artificial saliva (20 mM NaHCO3, 3 mM
NaH2PO4, 1 mM CaCl2, pH 7.0), rinsed with deinized water for 3 min. Artiicial caries: enamel lesions were created with an acidiied gel (1 mM Ca, 0.6 mM PO4, 0.1 mM F, pH 4.25) and evaluated by PLM.
Treatment: enamel with caries-like lesions were treated again for 14 d as described above, returned to acidiied gels for progression of the lesions and sections for PLM were obtained again. This was repeated once more.
Ref: Hicks and Flaitz39 (2000)
Artiicial caries: 13 mL of 0.1 M lactic acid, 0.2% poliacrilic acid (Carbopol C907), 50% saturated hydroxyapatite, pH 5.0 for 72 h, according to White110 (1987).
Human enamel SMH The new dentifrice with ion-exchange resin (calcium, phosphate, luoride and zinc) has the same effect than the conventional dentifrice in de/remineralisation
Treatment: 1 min, 10 mL slurry 1:3 in human saliva, 4 x/day (0.6 cm diameter) Re: 15 mL natural saliva, 37ºC, 1 h, under agitation
De: 3 h in the same solution for producing artiicial caries Total: 16 days (except weekends)
Ref: Torrado, et al.102 (2004)
Artificial caries: 0.2% carbopol C907, 0.1 M lactic acid 50% saturated with calcium phosphate, pH 5.0 for 44 h
Bovine enamel S M H / p H o f d e m i n e r a l i z i n g solutions after the third dentifrice treatment
Dentifrice containing both F and sanguinaria was more effective than dentifrice containing F alone on remineralization of enamel lesion and on the pH of de solution. NaF dentifrices were more effective than MFP dentifrices.
Preparation: specimens placed in natural saliva for 24 h for pellicle formation/salivary mineral salts KCl, K2HPO4, NaCl, MgCl2 and
CaCl2 were added to TSB containing 10% sucrose/Specimens were placed in 20 mL of TSB De-Re solution containing 2 mL of S. sobrinus (B13) cultured for 24 h/Culture for 24 h (twice)
(3 mm diameter)
pH-cycling (15 days): 2 min in slurry (1:2 saliva), 2 h Re (50% stimulated human saliva and 50% artiicial saliva), 2 h De (TSB with mineral salts and sucrose). This was repeated 3 times, but in the last time Re lasted 6 h and De 10 h
Ref: Hong, et al.40 (2005)
pH-cycling (14 days, 37oC): Human enamel
(4x4 mm)
SMH The whitening toothpastes evaluated showed effect similar to regular, nonwhitening toothpastes.
De: 6 h/day (24 ml, 2 mM Ca, 2 mM PO4, 0.075 M acetate, pH 4.3)
Treatment: dentifrice slurry 1:3 in water, 5 ml 10 min
Re: 17 h/day and overnight/ on the weekends (24 ml, 1.5 mM Ca, 0.9 mM PO4, 0.15 mM KCl, 20 mM HEPES buffer, pH 7.0) according
to Featherstone et al.29 (1986)
Ref: Watanabe, et al.105 (2005)
Artiicial caries: 2.2 mM CaCl2, 2.2 mM KH2PO4, 0.05 M acetic acid,
pH 4.4, 96 h, 10 ml, 150-200 µm deep
Human enamel PLM and TMR Both test Asiatic dentifrices remineralized initial carious lesions. However, the remineralizing potential of Colgate Total was higher.
pH-cycling (10 d):
Treatment : slurry (1:3), 5 ml, 1 min De: same as artiicial caries, 10 ml, 3 h
Re: 1.5 mM CaCl2, 0.9 mM NaH2PO4, 0.15 M KCl, pH 7.0, for 2 h
Treatment: slurry (1:3), 5 ml, 1 min/demineralization solution 3 h/treatment with slurry (1:3), 5 ml 1 min/remineralizing solution overnight
Ref: Rana, et al.76 (2007)
Artiicial caries (for Re only): 1:1 8% methyl cellulose/acid lactic gel system at 37°C, pH 4.6 for 10 days
Bovine enamel Analysis of total Ca in acidic buffer with the electrode for De and % SMH change for Re
In de and remineralization studies, the silica based blue covarine whitening dentifrice was similar to the conventional dentifrice.
Re pH-cycling (6 x/day for 8 days. Neutral buffer overnight/ weekend)
Treatment: slurry (1:3) for 5 min
De: acidic buffer (1.5 mM CaCl2.2H2O, 0.9 mM KH2PO4, 130 mM
KCl, 50 mM acetic acid, pH 5.0) for 30 min/neutral buffer (1.5 mM CaCl2.2H2O, 0.9 mM KH2PO4, 130 mM KCl, 20 mM HEPES ) for
10 min based on Gibbs et al.34 (1995)
De pH-cycling (12 times, 2ml each solution): slurry (1:3) for 5 min/ acidic buffer (1.5 mM KH2PO4, 50 mM acetic acid, pH 5.0) for 60
min/neutral buffer (1.5 mM K H2PO4, 20 mM HEPES, pH 7.0) for
1 min based on Page69 (1991)
Ref: Joiner, et al.45 (2008)
Figure 5- pH-cycling studies comparing the impact of new active principles on the anti-caries eficacy of luoride dentifrices
PROTOCOL DENTAL TISSUE R E S P O N S E
VARIABLE
CONCLUSION
pH-cycling (3d at 37°C): Human dentin SMH The cariostatic effect shown by luoride-containing dentifrice could enhance that shown by Ketac-Fil and Fuji II LC, and could mask that shown by F2000.
De: (1h) 2 mM Ca, 2 mM phosphate, acetate 74 mM, pH 4.3 (5x5x2mm) Re: (23 h) 1.5 mM Ca, 0.9 mM phosphate, 20 mM TRIS, pH 7.0
Treatment: Slurry (1:3 in water) 2X5 min/day (after de and after re) According to Featherstone et al.29 (1986)
Ref: Hara, et al.36 (2002)
Artiicial caries: According to White108 (1987) but 0.2% Carbopol 2050
was used
Bovine enamel % SMH recovery and fluoride concentration in enamel (acid biopsy)
Although a single F-varnish application is able to increase luoride concentration in enamel presenting early caries lesion, it does not improve the capacity of luoride dentifrice used regularly in enhancing the enamel surface rehardening. Re pH-cycling (12 d, 37°C): according to White108 (5x5 mm)
De: 2 h/day, solution for lesion preparation, 0.037 ppmF
Treatment: for dentifrices, 4 x/day, 50 ml slurry (1:3), 1 min; for varnish, single application, removal after 24 h
Re: artiicial saliva according to Ten Cate and Duijsters87 (1982), 0.049
ppm F, for the remaining of the period Ref: Maia, et al.57 (2003)
pH-cycling (14 d with 10 cycles. In days 6, 7, 13 and 14 only Re ): According to Featherstone, et al.29 (1986)
H u m a n e n a m e l (4x4x3 mm)
Visual examination by 5 examiners scoring the presence and severity of caries-like lesions according to a scale ranked 0 to 3
The association of restorative materials and F dentifrice yielded higher cariostatic effect, except for the conventional glass ionomer cement, whose cariostatic effect was not inluenced by the type of dentifrice
De: 2 mM Ca, 2 mM PO4, 0.075 M acetic acid, pH 4.3 15 ml for 6 h Treatment: dentifrice slurry (1:3) 5 ml for 5 min
Re: (1.5 mM Ca, 0.9 mM PO4, 50 mM KCl, 20 mM Tris, pH 7.0) 15 ml
for 18 h
Ref: Rodrigues, et al.77 (2005)
Artiicial caries: 0.05M acetate buffer, 50% saturated with HAP, 48 h, 37o C, 6.25 mL/mM2
Human enamel F a n a l y s i s ( d e -r e s o l u t i o n s ) a n d quantitative PLM and CSH (samples)
All treatment reduced the demineralization progress in enamel. However, the laser irradiation did not improve the effect of F.
Treatment: laser (once), F dentifrice slurry 1:3 water (2 x/day,5 min, under agitation, before De and Re), F mouthrinse (once, 1 min, under agitation, before De)
(4 mm2)
pH-cycling: 10 d, 37oC
De: 5 mL/mm2, 2 mM Ca, 2 mM PO
4, 75 mM acetate buffer, pH 4.6, 3 h/day
Re: 2.5 mL/mm2, 1.5 mM Ca, 0.9 mM PO
4, 150 mM KCl, 20 mM cacodylic
buffer, pH 7.0, 21 h/day, after 5 d and at the end of experiment- 2 d Both solutions were changed daily
Ref: Steiner-Oliveira, et al.81 (2008)
Figure 6- pH-cycling studies comparing the association between luoride dentifrices and other treatments for caries
Figure 7- Factors that affect the formation of subsurface lesions (caries-like) and surface-softened lesions (erosion-like)
Figure 8- Mineral distribution in low-R and high-R caries lesions. High-R lesions give better discrimination among the
treatments under study and seem to be more appropriate to compare the eficiency of remineralizing systems, such as luoridated dentifrices. Low-R lesions are more appropriate when physiological mineral distribution is required. Modiied
from Lynch, et al.54 (2007)
init ial dem ineralizat ion98, as t he sat urat ion m ight be r eached w it h t im e, depending on t he volum e an d v iscosit y of t h e d em in er alizat ion solu t ion r elat iv e t o t h e ar ea of en am el ex p osed t o it . Th ese fact or s in t er act w it h each ot h er, m ak in g dificult the establishment of their ideal individual charact erist ics in a pH- cycling m odel. The form at ion of t h e su r f ace lay er m igh t be also af f ect ed by
agit at ion, w her eas a higher agit at ion incr eases t he risk of surface dissolut ion ( Figure 7) . I t is im port ant t o consider t hese fact or s because t he t hickness of the surface layer may have inluence on subsequent de- r em ineralizat ion54.
∆Z at baseline have a marked decrease in further m ineral loss and a concom it ant incr ease in fur t her m ineral gain. The decr ease in dem ineralizat ion of lesions with higher ∆Z at baseline may be partially a r esult of decr eased int r insic solubilit y t hr ough modiied chemical composition such as loss of car bonat e and m agnesium . On t he ot her hand, t he t endency t owar ds m ineral gain m ight be due t o t he fact t hat larger, m ore porous lesions m ight be m ore easily r em ineralized t han sm aller and less por ous lesions83. This m ay explain t he t endency t oward net remineralization with increasing ∆Z at baseline in pH- cycling r egim es55. Fur t her m or e, shallow lesions ar e m or e pr one t o dem ineralizat ion t han deeper on es b ecau se in d eep er lesion s t h e d issolv ed m ineral fr om t he deeper por t ions m ight under go r epr ecipit at ion dur ing out war d diffusion61,78. On t he ot her hand, t he r em ineralizat ion rat e is low er in deeper lesions due t o t he longer dist ance for ionic diffusion befor e m ineral deposit ion can occur54,61.
I n addit ion t o t h e in it ial m in eral loss of t he artiicial caries lesion, its mineral distribution is also very important. Low-R (R=∆Z/depth) lesions ar e m or e appr opr iat e w hen physiological m ineral dist r ibut ion is r equir ed, w hile high- R lesions give bet t er discr im inat ion am ong t he t r eat m ent s under st udy and seem t o be m ore appropriat e t o com pare the eficiency of remineralizing systems5 4, such as luoridated dentifrices (Figure 8). Thus, the com par ison of t he r esult s fr om st udies in w hich lesions w it h differ ent charact er ist ics at baseline w e r e e m p l o y e d m u st b e d o n e w i t h ca u t i o n . Furthermore, luoride dose-response studies should be car r ied out w it h lesions of var ious degr ees of severity before conclusions on optimal luoride eficacy are drawn88.
Re spon se Va r ia ble s in pH - Cyclin g M ode ls t o Ev a lu a t e Flu or ida t e d D e n t ifr ice s
Many response variables can be em ployed in pH-cycling models to evaluate the eficacy of luoride dent ifr ices. Som e of t hem ( color im et r ic m et hods and at om ic absorpt ion spect rom et ry-AAS) evaluat e t he ions ( m ainly calcium and phosphat e) r eleased in t o t h e de- an d r em in er alizin g solu t ion s. Also the calcium luoride-like material and luorapatite form ed ont o t he dent al subst rat es as a consequence of t he t r eat m ent s can be r em oved by alkali or acid biopsies and luoride can be analyzed using an ion-speciic electrode. From these biopsies, the amounts of calcium and/ or phosphat e can be also evaluat ed by t he m et hodologies descr ibed above.
Regar ding t he analysis of t he sam ples, dept h-r el at ed p h-r o p eh-r t i es o f ah-r t i f i ci al l esi o n s can b e d e scr i b e d q u a n t i t a t i v e l y b y m i n e r a l co n t e n t a n d h a r d n e ss p r o f i l e s5 6 a n d q u a l i t a t i v e l y b y polarized light m icroscopy- PLM39 ( m ore frequent ly) a n d sca n n i n g el ect r o n m i cr o sco p y - SEM ( l ess
fr equent ly)114.
Tr an sv er se m icr or ad iog r ap h y ( TMR) can b e r egar ded as t he “ gold st andar d” for t he evaluat ion of m ineral dist r ibut ion in Car iology r esear ch. This t echnique pr ovides a quant it at ive m easur em ent of t he am ount of m ineral, lesion dept h and sur face layer t hickness31,48.
Mor e r ecen t ly, m icr ocom p u t ed t om og r ap h y ( Micr o- CT) has been used t o st udy t eet h. Car ious lesion s2 3, q u an t if icat ion of m in er al d ist r ib u t ion i n f i s s u r e e n a m e l2 4 a n d e n a m e l d e - a n d r em ineralizat ion21 have alr eady been st udied by t his m et hod. I t shows prom ise as a non- dest ruct ive m et hod of validat ion for t he pr esence and dept h of t oot h de- rem ineralizat ion21. Precise m easurem ent s of attenuation coeficients and greater sensitivity t o ch an g es in m in er al w it h t im e an d p osit ion are provided by this technique. These beneits allow m ineral cont ent m easur em ent s fr om int act enam el t o ex t ensiv e dem ineralizat ion, including a lon g it u d in al t r ack in g of lesion d ev elop m en t . Besid es, it allow s com p lem en t ar y an aly ses of luoride, calcium and phosphorus present in the enam el.
Hardness relects the mechanical resilience of t he subst rat e t o t he penet rat ion of an indent er. Sur face har dness em ployed w it h a r educed load ( 25- 50 g) pr esent s a good sensit ivit y t o evaluat e ear ly ch an g es ( b ot h d e an d r em in er alizat ion ) in t he out er m ost layer of enam el and t o pr edict t he out com e of an ant i- car ies t r eat m ent116. I t is im por t ant t o highlight t hat w hen sur face har dness is int ended t o be used as r esponse var iable in r oot caries m odels, t he lengt h of t he pH- cycling m ust be short ened ( from 10 t o 3 days) , as well as t he lengt h of t he dem ineralizat ion per iod ( fr om 6 t o 1 h) in order t o enable t he surface hardness of t he creat ed lesion t o be assessed36. Cr oss- sect ional har dness ( CSH) can be r egar ded as an indir ect m et hod for evaluat ion of t he m ineral dist r ibut ion because t he t echnique, in fact , evaluat es t he r esilience of t he dent al subst rat e t o t he penet rat ion of an indent er under a giv en load. Despit e differ ent equat ions have been pr oposed and used t o indir ect ly der ive t he m ineral volum e fr om t he har dness dat a31,48, t h e cor r elat ion b et w een h ar d n ess an d m in er al v olum e is differ ent depending on t he deepness of t h e lesion2 1 , 5 6 , 8 7. Addit ion ally, t h e size of t h e indent at ion in dem ineralized t issues only allow s t h e m easu r em en t of cr oss- sect ion al h ar d n ess a t d i st a n ces o f a t l ea st 2 0 µ m b et w een t w o indent at ions, which m ight result in inaccuracy when t he int egrat ed m ineral loss is calculat ed21.
on t he degr ee of hydrat ion, w hich, in t ur n, m ight com pr om ise t he analyt ical r esult s7.
Recent ly, differ ent m et hodologies t o pr oduce artiicial carious enamel lesions (2 gels, 2 buffered solut ions and a pH- cycling pr ot ocol) w er e t est ed using TMR and cross- sect ional hardness as response var iables. One int er est ing r esult of t his st udy was t hat bot h for m ulas t o conver t har dness t o m ineral v o l u m e3 1 , 4 8 p r esen t ed ov er al l h i g h co r r el at i o n b et w een t h em a n d l o w co r r el a t i o n w i t h TMR dat a, show ing t hat t he conver sion of t he cr oss-sect ional har dness t o m ineral volum e seem s t o be inadequat e. Thus, t he dat a should be expr essed as hardness num bers. The result s also indicat e t hat t he conver sion of har dness t o m ineral volum e should not be used, since t he for m ulas and t he TMR dat a show ed differ ent r esult s w hen t hey w er e used t o compare the ive methodologies at each depth. This inding gives more support to the fact that t he r esult s ar e dependent on t he pr ot ocol used for creating artiicial lesions56.
ADA Proile Applications of Dentifrices in pH - Cy clin g M ode ls
Regar dless t he t ype of m odel em ployed, it m ust m eet t he suggest ed ADA guidelines associat ed wit h t opical evaluat ion of dent ifr ices and det er m inat ion of eficacy in products1, 2, 17, 73, 74. The m odel m ust p r eser v e t w o im p or t an t p r op er t ies t h at r elat e it t o t he car ies pr ocess and t o t he inv est igat or per for m ing t he exper im ent : validit y and r eliabilit y. The validity of a model can be deined as the degree of success w it h w hich t he m odel act ually pr ovides infor m at ion about t he phenom enon or pr ocess it is being used t o st udy, i.e., t he pH- cycling pr ot ocol should be able t o sim ulat e as closely as possible t he clinical sit uat ion.
Reliabilit y is r elat ed t o t he m anner by w hich a n i n v est i g a t o r o b t a i n s m ea su r em en t s i n a n ex p er im en t . Th e r eliab ilit y of a m easu r em en t t echnique assesses t he degr ee t o w hich sim ilar v alu es w ou ld t en d t o b e ob t ain ed if r ep eat ed m easurem ent s were m ade on t he sam e t est sam ple, under near ly ident ical condit ions73. Fur t her m or e, a pH- cycling m odel m ust show dose- r esponse effect differ ent iat ing dent ifr ices cont aining 0, 250 and 1,000 or 1,100 ppm F. I t is not m andat or y t hat t he m odels ar e able t o differ ent iat e t he ant i- car ies effect of a low - F den t ifr ice con t ain in g 5 0 0 - 5 5 0 ppm F fr om t he convent ional 1,000- 1,100 ppm F. However, it is recom m ended t o include a 500 ppm F t reat m ent group t o obt ain a “ m ini dose- response”74.
Con t r ols
A working group report on laborat ory m odels for car ies91 recommended that for tests of luoridated dent ifr ices in t he t radit ional concent rat ion range ( 0 - 1 , 1 0 0 ppm F) , ex per im en t al gr ou ps t r eat ed
w it h cont r ols of 0, 250 and 1,000 or 1,100 ppm F ( a clinically pr oven “ gold st andar d” ) ar e r equir ed, since clinical data are available on luoride eficacy in t his concent rat ion range. The pur pose of t he 2 5 0 ppm F den t ifr ice w ou ld be t o ser v e as an int er m ediat e “ dose- r esponse” cont r ol t hat should generally produce result s t hat are 10 t o 20% bet t er t han t he placebo, but not as effect ive as t he 1,000 ppm F “ gold st andard”. The 250 ppm F dent ifrice for t est s can be m ade especially by t he m anufact ur er or by dilut ion of t he 1,000 ppm F dent ifr ice, and t his should t hen be t est ed for bioavailabilit y. For st udies wit h product s in ot her concent rat ion ranges, ot her cont r ols should be included, including t he concent rat ion range t est ed. Tr eat m ent should be done wit h slurries of 1 in 3 ( or 1 in 4) weight / weight dilut ions of t he t oot hpast es in wat er or saliva91.
St a t ist ica l Con side r a t ion s
A w or king gr oup r epor t on laborat or y m odels for car ies91 agr eed t hat t he PCK ( Pr oskin- Chilt on-Kingm an)74 t est , or iginally designed for int ra- oral st udies73, m ay also be an appr opr iat e st at ist ical t est f or in v it r o pH- cy clin g m odels t o evalu at e products for “equivalence”. By deinition, a test p r od u ct is con sid er ed eq u iv alen t t o a con t r ol pr oduct if it s effect is bet w een 90% and 110% of t hat of t he cont r ol; t hat is, if t he rat io of t r ue m ean effect iveness scor es ( t est over cont r ol) is bet w een 0.9 and 1.1. This range of values is t he “ range of equivalence”73.
To est ablish t he equivalence of a t est pr oduct t o a cont r ol, t he guidelines specify t w o m et hods. The irst involves either a conidence interval or, alt er nat ively, t he use of t w o one- sided hypot hesis t est s. The second m et hod is t he “ power rule”. Det ails on how t o conduct t hese t est s w er e descr ibed by Pr oskin73 ( 1992) .
3 . 5 p H - Cy clin g Pr ot ocols t o Ev a lu a t e t h e Anti-Caries Eficacy of Fluoride Dentifrices
solut ion by ar ea of t he sam ple. This is im por t ant in f or m at ion t h at sh ou ld b e st an d ar d ized in all paper s. Also t he lengt h of t he t r eat m ent s spans a w ide range ( m ainly 7- 15 days) and m any r esponse var iables can be used t o assess t he r esult of t he t r eat m ent s ( m ainly chem ical analysis of solut ions and physical analysis of t he sam ples) .
One of t he m ost oft en used pH- cycling prot ocols is t he one described by Feat herst one, et al.29 ( 1986) for human enamel, which was modiied from the one pr oposed by t en Cat e and Duij st er s87 ( 1982) . The m odel is of part icular int erest because it sim ulat es in
vivo high caries risk condit ion ( lesions form ed around
or t hodont ic banding follow ing 1 m ont h in vivo)67 and sim ult aneously m easur es t he net r esult of t he inhibit ion of dem ineralizat ion and t he enhancem ent of r em ineralizat ion. I n t his m odel, t he dy nam ic cycles of de- and r em ineralizat ion ar e sim ulat ed b y seq u en t ially im m er sin g en am el sp ecim en s in acid ic ( d em in er alizin g ) an d su p er sat u r at ed ( r em ineralizing) buffer solut ions. Dent ifr ice use is sim ulat ed by im m er sing specim ens in slur r ies dur ing t he de- and r em ineralizat ion st ages. Topical eficacy is subsequently evaluated in terms of the abilit y of a t est pr oduct t o lim it car ies pr ogr ession as a r esult of enhanced r em ineralizat ion and/ or dim inished dem ineralizat ion. The dem ineralizat ion st age ( 6 h) uses an acid buffer cont aining 2 m M Ca ( Ca( NO3)2) , 2 m M PO4 ( KH2PO4) and 75 m M acet at e at pH 4. 3. The r em ineralizat ion solut ion ( 17 h) cont ains calcium and phosphat e at a know n degr ee of sat urat ion ( 1.5 m M Ca and 0.9 m M PO4) t o m im ic t he r em ineralizing pr oper t ies of saliva, 130- 150 m M KCl ( t o pr ov ide back gr ound ionic st r engt h) and 100 m M TRI S or 20 m M cacodylat e buffer at pH 7. 0. This solut ion appr ox im at es t he m ineral ion com posit ion and super sat urat ion of saliva as or igin ally r epor t ed by t en Cat e an d Du ij st er s8 7 ( 1982) . Som e aut hor s ( Figur es 3- 6) changed t he exposur e t im e in de- ( 3 h, 6 h or 17 h/ day) and r em ineralizing ( 6 h or 17 h/ day) solut ions or t he concent rat ions of t he ions, accor ding t o t he focus of t he st udy ( de or r em ineralizat ion) . When t he focus is m ore on rem ineralizat ion, t he sam ples st ay less t im e in t he dem ineralizing solut ion, and t his solut ion is pr epar ed w it h low er concent rat ion of acid and ions and a higher pH. On t he ot her hand, t he t im e in t he r em ineralizing solut ion is higher, but t he concent rat ion of ions and t he pH r em ain unchanged ( Figur es 3- 6) .
Anot her com m on pH- cycling m odel for bovine enam el is r ecom m ended by t en Cat e84 ( 1993) . I n t his case, t he dem ineralizing solut ion cont ains 1.5 m M Ca ( CaCl2) , 0. 9 m M PO4 ( KH2PO4) , 0. 050 M acet at e at pH range of 4.6- 5.0. The rem ineralizat ion solut ion cont ains calcium and phosphat e at a known degr ee of sat urat ion ( 1.5 m M Ca and 0.9 m M PO4) t o m im ic t he r em ineralizing pr oper t ies of saliva,
130 m M KCl ( t o provide background ionic st rengt h) and 20 m M HEPES or cacodylat e buffer at pH 7.0. The cycles, in t his case, ar e m or e fr equent ( 6x 0.5 h in De/ day and 6x 2.5 h in Re/ day) . Addit ionally, the addition of luoride (0.03-0.06 ppm) into both de- and r em ineralizing solut ions3,11,75,86,103 has been pr oposed. For pr im ar y t eet h, it is r ecom m ended t o add higher luoride concentrations in the de- and r em ineralizing solut ions ( 0.25 ppm F)94.
Usu ally, t h e sam p les st ay in r em in er alizin g solu t ion s n ot on ly b et w een t h e d em in er alizin g challenges, but also overnight , on t he weekends and som et im es all day in t he last days. I n som e prot ocols t he de- r em ineralizing solut ions ar e changed daily, w hile in ot her s t hey ar e not . This change can be m ade m an u ally or by cu st om - m ade r obot s8 4 , 8 8. Anot her im por t ant point it is t hat in som e paper s, t he pH cycles ar e per for m ed for 3 days w it hout t he F dent ifr ice ( slur r y) t r eat m ent86 t o allow baseline values of calcium upt ake and loss t o be det erm ined. Then, t he t r eat m ent is per for m ed eit her befor e or aft er t he dem ineralizat ion challenge93, 1 t o 3 t im es per day, for 1 t o 5 m in, under agit at ion, dur ing 7 t o 14 days.
While pH- cycling m odels using lact ic or acet ic acids ar e t he m ost com m on appr oaches for t est ing luoride dentifrices, the use of biotic models has been pr oposed, since m icr obial act ivit y- dependent pH-cycling has been shown to be of beneit in t e st i n g d e n t i f r i ce s co n t a i n i n g Na F a n d p l a n t ex t r act s, su ch as san gu in ar ia, con sider in g t h at t hese plant ex t ract s could affect t he act iv it y of m icr oor ganism s m or e t han does NaF40. How ever, t hese m odels are of m uch m ore com plex execut ion, w hich r est r ict s t heir use.
“ pulses” t hat occur aft er t r eat m ent w it h dent ifr ices m ight facilit at e secondary nucleat ion, increasing t he int er nal sur face ar ea of dent in for cr yst allizat ion.
While clinical trials show a beneicial effect of 5,000 ppm F over 1,100 ppm F dent ifr ices t o ar r est r oot car ious lesions9,53, t his was not t est ed in pH-cycling st udies. Such st udies could help t o clar ify the mechanism of action of high-luoride dentifrices on root caries arrest m ent . Addit ionally, t he effect of luoride is most pronounced during demineralization t han dur ing r em ineralizat ion for bot h enam el and dent in86.
Also appropriat e conclusions about pH- response of NaF d en t if r ices3 , 1 1 can b e d r aw n f r om p H-cycling studies (Figure 3). Low-luoride (500 ppm) acidic ( pH 4.5 or 5.5) NaF dent ifr ices have been show n in pH- cycling st udies t o be as effect ive as 1 , 1 0 0 ppm F n eu t r al NaF den t ifr ices. Th is w as conirmed in clinical studies showing that a 550 p p m F p H 4 . 5 d en t if r ice led t o sim ilar p laq u e luoride concentrations as the 1,100 ppm F pH 7.0 dent ifr ice12 and t hat bot h dent ifr ices led t o sim ilar car ies incr em ent s in a 20- m ont h clinical t r ial w it h 4- 5- year- old childr en ( unpublished dat e) .
One aspect t hat should be consider ed in st udies involving testing of MFP or amine luoride (AmF)-based dent ifr ice for m ulat ions is t he solut ion used t o pr epar e t he dent ifr ice slur r y ( Table 2) . I n t hese cases, it is im por t ant t o use nat ural saliva t o m ake t he dilut ion1 4. For MFP- based for m ulat ions, t his appr oach is necessar y because salivar y enzym es assist in t he hydrolysis of t he covalent ly bound MFP to release luoride ions. It is important that the m odel is conduct ed at 37°C because it has been shown t hat when NaMFP slurries were prepared wit h fr esh hum an saliva at 21°C, only m inim al am ount s of the total luoride (less than 10%) were available as ionic luoride25. Since t he enzym at ic syst em is eficient in the in v iv o condit ion, no differ ences
in t h e an t i- car ies ef f icacy b et w een d en t if r ices for m ulat ed w it h NaF or MFP occur in t he clinical sit uat ion, as r evealed by r ecent m et a- analyses16,59. The var iable sim ulat ion of condit ions necessar y for MFP hydr olyt ic act ivit y pr obably explains t he lar ge range of effect s seen in in v it r o com par isons of NaF and MFP6,20,25,35,40,42,43,49,62,85,101,107- 109,111- 113. I t has recent ly been shown t hat t he m inor inhibit ory effect of a NaMFP for m ulat ion ( 1,000 ppm F) on lesion for m at ion of dent al enam el was a r esult of t he low concentration of free luoride ion (only 30 ppm)101. Am F, in par t icular, has a ver y low pH w hen dilut ed w it h wat er ( 4.96) rat her t han saliva ( 6.37)14. When
in vit r o st udies use wat er inst ead of saliva as t he
product diluent s, result s for Am F are generally m ore favorable65,101. This effect is generally considered t o be an ar t ifact of st udy design rat her t han pr oduct effect iveness w hen com par ed t o pr ot ocols t hat use nat ural saliva for t his pur pose14.
I n t he last decades t here has been a proliferat ion of dentifrices directed toward speciic needs of the populat ion13. Such designer dent ifrices include t hose dir ect ed against t ar t ar, gingivit is, enam el and r oot caries, root sensit ivit y, halit osis, and t obacco st ains, as w ell as t hose w it h ext ra- w hit ening abilit ies, and t hose consider ed t o be “ nat ural” dent ifr ices and dent ifr ices for childr en. Despit e t he var ious t ypes of toothpastes, luoride has been maintained in over 95 per cent of t he dent ifr ice for m ulat ions39. pH- cycling st udies have been em ployed t o evaluat e if t he addit ion of differ ent act ive pr inciples w ould interfere with the anti-caries action of luoride ( Figur e 5) . Overall, t he addit ion of act ive pr inciples w it h differ en t pu r poses su ch as py r oph osph at e ( a n t i ca l cu l u s)3 0 , 7 1, b l u e cov a r i n e4 5, ca r b a m i d e p er ox id e1 0 5 an d sod iu m h ex am et ap h osp h at e7 2 ( w h i t e n i n g ) , sa n g u i n a r i a ( a n t i p l a q u e )4 0, i o n -e x ch a n g -e r -e si n s1 0 2, t r i cl o sa n ( a n t i b a ct e r i a l ) com bined w it h PVM/ MA39,76 or zinc cit rat e84 have not j eopar dized t he car ies- pr event ive pr oper t ies of the luoridated dentifrices in pH-cycling studies. Var iat ions in m odel sensit ivit y can be obser ved in
in vit r o assessm ent s of t he act ivit y of ant icalculus
dent ifrices107, where some protocols show signiicant inhibit ion of r em ineralizat ion w it h t he addit ion of tartar control inhibitors to luoride dentifrices, while ot her s show no negat ive effect s w it h t he addit ion of t hese inhibit ors30,89,93,107,115. However, clinical51,52,82 and in sit u63 st udies w it h t ar t ar cont r ol dent ifr ices conirm that mineralization inhibitors do not appear t o affect car ies pr ot ect ion of dent ifr ices, in- line w it h dat a r epor t ed fr om som e pH- cycling st udies. For w h it en in g d en t if r ices, t h er e is on ly scan t y information on their anti-caries eficacy and there is st ill a need for furt her evaluat ion of bot h different t ypes of com pounds and lesions in pH- cycling and
in sit u st udies. On t he ot her hand, t he addit ion of
bioavailable am or phous calcium and phosphat e in a NaF dent ifr ice seem s t o cause a t r end t owar d further reduction in lesion depth over luoridated dent ifr ice in a pH- cycling m odel sim ulat ing car ies for m at ion and pr ogr ession39.