Ar
ti
cl
e
J. Braz. Chem. Soc., Vol. 21, No. 10, 2000-2004, 2010. Printed in Brazil - ©2010 Sociedade Brasileira de Química 0103 - 5 05 3 $ 6 .00+ 0.00
* e-mail: lh meller@ uf p a.b r
Fatty Acid Proiles and Tocopherol Contents of Buriti (Mauritia lexuosa), Patawa
(Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis)
and Inaj a (Maximiliana maripa) Fruits
Antonio M. da Cruz R odrigues,a S ylvain D arnetb and L uiza H . Meller da S ilva*,a
aInstituto de Tecnologia and bInstituto de Ciências Biológicas, Universidade Federal do P ará,
6 6 .0 7 5 - 9 0 0 Belém- P A , Brazil
O b uriti, tucumã, inaj á, mari e p atauá são p almeiras endêmicas da reg ião A mazônica. A s p olp as destes f rutos são tradicionalmente consumidas p ela p op ulação local, mas ainda não g anh aram os mercados nacionais e internacionais. A comp osição nutricional em ácidos g rax os f oi determinada p or cromatog raia g asosa ( C G ) e a de tocof erol determinada p or cromatog raia liq uida de alta eiciência ( C L A E ) . A s p olp as se mostraram b astante energ éticas, com um alto teor de óleo q ue v ariou entre 31,0 a 41,8 % . O s ácidos g rax os q ue ap resentaram maiores concentrações f oram o
oleico ( C 18 : 1) e o p almítico ( C 16 : 0) , p ara todas as p olp as estudadas. A p olp a de b uriti f oi a q ue ap resentou maior teor de v itamina E sendo considerada uma ótima f onte de tocof erol. O α-tocof erol
f oi o tocof erol p redominante, com ex ceção da p olp a de b uriti. O s dados indicaram q ue as f rutas estudadas são b oas f ontes de ácidos g rax os insaturados e tocof eróis.
A mazonian f ruits are rich in f at b ut h av e a f atty acid p roile th at may b e b eneicial in relation to risk of coronary h eart disease. A mazonian f ruits also contain oth er p otentially cardiop rotectiv e constituents including tocop h erols. T ocop h erol p roiles w ere determined b y h ig h p erf ormance
liq uid ch romatog rap h y ( H PL C ) , and f atty acid p roiles w ere determined b y g as ch romatog rap h y ( G C ) . I n th e p resent study , th e total oil content, f atty acid comp osition and tocop h erol content of
th e p ulp s of iv e A mazonian f ruits ( b uriti, p ataw a, tucuma, mari and inaj a) w ere measured. T h e total oil content of th e f ruits rang ed f rom 31.0 to 41.8 % . T h e maj or f atty acid in all of th e f ruits w as oleic acid ( C 18 : 1) , th oug h sub stantial lev els of p almitic acid ( C 16 : 0) w ere p resent. L inoleic acid ( C 18 : 2) w as th e main p oly unsatured f atty acid ob serv ed. α-T ocop h erol w as th e most p rev alent
tocop h erol ex cep t in b uriti p ulp . Buriti and mari p ulp h av e a h ig h content in α-tocop h erols w ith 29 7 and 15 5 µg g-1 of dry matter. O ur data indicate th at all iv e of th ese A mazonian f ruits are g ood
sources of unsaturated f atty acids and tocop h erols.
K eywords: A mazonian f ruits, f atty acids, v itamin E , A recaceae, I cacinaceae
Introduction
T h e A mazonian reg ion h ouses a larg e v ariety of
f ruit crop s, some of w h ich h av e p otentially p romising
h ealth and nutritional p rop erties. I n p articular, buriti
(M auritia lex uosa) , p ataw a (O enocarp us bataua) , tucuma
(A strocary um vulgare) and inaj a (M ax imiliana marip a)
h av e sig nif icant nutritional v alue and are ap p reciated
b y th e p op ulation of th is reg ion.1 T h ese f ruits b elong to
th e f amily A recaceae, and are distrib uted th roug h out th e
A mazon and north ern South A merica. T h e mesocarp is
comestib le and nutritious, containing h ig h q uality oil.2
M ari (P oraq ueiba p araensis) , b elong s to th e f amily
Icacinaceae, and is a nativ e sp ecies, ex clusiv e to Pará State,
common th roug h out th e estuary of th e low A mazon. T h e mesocarp and ep icarp sh ell are edib le, and are also used
to p roduce oil and w ine.3
A mazonian f ruits are rich in f at, b ut more th an 6 1% of th ese f ats are unsaturated and could b e considered h ealth y f ats w ith cardiop rotectiv e p otential. A mazonian f ruits also h av e h ig h lev els of tocop h erols, w h ich are p resent in th e
unsap oniiab le lip id f raction of f oods.4 T ocop h erols are
sy nth esized in p h otosy nth etic microorg anisms and p lants
and are most concentrated in p lant seeds.5
Vitamin E is an imp ortant natural antiox idant in f oods,
esp ecially th ose rich in p oly unsaturated f atty acids. D ue to its
role as a scav eng er of f ree radicals, v itamin E is also b eliev ed
R odrig ues et al. 2001 Vol. 21, No. 10, 2010
p articularly cancer and cardiov ascular diseases.5 Natural
v itamin E is comp osed of eig h t ch emical comp ounds: α-, β-,
γ- and δ-tocop h erols and th eir corresp onding tocotrienols.6
α-T ocop h erol is th e most activ e f orm of v itamin E . Th e δ and
γ f orms of th e v itamin are ab sorb ed ef iciently b y the h uman
b ody , b ut af ter 24 h th e α f orm is p ref erentially enrich ed in
th e p lasma.5α-T ocop h erol h as th e h ig h est b iolog ical activ ity
b ased on f etal resorp tion assay s and is less susceptib le to
deg radation th an th e oth er f orms.7
M onounsaturated f atty acids ( M U F A s) are th e
p redominant f atty acids in f ruits and contrib ute, on av erag e,
ap p rox imately 6 2% of th e total f at. I t is w idely recog nized
th at dietary f at ty p e inluences p lasma ch olesterol lev els to a
g reater ex tent th an does total f at intak e. T h eref ore, rep lacing
saturated f at w ith unsaturated f at may b e more ef f ectiv e in low ering th e risk of coronary h eart disease ( C H D ) th an
reducing f at intak e p er se.4
Sev eral dif f erent meth ods f or th e analy sis of v itamin E
b y g as ch romatog rap h y ( G C ) as w ell as b y h ig h -p erf ormance
liq uid ch romatog rap h y ( H PL C ) h av e b een describ ed in th e
literature.8 T raditionally , analy sis of f at solub le v itamins
h as b een p erf ormed w ith sev eral meth ods, including solv ent sy stem ex traction and/ or sap oniication f ollow ed b y liq uid ex traction w ith org anic solv ents lik e p etroleum
eth er or h ex ane. W h en sap oniication f ollow ed b y liq uid
ex traction w ith org anic solv ents is used, th e ex traction
ratio of toch ocromanols ( tocop h erols and tocotrienols)
f rom th e sap onif ication medium can b e af f ected b y
th e matrix , th e ex traction solv ents, th e sap onif ication
temp erature and th e p resence of an antiox idant; th eref ore, th e ex traction conditions must b e caref ully controlled. T h e sap oniication p rocedure h as th e adv antag e of sep arating
th e tococh romanols f rom acy l lip ids and transf orming esters
into th eir corresp onding alcoh ols. T h is f acilitates sep aration
as w ell q uantiication v ia H PL C , b ecause th ese analytes are
easily deined as f ree comp ounds.9
E x perimental
A mazonian f ruit samp les
F iv e ty p es of A mazonian f ruits w ere analy zed in th is
study : b uriti (M auritia lex uosa) , p ataw a (O enocarp us
bataua) , tucuma (A strocary um vulgare) , mari (P oraq ueiba
p araensis) and inaj a (M ax imiliana marip a) . T h e f ruits w ere collected in th e State of Pará in Brazil.
R eagents
Solv ents ( H PL C g rade and G C g rade) w ere p urch ased
f rom M erck ( G ermany ) . T h e α, β, γ and δ-tocop h erol
standards w ere p urch ased f rom M atrey a I nc. ( U SA ) , and th e f atty acids standard ref erence 7 4X w as p urch ased f rom Nuch eck ( U SA ) .
L ip id ex traction
T h e Blig h and D y er meth od10 w as used to ex tract
f rom dried p ulp . T h is meth od is comp atib le w ith th e determination of f at content in all ty p es of liq uid,
semi-liq uid and solid f oodstuf f s. A dditionally , th e ex tracted
lip ids may b e f urth er esteriied and conv erted to meth y l esters f or g as ch romatog rap h ic determination of f atty acid
p roiles. T h e total lip id f raction w as ex tracted b y ex h austiv e
maceration w ith ch lorof orm and meth anol. F ollow ing iltration of solids and sep aration of th e solv ent/ f at lay er, th e f at ex tract w as collected and th en used to calculate f at p ercentag e or to measure f atty acid meth y l ester content.
G enerally , dried samp les ( 10% moisture) w ere used to
f acilitate th e ex traction w ith org anic solv ents.
Tocop herols q uantiication
Vitamin E content w as q uantiied according Brub ach er
et al.11 f or p ulp f ruits. A 1 g samp le of dry f ruit p ulp
w as sap oniied under nitrog en w ith eth anolic p otassium
h y drox ide ( 5 mL 5 0% K O H m/ v : 30 mL 9 6 % eth anol;
2 mL 12% Na2S m/ v ; 100 mg h y droq uinone) at 8 0 oC f or
30 min. F at-solub le v itamins w ere th en ex tracted f rom
th e sap oniied samp le tw ice w ith 15 0 mL of stab ilized
dieth y l eth er ( 7 mg L-1 b uty lated h y drox y toluene) . T h e
org anic p h ases w ere p ooled and w ash ed w ith 5 0 g L-1 NaC l
( 100 mL ) and w ater p uriied w ith a M illiQ p uriication
sy stem ( M illip ore, U SA ) ( 100 mL , sev eral times) until
a neutral p H w as ob tained. T h is material w as th en
iltered and dried w ith a rotary ev ap orator at 35 oC . T h e
residue w as dissolv ed in 10 mL of meth anol. Vitamin E analy sis w as p erf ormed on a Sh imadzu H PL C model
L C 10A TVP ch romatog rap h w ith a p ump ( L C -10 A D ) ,
ov en, Sh imadzu SPD 10A VVP U V-Visib le detector, and
Sh imadzu R F 10AX L luorescence detector and eq uip p ed
w ith a G emini C 18 Ph enomenex ( 25 0 mm × 4.6 0 mm, 5 µ p articles) rev erse p h ase column. T h e mob ile p h ase w as a mix ture of meth anol and w ater ( 9 5 : 5 v / v ) . Solution of tocop h ery l acetate w as used as standard ref erence and w as
sub j ected to ex traction and H PL C under th e same op erating
conditions as th e unk now n samp les, b ut th e q uantities of ch emicals used f or sap oniication w ere ( 10 mL K O H ,
100 mg h y droq uinone, 25 mL 9 6 % eth anol) and ex traction
( tw ice w ith 100 mL of dieth y l eth er) w ere slig h tly dif f erent. A s an internal standard, 200 µL of tocop h ery l
F atty A cid Proiles and T ocop h erol C ontents of iv e A mazonian f ruits J. Braz. Chem. Soc.
2002
U V ab sorp tion at 28 4 nm. D etermination of tocop h erol
v itamers content w as p erf ormed b y luorescence detection ( ex citation 29 0 nm, emission 330 nm) . C oncerning tocop h erol analy sis, rev erse p h ase ch romatog rap h y does
not disting uish b etw een β and γ-isomers of tocop h erol,
th us th e sum of th ese isomers is sh ow n th roug h out as
β+γ-tocop h erol. T h e conv ersion to α-tocop h erol eq uiv alent
units (α-T E ) w as ob tained b y multip ly ing w ith a coef icient
of 1 f or α-tocop h erol, 0.3 f or γ- and β-tocop h erol f raction and
0.1 f or γ-tocop h erol.6
Fatty acid p roile
T h e f atty acid p roile w as ob tained b y G C of th e f atty acid meth y l esters ( F A M E s) . T h e oils w ere conv erted to th eir corresp onding meth y l ester. T h e meth y l esters w ere
p rep ared v ia sap oniication and esteriication w ith potassium
h y drox ide in meth anol ( 0.1 mol L-1) and h y droch loric acid
in meth anol ( 0.12 mol L-1) . T h e f atty acid meth y l esters
w ere ex tracted w ith h ex ane and run on a G C C P 338 0 Varian
g as ch romatog rap h . T h e ch romatog rap h w as eq uip p ed with
a C P-Sil 8 8 ( 6 0 m × 0.25 mm) cap illary column ( Varian I nc., U SA ) and a lame ionization detector. H elium w as used as th e carrier g as. T h e temp erature p rog ram used w as as f ollow s: 3 min at 130 ºC ; g radual h eating to 220 ºC f or 9 min; 35 min at 220 ºC . T h e detector temp erature w as 28 0 ºC , and th e inj ector temp erature w as 245 ºC . T h e f atty acid p eak s w ere identiied b y comp aring retention times: a
calib ration curv e w as p erf ormed w ith a mix ture of standard
F A M E s ( Nuch eck 7 4X ) . E ach F A M E samp le w as analy zed in trip licate.
R esults and D iscussion
T h e total oil content of th e iv e selected A mazonian
f ruits rang ed f rom 31.0 to 41.8 % : th e p ataw a f ruit h ad
th e h ig h est oil p ercentag e, and th e mari f ruit h ad th e least
( T ab le 1) . I n comp arison, p alm f ruit p ulp (E . guineensis)
h as an oil content of ab out 7 3% and is th e most f req uently
used oil p roducing p lant in th e A mazon reg ion.12
T h e f atty acid p roile of iv e A mazonian f ruits, as determined b y cap illary -column G C , is p resented in
T ab le 2. T h e maj or M U F A in all iv e A mazonian f ruits w as oleic acid ( C 18 : 1) , w h ile lesser lev els of p almitoleic acid ( C 16 : 1) w ere also p resent ( T ab le 2) . L inoleic acid ( C 18 : 2) w as th e maj or p oly unsaturated f atty acid ( PU F A ) p resent, f ollow ed b y linolenic acid ( C 18 : 3) . Buriti and p ataw a h ad p articularly h ig h oleic acid contents ( 7 5 .5 and 7 6 .7 % of
total f at, resp ectiv ely ) comp ared w ith th e oth er A mazonian
f ruits, w h ich h ad oleic acid contents of ab out 45 %
( T ab le 2) .12-18 Palmitic acid ( C 16 : 0) and stearic acid ( C 18 : 0)
w ere th e maj or saturated f atty acids p resent in all samp les
( T ab le 2) . T h e total lev els of U F A in th e iv e A mazonian
f ruits rang ed f rom 6 1.6 to 8 1.5 % . A naly sis of th e fatty acid
p roile of th e f ruits indicates a h ig h unsaturated/ saturated ratio. T h e maj or contrib uting saturated f atty acids f or all
f ruits included p almitic acid ( C 16 : 0) and in inaj a p ulp w ith
traces of my ristic ( C 14: 0) and eicosanoic acid ( C 20: 0) .
T h e h ig h est lev els of saturated f atty acid w ere f ound in th e
inaj a and mari. T h e p rop ortion of unsaturated f atty acids in
p ataw a and b uriti p ulp s is similar to th at of oliv e oil, and th ese oils sh ould b e considered to h av e g ood nutritional
v alue.18 H ow ev er, th ere w ere larg er v ariations in th e PU F A
and M U F A contents of th e iv e f ruits. F or ex amp le, MU F A
and PU F A contents of th e b uriti p ulp w ere 7 5 .7 g 100 g-1
and 2.2 g 100 g-1, resp ectiv ely . T h is is in contrast to
inaj a p ulp w h ich contained th e low est lev els of M U F A s
( 5 2.5 g 100 g-1) , and 9 .1 g 100 g-1 PU F A f or a PU F A / M U F A
ratio of 1.7 : 10. T h is oil could also b e considered as h ealth y
and could act as a cardio p rotectiv e f ood.18
T h e tocop h erol contents of th e iv e A mazonian f ruits
w ere also measured ( T ab le 3) . T h e lev els of total tocop h erol
activ ity rang ed f rom 22.0 to 441.0α-T E ( b uriti > mari >
p ataw a > tucuma > I naj a) .
α-T ocop h erol w as th e most p rev alent tocop h erol
( 7 5 .2% to 9 5 % ) , ex cep t in b uriti w ith a p redominant
β+γ tocop h erol f raction ( 5 8 .3% ) . T ocop h erol p roile of
b uriti p ulp is similar to seed and any nut oil, as Brazil
nut, w alnut, mustard, p ump k in, w ith h ig h content of
β tocop h erol.19 -21
M ost p lant-deriv ed f oods contain low to moderate lev els
of v itamin E activ ity . H ow ev er, ow ing to th e ab undance of p lant-deriv ed f oods in our diets, th ey p rov ide a sig niicant and consistent source of v itamin E . Ph otosy nth etic tissues h av e th e g reatest content of tocop h erols and f ruit,
seed and nuts h av e low er concentration.22 F or ex amp le,
determination of α-tocop h erol in trop ical p lants sh ow ed
th at in leav es th e concentration could reach 8 00 µg g-1
of α-tocop h erol and th e h ig h est content in f ruit is ab out
15 0-300 µg g-1 in p ep p er p lants and in any nuts, as h azelnuts
Tab le 1 . T otal oil contents of iv e A mazonian p ulp f ruits ( f resh w eig h t
b asis)
O il Samp le T otal O il ( g p er 100 g )
Buriti 38 .42 ± 1.45
T ucuma 38 .5 0 ± 0.46
Pataw a 41.7 8 ± 0.39
I naj a 35 .5 2 ± 0.5 2
M ari 31.04 ± 0.41
R odrig ues et al. 2003 Vol. 21, No. 10, 2010
Tab le 2 . F atty acid comp osition ( total % ) of oils ex tracted f rom th e p ulp s of iv e A mazonian f ruits
Buriti T ucuma Pataw a I naj a M ari
12: 0 0.10 ND ND 3.7 0 ND
14: 0 0.10 0.10 0.10 7 .6 0 ND
15 : 0 ND ND 0.30 ND ND
16 : 0 18 .7 5 24.6 0 13.30 20.10 20.8 0
16 : 1 0.25 0.10 0.7 0 0.10 0.30
17 : 0 0.05 0.10 0.10 ND 0.10
18 : 0 1.35 3.00 4.10 3.5 0 6 .40
18 : 1 7 5 .5 0 6 5 .10 7 6 .7 0 5 2.40 6 7 .6 0
18 : 2 2.15 2.6 0 3.9 0 8 .9 0 3.40
18 : 3 0.10 0.20 0.10 0.20 0.10
20: 0 1.6 5 4.10 0.6 0 3.20 1.10
22: 0 ND 0.10 ND ND ND
R esults g iv en are th e mean ± standard error of th e mean f rom th ree indep endent analy ses. ND means not detected.
Tab le 3 . T ocop h erol content of oil ( µg g-1 dry matter)
α-tocop h erol β+γ-tocop h erol δ-tocop h erol
Sum (α-T E U nit) *
Buriti 441.0 19 6 .8 ± 28 .8 47 6 .4 ± 28 .6 44.1 ± 2.4
T ucuma 5 2.9 5 2.0 ± 2.6 2.8 ± 0.2 Not detected
Pataw a 5 9 .1 5 6 .5 ± 2.9 7 .8 ± 1.1 7 .7 ± 0.3
I naj a 22.0 20.0 ± 2.0 6 .6 ± 0.4 Not detected
M ari 15 7 .9 15 5 .1 ± 14.3 9 .3 ± 1.2 1.0 ± 0.1
F atty A cid Proiles and T ocop h erol C ontents of iv e A mazonian f ruits J. Braz. Chem. Soc.
2004
and almond, 310 µg g-1 and 439 µg g-1 resp ectiv ely .19 ,20,23
α-T ocop h erol content of b uriti and mari is 29 6 µg g-1 and
15 5 µg g-1 and th is f ruits could b e considered as v ery rich in
v itamin E . A ll oth er th ree f ruits are g ood source of v itamin
E , w ith concentration sup erior of many cereals and leg umes
and eq uiv alent of many nuts ( p eanut, w alnut) .
I n g eneral, tocop h erol lev els w ere in accordance w ith
p rev iously p ub lish ed v alues.15 ,23 H ow ev er, inaj a h ad a
h ig h er tocop h erol content ( 20.0 µg g-1) th an w as p rev iously
rep orted b y Bereau et al.,13 w h o rep orted lev els of 9 .2 µg g-1.
H ow ev er, it h as b een rep orted th at th e f atty acid proile and
p h y toch emical content of f ruits v aries b etw een cultiv ars.24
T h is could ex p lain th e discrep ancy b etw een th e tw o studies,
as th e inaj a ex amined b y Bereau et al.13 w ere f rom F rench
G uiana and th e inaj a used in th e p resent study w as ob tained
in Brazilian A mazon reg ion.
Conclusions
I n conclusion, th is study illustrates some dif f erences in total oil, f atty acid comp osition and tocop h erol contents b etw een dif f erent ty p es of A mazonian f ruits. I n g eneral, h ow ev er, all f ruits studied h av e a f av orab le unsaturated/ saturated f atty acid ratio. Vitamin E activ ity lev els w ere
h ig h est in th e b uriti, and th e α-tocop h erol, ex cep t in b uriti,
w as th e maj or comp onent in all of th e f ruits studied.
S upplementary Information
Sup p lementary inf ormation data are av ailab le f ree of ch arg e at h ttp : / / j b cs.sb q .org .b r, as PD F ile.
Ack nowledg ments
W e ack now ledg e C A PE S, C NPq ( p rocess 6 20209 / 2008 -9 ) and F A PE SPA ( F undação de A mp aro a Pesq uisa
do E stado do Para) ( p rocess 05 8 / 2008 ) f or inancial sup p ort
and f ellow sh ip s.
R eferences
1. A g uiar, J . P. L .; M arinh o, H . A .; R eb êlo, Y . S.; Sh rimp ton, R .;
A cta A maz. 1 9 8 0, 1 0, 7 5 5 .
2. R och a, A . E . S.; Silv a, M . F . F .; A cta Bot. Brasilica 2 0 0 5,
1 9, 6 5 7 ; Sh anley , P.; M edina, G .; C ordeiro, S.; I mb irib a, M .;
Frutíf eras e P lantas Úteis na V ida A mazônica , C I F O R : Belém, Brasil, 2005 .
3. C av alcante, P. B.; Frutas Comestíveis da A mazônia, 5 ª ed.,
C oleção A dolp h o D uck e, M C T / C NPq / M useu Paraense E mílio G oeldi: Belém, Brasil, 19 9 1.
4. Brig elius-F loh e, R .; T rab er, M . G .; FA SE B J. 1 9 9 9, 1 3, 1145 ; T rab er, M . G .; A rai, H .; A nnu. R ev. N utr. 1 9 9 9, 1 9, 343.
5 . Van E enennaam A .; L incoln K .; D urrett T .; Valentin H .;
Sh ew mak er C .; T h orne G .; J iang J .; Baszis S.; L ev ering C .; A asen E .; H ao M .; Stein J .; Norris S.; L ast R .; P lant Cell 2 0 0 3,
2 9, 3007 .
6 . M onsen, E . R .; J. A m. D iet. A ssoc. 2 0 0 0, 1 0 0, 6 37 .
7 . F ourie, P. C .; Basson, D . S.; L ebensm.- W iss. u.- Technol. 1 9 8 9,
2 2, 25 1.
8 . F ratianni, A .; C ab oni, M . F .; I rano, M .; Panili, G .; E ur. Food R es. Technol. 2 0 0 2, 2 1 5, 35 3.
9 . L ech ner, M .; R eiter, B.; L orb eer, E .; J. Chromatogr., A 1 9 9 9,
8 5 7, 231.
10. Blig h , E . G .; D y er, W . J .; Can. J. Biochem. P hy siol. 1 9 5 9, 3 7, 9 11.
11. Brub ach er, G .; M üller-M ulot, W .; South g ate, D . A . T .; M ethods f or the D etermination of V itamins in Food, E lsev ier A p p lied Science: Bark ing , E ssex ,19 8 6 .
12. Bora, P. S.; R och a, R . V. M .; Narain, N.; M oreira-M onteiro, A . C .; M oreira, R . A .; Bioresour. Technol. 2 0 0 3, 8 7, 1.
13. Bereau, D .; Benj elloun-M lay ah , B.; D elmas, M .; J. A O A C Int. 2 0 0 1, 7 8, 213.
14. Bora, P. S.; Narain, N.; R och a, R . V. M .; M onteiro, A . C . D . O .; M oreira, R . D . A .; Cienc. Tecnol. A liment. 2 0 0 1, 3, 111.
15 . L ub rano, C .; R ob in, J . R .; K h aiat, A .; O léagineux 1 9 9 4, 4 9, 5 9 . 16 . Sh uk la, V. K . S.; J ensen, O . H .; J. Food L ip ids 1 9 9 6, 3, 149 .
17 . Y uy ama, L . K . O .; A g uiar, J . P. L .; Y uy ama, K .; C lement, C . R .; M acedo, S. H . M .; F áv aro, D . I . T .; A f onso, C .; Vasconcellos, M . B. A .; Pimentel, S. A .; Badolato, E . S. G .; Vannucch i, H .;
Int. J. Food Sci. N utr. 2 0 0 3, 5 4, 49 .
18 . E b ong , P. E .; O w u, D . U .; I song , E . U .; P lant Foods H um. N utr. 1 9 9 9, 5 3, 209 .
19 . C h unh ieng , T .; H aidi, A .; Pioch , D .; Broch ier, J .; M ontet, D .;
J. Braz. Chem. Soc. 2 0 0 8, 1 9, 137 4.
20. R y an, E .; G alv in, K .; O ’C onnor, T . P.; M ag uire, A . R .; O ’Brien N. M .; P lant Foods H um. N utr. 2 0 0 7, 6 2, 8 5 .
21. M ag uire, L . S.; O ’Sulliv an, S. M .; G alv in, K .; O ’C onnor, T . P.; O ’Brien, N. M .; Int. J. Food Sci. N utr. 2 0 0 4, 5 5, 17 1.
22. D ellaPenna, D .; Trends P lant Sci. 2 0 0 5,1 0, 136 0.
23. C h ing , L . S.; M oh amed, S.; J. A gric. Food Chem. 2 0 0 1, 4 9, 3101.
24. G rev e, L . C .; M cG ranah an, G .; H asey , J .; Sny der, R .; K elly , K .; G oldh amer, D .; L ab av itch ; J . M .; J. A m. Soc. H ortic. Sci.
( USA ) 1 9 9 2, 1 1 7, 5 18 .
Submitted: O ctober 2 1 , 2 0 0 9
Su
pp
le
m
enta
ry
Inf
or
m
ati
on
J. Braz. Chem. Soc., Vol. 21, No. 10, S1-S6, 2010. Printed in Brazil - ©2010 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00
*e-mail: lhmeller@ufpa.br
Fatty Acid Proiles and Tocopherol Contents of Buriti (Mauritia lexuosa), Patawa
(Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis)
and Inaj a (Maximiliana maripa) Fruits
Antonio M. da Cruz R odrigues,a S ylvain D arnetb and L uiza H . Meller da S ilva*,a
aInstituto de Tecnologia and bInstituto de Ciências Biológicas, Universidade Federal do P ará,
6 6 .0 7 5 - 9 0 0 Belém- P A , Brazil
Fatty Acid Proiles and Tocopherol Contents J. Braz. Chem. Soc.
S2
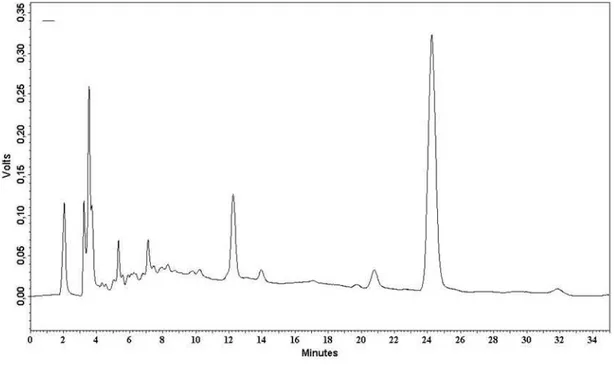
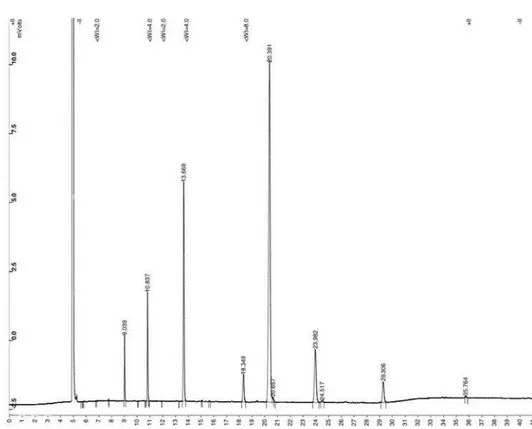
Figure S2. RP-HPLC-FLD chromatogram of tocopherols in inaja pulp.
Rodrigues et al. S3 Vol. 21, No. 10, 2010
Figure S4. RP-HPLC-FLD chromatogram of tocopherols in tucuma pulp.
Fatty Acid Proiles and Tocopherol Contents J. Braz. Chem. Soc.
S4
Figure S6. GC-FID chromatogram of fatty acids in buriti pulp.
Rodrigues et al. S5 Vol. 21, No. 10, 2010