AACL BI OFLUX
Aqu a cu lt u r e , Aqu a r iu m , Con se r va t ion & Le gisla t ion
I n t e r n a t ion a l Jou r n a l of t h e Bioflu x Socie t y
Sor pt ion k ine t ics of Pb
2 +, Cu
2 +a n d Zn
2 +ion s by
n a t u r a l ze olit e s fr om M a r a m u r e s Coun t y
( n or t he r n Rom a n ia ) : pot e n t ia l a pplica t ion in
w a st e w a t e r re u se
I rina Sm ical
Facult y of Environm ent al Science, Babeş- Bolyai Universit y Cluj - Napoca, 30 Fânt ânele
St reet , Cluj - Napoca Cit y, Cluj Count y, Rom ania, European Union; e- m ail: irinadan2003@yahoo.com
Abst r a ct. The sorpt ion kinet ics of Pb2+, Cu2+ and Zn2+ ions by natural zeolite tuff from Maramureş
Count y ( nort hern Rom ania) was st udied using agit at ed bat ch laborat ory apparat us at t wo t em perat ure levels of 25 0
C and 50 0
C. The sorpt ion kinet ics of st udied heavy m et als ions by t he nat ural zeolit ic t uff was est ablished by fit t ing of experim ent al dat a using various m odels applied in t he kinet ic st udies of heavy m et als sorpt ion ont o ions exchangers ( pseudo- order 1, pseudo- order 2 and Weber- Morris) . From all of t hese t he best result s were recorded for t he pseudo- order 1 m odel use. The int eract ion bet ween m et al ions and adsorbent surface is m ade on a single adsorpt ion sit e and t he adsorpt ion m echanism is a diffusive one t hrough t he adsorbent pores.
Ke y W or ds: kinet ics, nat ural zeolit e t uff, adsorpt ion, heavy m et als, wat er reuse.
Re zu m a t. Datele prezentate în lucrare, axate pe interpretarea şi prelucrarea rezultatelor experimentale utilizând diferite modele pentru determinarea cineticii de adsorbţie, relevă faptul că tuful zeolitic natural din zona Călineşti- Bârsana, judeţul Maramureş, are proprietăţi ridicate de reţinere a metalelor grele din apele uzate. Conform rezultatelor interpretării acest proces se derulează cel mai
bine la t em perat uri m ai ridicat e. Dint re m odelele cinet ice ut ilizat e ( pseudo- ordin 1, pseudo- ordin 2 şi
Weber- Morris) cele m ai bune rezult at e au fost obţinute cu ajutorul modelului cinetic de pseudo- ordinul 1. Acest lucru indică faptul că interacţiunea dintre ionii metalici şi suprafaţa adsorbantă se realizează pe câte un singur site de adsorbţie. Pe de altă parte, datele obţinute aplicând modelul cinetic Weber-Morris aratăcă că mecanismul de adsorbţie este de tip difuziv în porii adsorbantului.
Cu v in t e ch e ie: cinetică, tuf natural zeolitic, adsorbţie, m et ale grele, reciclarea apei.
I n t r odu ct ion. The adsorption abilit y and t he high ion exchange of zeolit es offer t he possibilit y of a high usage of t he zeolitic t uff in various fields of econom y, anim al healt h and environm ent prot ection ( Kovo & Edoga 2005; Nicula et al 2010; Sm ical et al 2010ab) . The very long and still inexhaustible list of t he convent ional and unconvent ional application of t he zeolite volcanic t uff, recom m end t his raw m at erial as one of t he m ost im port ant in t he non- m et alliferous dom ain.
I n t he last half cent ury considerable effort s have been m ade and t echnologies for zeolit es usage have been developed in various applications ( Wise 2005) . The ion exchange by using nat ural zeolit es m ay be considered one of t he m ost at t ractive m et hods because t hey are abundant and t he cost price is low. I n addition, nat ural
zeolit es could be deposited and regenerat ed cheaply ( Bailey et al 1999) . The m ost
applications of nat ural zeolit es are based on t heir st ruct ural charact eristics, excellent sorbent s propert ies and t heir large specific surface ( Çoruh 2008) .
The cations adsorbtion, positively charged, and the com plex form ing are possible due t o t he various form s of t he cryst al faces, im perfection of surface, breaking t he links and t he m argins as well as t o t he am phot eric nat ure of t he hydroxil grouping [ ( Al/ Si) -OH) ] ( Trgo & Peric 2003; Alt in et al 1998) .
Our present experim ent al dat a and t heir processing result s give inform at ion about t he adsorption rat e of t he Pb2+, Cu2+ and Zn2+ from synt hetic solution by nat ural zeolitic
M a t e r ials an d Me t h od. The zeolit e t uff used in the experim ent al research was sam pled
from a quarry situated on the Călineşti-Văleni area (Maramureş County, northern
Rom ania, European Union) .
This nat ural zeolit e t uff deposit is on t he right slope of t he Valea Mijlocie st ream and close t o it s confluence wit h Valea Morii st ream (see Fig. 1) .
Aft er grinding and granulom et ric classification of zeolit e t uff, t he m at erial was prepared
by washing of it wit h distilled wat er followed by filt rat ion and drying in a heat ing (
Binder-Germ any) during 48 hours at t em perat ure of 105 0C.
I n order t o r un t he experim ent an am ount of 1 g of zeolite t uff ( d= 0.037 m m ) was
cont act ed wit h a volum e of 50 m L of Pb2+, Cu2+ and Zn2+ synt het ic solution, t hrough cont inuously agit ation during 24 hours ( enough t o reach t he equilibrium ) , in t herm ost atic
conditions at 25 0C and 50 0C, using an orbit al shaker (Heidolph Unim ax 1010 –
Germ any) .
The zeolit e t uff agit ation t oget her wit h t he cont act solution was m ade in polyet hylene phials of 75 m L each one.
The synt hetic solutions cont aining Pb2+, Cu2+ and Zn2+ ions were prepared using
salts of CuSO4ּ5H2O, Pb( CH3COO)2∙3H2O, respect ively ZnSO4∙7H2O p.a., m ade by E.
Merck, Darm st adt ( Germ any) and t he solutions initial pH was fix at 4.0 using a solution
of H2SO4 p.a., respectively CH3COOH.
The pH was m easured wit h a digit al pH m et er Consort P901 ( Belgium ) and for salts dissolving distilled wat er was used being obt ained wit h Fist reem Cyclon ( Unit ed Kingdom ) distiller.
I n order t o m ake a kinetic st udy on Pb2+, Cu2+ and Zn2+ ions adsorption by nat ural
zeolite tuff from Călineşti- Bârsana area, t he following working conditions were m et : Tem perat ure of 25 0C
Tem perat ure of 50 0C
I nit ial m et al concent ration in solution of 100 m g/ L
Fig. 1. The natural zeolite tuff quarry in Călineşti – Bârsana
I nit ial solutions pH of 4.0 m ixing ratio R= S/ L= 1/ 50
At different periods, sam ples of solution were collected, cent rifuged and subj ect ed t o procedures of est ablishing t he heavy m et als concent rat ion by at om ic spect rom et ry m et hod using an at om ic adsorption spect rom et er Perkin Elm er AAS800 ( Shelt on - USA) .
By st udying t he kinetics of adsorption process t he rat e was observed as well as t he m echanism of reaction. The adsorption rat e is generally given by equation ( Sent hil Kum ar & Gayat hri 2009) :
C
C
E
k
dt
da
( 1)where:
a = am ount adsorbed per gram of adsorbent ( m ol/ g) ; t = tim e at t he beginning adsorpt ion ( m in) ; k = a rat e const ant ( m in- 1) ; C = t he adsorbate concent ration in fluid phase ( m ol/ L) ;
CE = t he adsorbat e concent rat ion in fluid phase at equilibrium conditions ( m ol/ L) .
Fit t in g t h e e x per im e n ta l da t a u sin g t h e pse u do- or de r 1 , pse u do- or de r 2 a n d W e be r - M or r is m ode ls. The experim ent al dat a were fitt ed using t he m ain m odels for adsorpt ion kinetics, nam ely: pseudo- order 1, pseudo- order 2 and Weber- Morris.
The kinetics equat ions used for Pb2+, Cu2+ and Zn2+ ions adsorption ont o t he
zeolit e t uff m ight be writ t en in t he following int egrat ed form s:
for k in e t ics of pse u do- or de r 1 ( Nam asivayam & Holl 2004; Sun et al 2004; Acem ioglu & Alm a 2004) :
)
(
1 e t
t
k
q
q
dt
dq
q
e
q
t
ln
q
e
k
t
ln
( 2)where:
qt = t he adsorbed am ount of Pb2+, Cu2+ and Zn2+ ( m ol Pb2+/ g, m ol Cu2+/ g, m ol Zn2+/ g) qe = t he am ount of Pb2+, Cu2+ and Zn2+ adsorbed in equilibrium conditions ( m ol Pb2+/ g, m ol Cu2+/ g, m ol Zn2+/ g)
t = tim e ( m in)
k = t he adsorption const ant which involves t he activation energy ( g∙mol- 1 m in-1)
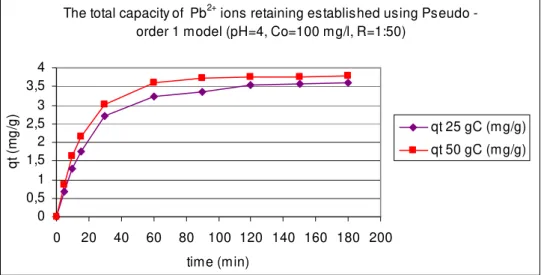
Fig. 2 The total capacity of retaining the Pb2+ ions by the natural zeolit e tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 1 kinetic m odel.
The total capacity of Pb2+ ions retaining established using Pseudo - order 1 model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5 3 3,5 4
0 20 40 60 80 100 120 140 160 180 200 time (min)
q
t (
m
g
/g
)
for k in e t ics of pse u do- or de r 2 ( Ho & McKay 2004; Ho 2004; Nam asivayam & Holl 2004; Sun et al 2004) :
2 2
(
e t)
t
k
q
q
dt
dq
e e
t
q
t
q
k
q
t
1
2 ( 3)where:
qt = t he adsorbed am ount of Pb2+, Cu2+ and Zn2+ ( m ol Pb2+/ g, m ol Cu2+/ g, m ol Zn2+/ g) qe = t he am ount of Pb2+, Cu2+ and Zn2+ adsorbed at equilibrium conditions ( m ol Pb2+/ g, m ol Cu2+/ g, m ol Zn2+/ g)
t = tim e ( m in)
k = t he adsorption const ant which involves t he activation energy ( g∙mol- 1 m in-1)
Fig. 3 The total capacity of retaining the Cu2+ ions by the natural zeolit e tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 1 kinetic m odel.
The total capacity of Cu2+ ions retaining established using Pseudo - order 1 model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5
0 20 40 60 80 100 120 140 160 180 200 time (min)
qt
(
m
g/
g)
qt 25 gC (mg/g) qt 50 gC (mg/g)
Fig. 4. The total capacity of retaining the Zn2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 1 kinetic m odel.
The total capacity of Zn2+ ions retaining established using Pseudo -
order 1 model (pH=4, Co=100 mg/l, R=1:50)
0 0,2 0,4 0,6 0,8 1 1,2 1,4 1,6 1,8
0 20 40 60 80 100 120 140 160 180 200 time (min)
qt
(
m
g/
g)
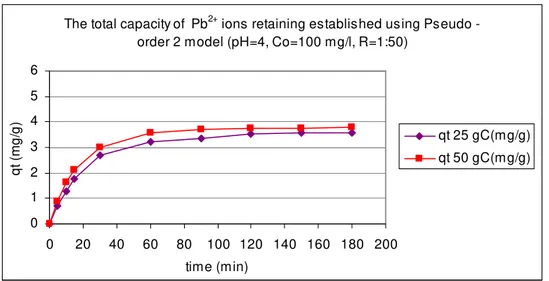
Fig. 5. The total capacity of retaining the Pb2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 2 kinetic m odel.
The total capacity of Pb2+ ions retaining established using Pseudo -
order 2 model (pH=4, Co=100 mg/l, R=1:50)
0 1 2 3 4 5 6
0 20 40 60 80 100 120 140 160 180 200 time (min)
q
t (m
g
/g
)
qt 25 gC(mg/g) qt 50 gC(mg/g)
Fig. 6. The total capacity of retaining the Cu2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 2 kinetic m odel.
The total capacity of Cu2+ ions retaining established using Pseudo -
order 2 model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5
0 20 40 60 80 100 120 140 160 180 200 time (min)
qt
(
m
g/
g) qt 25 gC(mg/g)
qt 50 gC(mg/g)
Fig. 7. The total capacity of retaining the Zn2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Pseudo-order 2 kinetic m odel.
The total capacity of Zn2+ ions retaining established using Pseudo - order 2 model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2
0 20 40 60 80 100 120 140 160 180 200 time (min)
q
t (
m
g
for t h e k in e t ic m ode l W e be r - M or r is ( in t r apa r t icle diffusion ) ( Weber Jr. & Digiano 1996; Mckay & Poot s 1980) :
5 . 0
0
k
t
I
q
t
p
( 4)5 . 0
t
k
q
t
i
( 5)2
12
e p p eq
k
d
D
t he effective diffusion coefficient ( 6)The experim ent al dat a fit ting using t he pseudo- order 1, pseudo- order 2 and Weber-Morris kinetic m odels and t he calculation using t he correlation coefficient s are presented in Tables 1- 3.
Fig. 8. The total capacity of retaining the Pb2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Weber-Morris m odel.
The total capacity of Pb2+ ions retaining established using
Weber-Morris model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5 3 3,5 4
0 20 40 60 80 100 120 140 160 180 200
time (min) q t ( m g /g )
qt 25 gC(mg/g) qt 50 gC(mg/g)
Fig. 9. The total capacity of retaining the Cu2+ ions by the natural zeolite tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Weber-Morris m odel.
The total capacity of Cu2+ ions retaining established using
Weber-Morris model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5 3
0 20 40 60 80 100 120 140 160 180 200
time (min) qt ( m g/ g)
Table 1 The param eters result ed from the experim ental data fitt ing using the
pseudo-order 1 kinet ic m odel
No. Metal ion A B r qe k1
1 Pb2+ 0.898066 -0.03194 0.982939 2.45485 0.031945
2 Cu2+ 0.118093 -0.02895 0.987578 1.125349 0.02895
3 Zn2+ -0.6281 -0.02578 0.95641 0.533604 0.025783
Table 2 The param eters result ed from the experim ental data fitt ing using the
pseudo-order 2 kinet ic m odel
No. Metal ion A B r qe k2
1 Pb2+ 31.22842 -167.227 0.673763 -0.00598 895.4975
2 Cu2+ 62.26858 -350.998 0.673183 -0.00285 1978.514
3 Zn2+ 85.60951 -449.809 0.512924 -0.00222 2363.384
Table 3 The param eters result ed from the experim ental data fitt ing using the
Weber-Morris kinet ic m odel
No. Metal ion A B r I o Kp De
1 Pb 0.747234 0.247777 0.923084 0.747234 0.247777 1.42E-13 2 Cu 0.589753 0.104472 0.872797 0.589753 0.104472 1.02E-13 3 Zn 0.339268 0.066838 0.876784 0.339268 0.066838 1.11E-13
D iscussion a nd Con clusion s. As it can be observed in all graphical represent ations t he best adsorption process of t he m et al ions by t he Călineşti- Bârsana zeolit e t uff is run at higher t em perat ures.
The best fit ting of experim ent al dat a was obt ained using pseudo- order 1 kinet ic m odel. This t hing shows t hat t he int eractions between t he m et al ions and t he adsorbent surface are m ade on a single adsorption sit e.
Fig. 10. The total capacity of retaining the Zn2+ ions by the natural zeolit e tuff
from Călineşti-Bârsana area, at tem peratures of 25 0C and 50 0C using
Weber-Morris m odel.
The total capacity of Zn2+ ions retaining established using
Weber-Morris model (pH=4, Co=100 mg/l, R=1:50)
0 0,5 1 1,5 2 2,5 3
0 20 40 60 80 100 120 140 160 180 200
time (min)
qt
(
m
g/
g) qt 25 gC(mg/g)
As it can be observed in Tables 1- 3, t he experim ent al dat a are fit ting wit h t he best correlation coefficient s in t he use of t he kinetic m odels: pseudo- order 1, pseudo- order 2 and Weber- Morris. The m edium of t he correlation coefficients are: 0.9756, 0.8909 and 0.6199, respectively.
Using Weber- Morris m odel t he values 1.42·10- 13 for Pb2+, 1.02·10- 13 for Cu2+ and
1.11·10- 13 for Zn2+, were obt ained. This m agnit ude orders indicate t hat t he adsorption
m echanism is a diffusive one t hrough t he adsorbent pores.
From t he t hree present ed kinetic m odels ( pseudo- order 1, pseudo- order 2 and Weber- Morris) t he best fit ting of t he experim ent al dat a was m ade using t he pseudo- order 1 kinetic m odel.
The int erpret ation results indicat e t hat t he int eract ion bet ween m et al ions and adsorbent surface was m ade on a single adsorption sit e and t he adsorption m echanism is a diffusive one t hrough t he adsorbent pores.
Re fe r en ce s
Acem ioglu B., Alm a M. H., 2004 Sorpt ion of copper ions by pine sawdust. Holz Roh
Werkst 6 2: 268- 272.
Altın O., Özbelge H. Ö., Dogu T., 1998 Use of general purpose adsorption isot herm s for heavy m et al- clay m ineral int eractions. Journal of Colloid and I nterface Science 1 9 8: 130–140.
Bailey S., Olin T., Bricka M., Adrian D., 1999 A review of pot entially low- cost sorbent s for
heavy m et als. Wat er Res 3 3: 2469–2479.
Çoruh S., 2008 The rem oval of zinc ions by nat ural and conditioned clinoptilolit es.
Desalinat ion 2 2 5: 41–57.
Ho Y. S., 2004 Pseudo isot herm s using a second order kinetic expression const ant .
Adsorpt ion 1 0: 151- 158.
Ho Y. S., Mc Kay G., 2004 Sorpt ion of copper from aqueous solut ions by peat. Wat er, Air
and Soil Pollution 1 5 8: 77- 97.
Kovo A. S., Edoga M. O., 2005 Pr oduct ion and charact erization of zeolit e from Ahako Clay in Kogi St at e, Nigeria. Leonardo Elect ronic Journal of Practices and Technologies 7: 31- 40.
Mckay G., Poot s V. J., 1980 Kinet ics and diffusion processes in colour rem oval from
effluent using wood as an adsorbent . J Chem Technol Biot echnol 3 0: 279- 292.
Nam asivayam C., Holl W. H., 2004 Chrom ium ( I I I ) rem oval in t annery wastewat ers using
Chinese Reed ( Miscant hus Sinensis) a fast growing plant. Holz Roh Werkst 6 2:
74-80.
Nicula M., Banat ean- Dunea I ., Gergen I ., Harm anescu M., Sim iz E., Pat ruica S., Polen T., Marcu A., Lunca M., Szucs S., 2010 Effect of nat ural zeolit e on reducing t issue bioaccum ulation and cadm ium ant agonism related t o som e m ineral m icro- and
m acronut rient s in Prussian carp (Carassius gibelio) . AACL Bioflux 3( 3) : 171- 179.
Sent hil Kum ar P., Gayat hri R., 2009 Adsorpt ion of Pb2+ I ons from Aqueous Solut ions ont o
Bael Tree Leaf Powder: I sot herm s, Kinetics and Therm odynam ics St udy. Journal of
Engineering Science and Technology 4( 4) : 381- 399.
Sm ical I ., Mihaly- Cozm ut a L., Costin D., 2010a Research concerning t he influence of several fact ors on Pb2+, Cu2+ and Zn2+ ions adsorpt ion by nat ural zeolit e t uff from
Maramureş count y, Nort hern Rom ania. AES Bioflux 2( 2) : 171- 180.
Sm ical I ., Mihaly- Cozm ut a L., Costin D., 2010b Use of nat ural zeolit es from Maram ures
count y ( Rom ania) in rem oval of Cu2+, Pb2+, Zn2+ ions from indust rial wastewat ers.
AES Bioflux 2( 2) : 181- 188.
Sun Q. Y., Lu P., Yang L. Z., 2004 The adsorpt ion of lead and copper from aqueous solution on m odified peat resin particles. Environm ent al Geochem ist ry and Healt h
2 6: 311- 317.
Trgo M., Peric J., 2003 I nt eract ion of t he zeolitic t uff wit h Zn- cont aining sim ulat ed
Weber Jr. W. J., Digiano F. O., 1996 Process Dynam ics in Environm ent al Syst em ; Environm ent al, Science and Technology Series, John Wiley and Sons: New York; p.89- 94.
Wise W. S., 2005 Encyclopedia of Geology - Minerals Zeolit es, Universit y of California – Sant a Barbara, Sant a Barbara, CA, USA, p.591- 601.
Received: 02 Oct ober 2010. Accept ed: 30 Decem ber 2010. Published online: 04 July 2011. Aut hor:
I rina Sm ical, Faculty of Environmental Science, Babeş- Bolyai Universit y of Cluj - Napoca, 30 Fânt ânele St reet , Cluj - Napoca Cit y, Cluj Count y, Rom ânia, irinadan2003@yahoo. com
How t o cit e t his art icle:
Sm ical I ., 2011 Sorpt ion kinet ics of Pb2+, Cu2+ and Zn2 + ions by nat ural zeolit es from Maram ures Count y