*Corresponding author. Tel.: #33-1-46835627; fax: # 33-1-46619334.
E-mail address:gillian.barratt@cep.u-psud.fr (G. Barratt).
Relationship between complement activation, cellular uptake and
surface physicochemical aspects of novel PEG-modi
"
ed nanocapsules
Vanessa Carla Furtado Mosqueira
, Philippe Legrand , Annette Gulik
, Olivier Bourdon
,
Ruxandra Gref , Denis Labarre , Gillian Barratt
*
Laboratoire de Physico-Chimi, Faculte& de Pharmacie, Universite& de Paris XI Sud, UMR CNRS 8612, 5 rue Jean-Baptiste Cle&ment, ChaLtenay Malabry Cedex 92296, France
Centre de Ge&ne&tique Mole&culaire*UPR A2420, Gif-sur-Yvette 91198, France
LPBC, UPRESA CNRS 7033, Universite& Pierre et Marie Curie, 4 place Jussieu, Case 318, 75252 Paris Cedex 05, France
Departamento de Farma&cia da Escola de Farma&cia, Universidade Federal de Ouro Preto, Rua Costa Sena, 171-Ouro Preto-35400000, Minas Gerais, Brazil Received 24 January 2000; accepted 24 January 2001
Abstract
The aim of our work was to examine the relationship between modi"cations of the surface of nanocapsules (NC) by adsorption or covalent grafting of poly(ethylene oxide) (PEG), and changes in their phospholipid (PL) content on complement activation (C3 cleavage) and on uptake by macrophages. The physicochemical characterization of the NC included an investigation of their properties, such as surface charge, size, hydrophilicity, morphology and homogeneity. This is the"rst time that such properties have been correlated with biological interactions for NC, a novel carrier system with a structure more complex than nanospheres. C3 crossed immunoelectrophoresis revealed the reduced activation for NC with longer PEG chain and higher density, although all formulations induced C3 cleavage to a lesser or greater extent. NC bearing PEG covalently bound to the surface were weaker activators of complement than plain PLA [poly(D,L-lactide)] NC or nanospheres (NS). Furthermore, the #uorescent/confocal microscopy of J774A1 cells in contact with NC reveal a dramatically reduced interaction with PEG-bearing NC. However, the way in which PEG was attached (covalent or adsorbed) seemed to a!ect the mechanism of uptake. Taken together, these results suggest that the low level of protein binding to NC covered with a high density of 20 kDa PEG chains is likely to be due to the steric barriers surrounding these particles, which prevents protein adsorption and reduces their interaction with macrophages. 2001 Elsevier Science Ltd. All rights reserved.
Keywords: Nanocapsules; Poly(D,L-lactide-co-ethylene oxide) copolymers; Complement activation; Cellular uptake; Physicochemical characterization; Surface properties
1. Introduction
When administrated intravenously, conventional col-loidal drug carriers are rapidly cleared from the blood-stream by the mononuclear phagocyte system (MPS), mainly represented by the Kup!er cells of the liver and spleen macrophages [1]. This removal from the circula-tion generally occurs through speci"c recognition by cellular receptors speci"c for plasma proteins bound to the carriers rather than the carriers themselves. The pat-terns of protein absorption that determine colloid recog-nition by macrophages has been extensively studied and
is a very useful tool to evaluate the ability of colloids that undergo delayed plasma protein adsorption [2}4] and thus reduced macrophage uptake. In particular, the complement system plays a major role in the immune system recognition of foreign particles [5]. The concept of surface modi"cation of particulate carriers, developed in the last 10 years in order to control the opsonization process, the speci"c and non-speci"c interaction of par-ticulate carriers with MPS and blood components, raises questions about the optimal surface properties of the carrier [1]. These properties have been modi"ed by adsorption or by covalent attachment of hydrophilic polymers at the colloid surface. Surface charge, size, hydrophilicity and the conformation of the polymer chains are other factors that in#uence their interactions with biological media [6}9].
Stealth威is a trademark of Sequus Pharmaceutical Inc.
Among the polymers able to drastically reduce interac-tion with blood proteins, thus also reducing complement activation, poly(ethylene oxide) (PEG) has been the most investigated. PEG is an uncharged, hydrophilic and non-immunogenic polymer that can be physically adsorbed onto or, preferably, covalently attached to the surface of hydrophobic colloids [8,10]. For example, the presence of the hydrophilic layer of PEG at the surface of the so-called Stealth威liposomes and nanospheres, results in reduced clearance by the MPS and thus prolonged residence time in blood after intravenous administration [10}12].
In contrast to Stealth威 liposomes [10] and nano-spheres [8,9,11,12], surface-modi"ed nanocapsules (NC) bearing adsorbed or covalent grafted poly(ethylene ox-ide) have not been thoroughly investigated as far as their physicochemical characteristics and their interactions with biological medium as a function of their components and surface properties are concerned. The advantages of NC obtained from polyesters such as poly(D,L-lactide) (PLA) or copolymers such as monomethoxypoly(D,L -lactide-co-ethylene oxide) (PLA-PEG) are their biode-gradability, high lipophilic drug payload in the oily core, low polymer content compared with nanospheres and low inherent toxicity [13,14]. These systems represent an alternative colloidal carrier to nanospheres or liposomes when the solubility of the drug is higher in the oil phase of nanocapsules compared to the polymer (or lipid bi-layer).
In previous work, a detailed investigation of the inter-actions between PEG surface-modi"ed NC and macro-phages was undertaken [13]. The hydrated PEG chains are #exible and decrease surface interactions with opsonins by steric repulsion. This e!ect is more depen-dent on the chain length and density of PEG on the particle surface. As a result, NC with a high PEG density showed a reduced interaction with J774A1 cells, with the best results being obtained with a longer PEG chain length (20 kDa) [13]. PLA-PEG diblock polymers are known to impair the complement activation when nano-spheres are incubated in human serum [7]. On the other hand, for PLA-PEG nanocapsules, the direct correlation between the physicochemical properties, such as surface hydrophilicity and complement activation at the NC surface has not yet been studied.
In the present work we have investigated the in#uence of some parameters of NC composition, such as the nature of the hydrophobic polymer block, chain length and density of PEG and the phospholipid (PL) content, on the physicochemical and biological properties of NC. In particular, some properties (size, PEG density, surface charge, surface morphology and hydrophobicity) of PEG-modi"ed and unmodi"ed NC were correlated with their interactions with biological components such as
human serum, complement proteins and macrophages. Complement activation was assessed by two-dimen-sional immunoelectrophoresis of complement compo-nent C3. Fluorescence microscopy of NC, labeled with a#uorescent oil dye, contributed to understanding the cellular pathways involved in NC interactions with phagocytic cells in vitro.
2. Materials and methods
Soy lecithin (Epikuron 170威, composed of approxim-ately 70% of soy phosphatidylcholine) was purchased from Lucas Meyer (France) and Poloxamer-188 (Syn-peronic F68威) from ICI (France). Miglyol 810 N was kindly provided by HuKlls (France). Poly(D,L-lactic acid) PLA with weight average molecular mass (Mw) of 42 kDa was supplied by Phusis (France), poly( -caprolac-tone) (PCL) Mw 42.5 kDa and poly(D,L-lactide-co -glycolide) PLGA (75 : 25 wt. ratio lactic/glycolic acid) 75}120 kDa by Sigma-Aldrich (France). The diblock copolymers PLA-PEG 45}5 (PLA 45 kDa and PEG 5 kDa), PLA-PEG 45}20 (PLA 45 kDa and PEG 20 kDa), PLA-PEG 2}5 (PLA 2 kDa and PEG 5 kDa), PLGA-Peg 45}5 (PLGA 75 : 25 45 kDa and PEG 5 kDa), PCL-PEG 45}5 (PCL 45 kDa and PEG 5 kDa) were synthesized and characterized as described [4]. DiD oil (1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine perchlorate) was supplied by Molecular Probes (The Netherlands). Density marker beads威, Percoll威, Gel-bond威"lms and Agarose were obtained from Pharmacia (Uppsala, Sweden). The solvents used were analytical grade and all other chemicals were commercially avail-able reagent grade. Water was puri"ed by reverse osmo-sis (MillliQ, Millipore威).
2.1. Nanocapsule preparation and characterization
Nanocapsules were prepared by the method described by Fessi et al., based on interfacial polymer deposition following solvent displacement [15] and was previously described in detail [13].
Table 1
In#uence of PEG chain length and lecithin content on physicochemical characteristics of nanocapsules NC formulation and polymer
blend composition
PEG %w/w
Lecithin0.75% w/v Lecithin 0.30% w/v
Mean size$SD (nm)
Polydispersity index
potential (mV)
Mean size$SD (nm)
Polydispersity index
potential (mV)
Naked PLA 0 218$52 0.130 !51.8$0.1 195$42 0.061 !47.7$0.5
PLA-POLOX * 211$61 0.282 !50.9$0.2 246$45 0.017 !35.1$0.1
PLA-PEG (45}5) 10 200$51 0.056 !51.0$1.1 223$45 0.050 !40.6$0.4
PLA-PEG (45}5)/ PLA-PEG (2}5) 2 : 1
20 277$95 0.217 !56.2$1.0 242$42 0.036 !41.9$2.2
PLA-PEG (45}5)/ PLA-PEG(2}5) 1 : 2
30 239$88 0.295 !50.0$0.7 255$44 0.021 !35.6$0.5
PLA-PEG (45}20)/PLA 1 : 2 10 220$71 0.174 !45.9$0.6 242$55 0.079 !36.3$0.7
PLA-PEG (45}20)/PLA 2 : 1 20 216$65 0.146 !45.9$0.6 240$26 0.022 !22.2$0.1
PLA-PEG (45}20) 30 193$59 0.159 !37.8$1.1 226$60 0.105 !5.4$0.2
w/w of total polymer concentration (6 mg/ml).
Lecithin is Epikuron 170威(with&70% of soy phosphatidylcholine).
Standard deviation of populations that were reported by the instrument (n"4) on a typical preparation. Monodispersed samples (below 0.3).
Blend ratio w/w.
(300,000 Da cut-o!membrane) to obtain naked PLA NS, containing only a minimum of poloxamer at their surface. The poloxamer content was not measured after dialysis.
Surface-modi"ed NC were prepared using diblock polymers (PLA-PEG 45}5, PLA-PEG 45}20, PLA-PEG 2}5) and PLA in various combinations as shown in Table 1 so as to obtain PEG contents of 10, 20 and 30% of PEG w/w with respect to total polymer in the acetone phase, with lecithin and Miglyol 810 N as above. No poloxamer was added to the aqueous phase in this case In order to prepare #uorescent NC, a hydrophobic #uorescent marker, DiD oil, was incorporated into the oil phase (83.3g/ml of NC suspension) at high loading yields ('99%) as described [13]. This probe has the advantage of possessing excitation and emission max-imum at much higher wavelengths than those of cell components. The size of the nanocapsules was deter-mined by quasi-elastic light scattering (QELS), with a Nanosizer (Coulter model N4 Plus, Coulter Electronics Inc., Hialeah, FL, USA) and potential measurements were carried out (Zetasizer 4, Malvern Instr., UK) after dilution of nanocapsules by a constant factor of 1 : 250 in 1 mM NaCl (the conductivity was constant at 100$ 5S/cm) except when otherwise stated.
2.2. Hydrophobic interaction chromatography
The hydrophobicity of the di!erent surfaces of NC with or without PEG was investigated by hydrophobic interaction chromatography (HIC), used as previously described for the assessment of surface modi"cation of polystyrene particles by ethoxylated surfactants [6]. The column was propyl-agarose (Sigma, France); its diameter
was 1.0 cm, the height 16 cm, the void volume 5.5 ml and the#ow rate 0.5 ml/min. The elution bu!er was PBS and 0.1% Triton X-100 in PBS was used as the washing solution, after elution of the "rst 25 ml. The injected volume was 200l and the concentration was adjusted to yield elution peaks with a maximum absorption of 0.9 at 350 nm.
2.3. Density gradient studies
Separation of particles was achieved by development in situ of colloidal silica (Percoll威 54% v/v in NaCl 0.15M, initial density 1.068 g/cm) gradients during cen-trifugation in a model SW41Ti rotor (Beckman) at 203C and 20800 g for 130 min. 11.8 ml of Percoll were added to 0.2 ml of initial colloidal suspension without prior concentration. In a separate tube, density marker beads威, of di!erent pre-determined densities were added under the same conditions as samples and used for ex-ternal calibration of the bands. Millimeter scaled paper strips were used to measure the distance from the top meniscus to the band limits. Particle densities were cal-culated from the curves plotting distance from the top versus the density of each band of marker beads. The encapsulated#uorescent dye (DiD) was a helpful tool to identify the separated bands.
2.4. Nanocapsule morphology by freeze-fracture scanning electron microscopy
liquid propane. Fracturing and shadowing, using Pt}C, were performed in a Balzers BAF 301 freeze unit. The replicas were observed in a Phillips 410 electron micro-scope.
2.5. Crossed immunoelectrophoresis of complement component C3
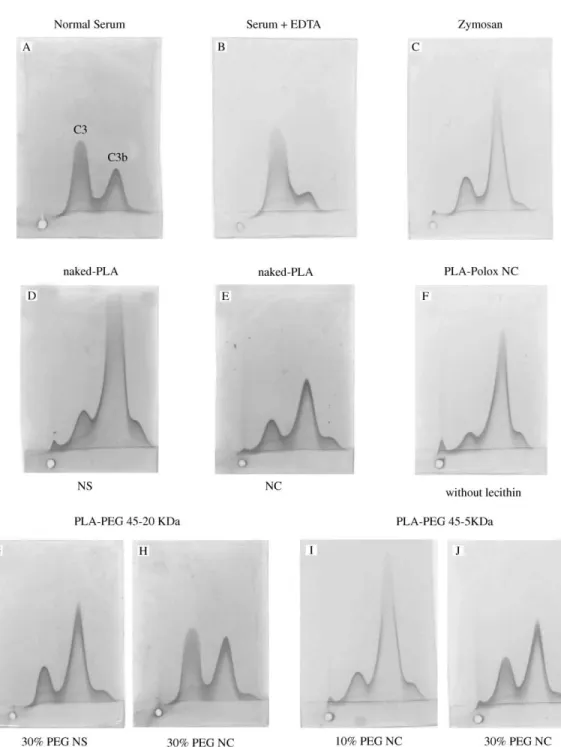
The speci"c activation of C3 complement component b y di!erent NC surfaces in human serum was assessed by comparative measurements of C3 cleavage, as previously described [16,17]. Human serum was obtained from healthy donors and stored at !803C until use. Nanoparticles were incubated for 1 h at 373C with hu-man serum diluted 1/4 in veronal-bu!ered saline contain-ing 0.15 mMCa and 0.5 mMMg ions (VBS>) with gentle agitation. To achieve a valid comparison of the di!erent NC and NS batches, sample volumes with an equal surface area (500 cm) were incubated with 100l of hu-man serum. The method of calculation of NC and NS surface area is described elsewhere [7,13]. All the samples (5l) were subjected to a"rst isoelectric focusing on 1% agarose gel. The second-dimension electrophoresis was carried out on Gelbond威"lms in agarose gel containing a polyclonal antibody to human C3, recognizing both C3 and C3b(complement C3 antiserum raised in goat, Sigma, France). The "lms were "nally stained with Coomassie blue (Sigma). The ratio between the heights of the C3 and C3bpeaks was calculated. Opsonized zymozan (Sigma), pretreated as described [18], was used as a positive control.
2.6. Fluorescence microscopy
The J774 A1 murine macrophage-like cell line was maintained as an adherent culture as previously de-scribed [13]. Cell viability was superior to 95% after 4 h incubation with particles as estimated by the MTT con-version test. One million J774A1 cells were previously seeded for 2 h onto sterile cover slips in 6-well plates (Costar) and incubated for 4 h at 373C with DiD-labeled NC diluted 20 times in RPMI/10% (v/v) FCS medium without phenol red (4200 ng of DiD/ml). Incubations at 43C were carried out in parallel. The cover slips were washed twice with ice-cold PBS and the living macro-phages were placed in the closed chamber with fresh RPMI medium without FCS. The cells were immediately observed with a 63;objective in Nikon epi#uorescence microscope (Optiphot-2, Japan) equipped with a Nipkow wheel coaxial-confocal attachment coupled to cooled camera (RTEA1317 K1CCD, Princeton Instruments). Fluorescence images were obtained with rhodamine"lter set (BP546, FT590, LP600). Micrographs were transfer-red to a computer for processing with Adobe Photo-shop威 4.0. Images were assembled and printed directly on photo quality glossy paper.
3. Results
3.1. Physicochemical characterization of nanocapsule surface
Table 1 shows the e!ect of PEG (content and chain length) and the soy lecithin concentration on NC size, polydispersity andpotential. The NC size was between 150 and 250 nm diameter depending on the formulation tested. Particle dimensions and polydispersities seemed to be more in#uenced by the amount of soy lecithin and by the blending of polymers, PLA-PEG 45}20 kDa and PLA, or PLA-PEG 45}5 kDa and PLA-PEG 2}5 kDa than by the characteristics of the polymers themselves. Lower polydispersities were obtained with lower amounts of lipids. The results shown in Table 1 are from one representative batch of each type of nanocapsule. In every preparation the same tendencies were observed: the use of blends of di!erent polymers led to slightly larger NC than those prepared from only one polymer type, whatever the blend of polymer used.
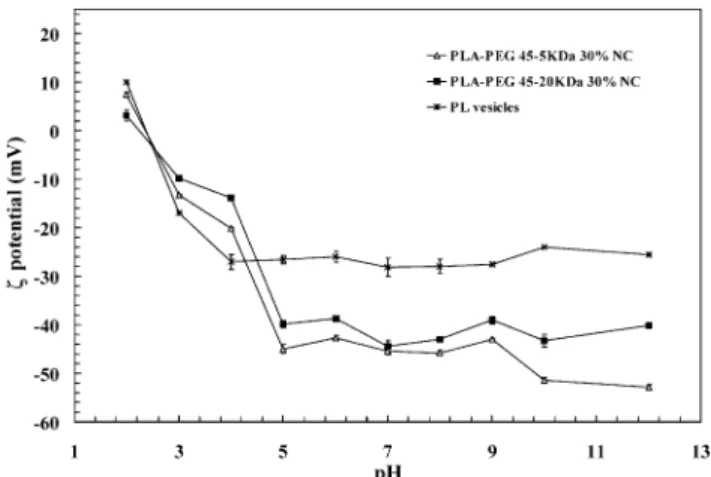
Electrical potential distributions on the surface of PEG NC were examined by measuringpotential. When the ionic strength and concentration of NC in the suspen-sion were kept constant it was possible to observe the e!ects of chain length, PEG content and soy lecithin content on thepotential. A strong in#uence of lecithin (PL) onpotential of NC was observed (Table 1). In fact, a high concentration of soy lecithin imparted a high negativepotential to the NC regardless of the polymer, the length and the content of PEG used. In this case, only PLA-PEG NC with PEG 20 kDa at 30% w/w content had an e!ect on surface charge compared with naked PLA NC (!38 to!51 mV). However, when lower amounts of soy lecithin (0.3% w/v) were used, increasing the PEG content led to potentials closer to zero for surface-modi"ed NC. This e!ect was much more pro-nounced for NC with PEG chains of 20 kDa Mw (!5 mV), showing an e$cient shielding of the negative surface charges.
This strong in#uence of lecithin is con"rmed in Fig. 1. Thepotential pro"le of di!erent PEG NC as a function of pH was similar to the pro"le of lecithin-based vesicles, and was related to the ionization of the phosphate (nega-tive charge at higher pH) and choline groups (posi(nega-tive charge at lower pH) of phosphatidylcholine. At higher pH values, NC have more negative potential values than lecithin vesicles, probably due to the ionization of free fatty acids from the oil and from lecithin mixture as previously discussed [19,20].
3.2. Hydrophobic interaction chromatography
Fig. 1. Thepotential of PEG surface modi"ed NC as a function of the pH. All the formulations were obtained with lecithin at 0.75% w/v. The potential measurement was performed after sample dilution 50 times in solutions of di!erent pH adjusted with NaOH and HCl at constant ionic strength. Results are the mean of three measurements of two sample dilutions (n"6)$SD.
Fig. 2. HIC chromatogram of di!erent NC formulations prepared by the solvent displacement method. The arrow represents the beginning of washing with Triton X-100. The phospholipid (PL) vesicles were prepared with 0.75% w/v of lecithin in water.
chromatography. The formulations containing lecithin (PL) and poloxamer showed little interaction with the column and were eluted rapidly. In contrast, the formula-tion without lecithin was the most retained on the col-umn and exhibited a larger area peak. Three additional peaks were also observed in this case even after washing step indicating higher hydrophobic interaction with col-umn. Naked-PLA NC, PLA-POLOX and PLA-PEG NC produced similar pro"les with little interaction be-tween particles and the gel matrix.
3.3. Nanocapsule homogeneity
Fig. 3 shows the density pro"les obtained from di! er-ent preparations after ultracer-entrifugation in Percoll威 gradient. This technique allows the heterogeneity of the
preparations to be assessed in terms of formation of di!erent systems in the same preparation process. In-creasing the amount of PEG reduces the polydispersity of the system and increases the homogeneity of the NC, with less formation of NS. Probably, the amphiphilic polymer containing PEG surrounds the oil droplets more e!ectively than the monoblocks of more hydropho-bic PLA polymer. NC prepared from blends of polymer (e.g. PLA and PLA-PEG) were more heterogeneous than the NC obtained from only one polymer, as evidenced by thicker bands. PLA NS are present as contaminants only in formulations obtained from blends, but in very small amounts. The bands corresponding to the lower densities are probably due to the presence of small amounts of uncovered droplets as nanoemulsions (NE), that can be directly compared with the NE formulation. PL vesicles are very polydisperse. The presence of these vesicles seen in higher proportions in NE and PLA-POLOX NC than in the other NC preparations. These results indicate that, under our conditions, PLA-PEG 20 kDa NC at 30% PEG and PLA-PEG 5 kDa NC at 10% PEG yield the most homogeneous formulations of NC. These are not prepared from blends of polymers.
3.4. Nanocapsule morphology
Freeze-fracture electron microscopy revealed the pres-ence of nanocapsules. Fig. 4 shows typical images. In both cases, PLA NC (not shown) and PLA-PEG NC, it was possible to obtain cross-fractured particles. This is the usual way of the propagation of the fracture of triglyceride microemulsions. However, we often noticed a plastic deformation on the border of the PLA-PEG NC which is probably related to the nature of the polymer.
3.5. Serum protein adsorption
Fig. 3. Ultracentrifugation of di!erent formulations loaded with DiD probe in a Percoll gradient. Gradient density was monitored using colored density marker beads in 0.15M NaCl. The starting density was 1.068 g/cm. NC were obtained as described in Section 2. For comparison, nanoemulsion, nanospheres and phospholipids vesicles were obtained in the same way without polymer, without lecithin or oil, and with 0.75% w/v of lecithin only, respectively. The gray-scale intensity of the bands is proportional to their intensity in Percoll威.
Fig. 4. Freeze-fracture electron microscopy. Visualization of naked PLA NC (A) and PLA-PEG NC (B). Bar"200 nm, the two micro-graphs are at the same magni"cation.Large NC of about 200 nm of diameter are clearly identi"ed. In (B) the border appears much more deformed than in (A). Both NC were prepared with a minimal concen-tration of lecithin (0.05% w/v).
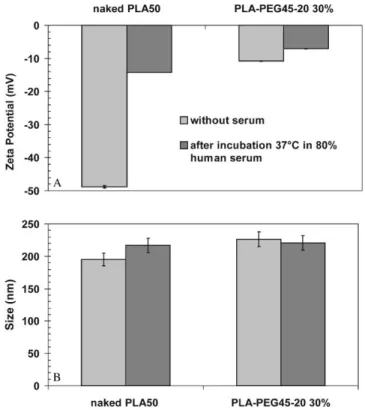
Fig. 5. E!ect of human serum protein binding onpotential (A) and size (B) of NC. To determine the e!ect of serum proteins onpotential, 100l nanocapsules were incubated in 400l of human serum for 30 min at 373C. The NC with or without serum were diluted 1 : 250 1 mMNaCl and the conductivity was adjusted to 160$6S/cm for direct comparison.
3.6. Assessment of complement activation
Evidence of C3 cleavage was obtained by measuring C3 and C3bmigration by crossed immunoelectrophoresis
Table 2
Relationship between PEG content, distance, chain length,potential and C3 activation of PEG and naked NC obtained from di!erent biodegradable polymers
Nanocapsule formulation and polymer composition
PEG % w/w potential (mV) DPEG-PEG
(nm) calculated
Ratio height C3/C3bpeaks
Naked PLA 0 !47.7$0.5 * 0.30
PLA-PEG 45}5 KDa 10 !43.5$0.5 4.17 0.42
PLA-PEG 45}20 KDa 30 !3.9$0.2 3.82 1.21
Naked PLGA (75 : 25) 0 !43.8$0.5 * 0.75
PLGA-PEG 45}5 KDa 10 !41.7$0.3 3.78 0.94
Naked PCL 0 !46.9$0.4 * 0.70
PCL-PEG 45}5 KDa 10 !39.9$0.3 4.02 0.70
Lecithin at 0.3% w/v.
w/w of total polymer concentration (6 mg/ml).
proportions of native and cleaved C3. Controls were carried out to estimate C3 cleavage in the absence of particles, in the absence of divalent ions in serum and in the presence of opsonized zymozan, a well-known com-plement activator. The relative sizes of the peaks of C3 and C3b fragments generated by incubation with naked PLA NC and PLA NS, POLOX NC and PLA-POLOX NC without lecithin were measured in order to compare with those generated by contact with PEG covalently bound to PLA NC.
Table 2 and Fig. 6 summarize the results obtained with the di!erent NC formulations. To assess the e!ects of hydrophobic cores composed of di!erent biodegradable polyesters, NC with increasing hydrophobicity related to the nature of side chain were prepared from PLGA, PLA and PCL. Table 2 shows the e!ect of these di!erent polymer blocks on complement activation in relation with their surface properties. NC diameter was between 180 and 270 nm and the polydispersity indexes of the populations were very similar (data not shown). The PEG distances and potential were close, except for PLA-PEG 45}20 kDa NC, which show lowerpotential values and the lowest complement activation. PLA, which has intermediate hydrophobicity, was the stronger activator in a NC system. The PCL and PLGA NC activate C3 less than those with a PLA polymer. More-over, the activation pattern for NC containing di!erent polyesters was not greatly altered by PEG at similar chain length and distance except for the higher chain length (20 kDa), as shown in Table 2 for PLA-PEG 45}20 kDa 30% NC.
A clear di!erence in activation between NS and NC is shown in Figs. 6D and E. Naked-PLA nanospheres have a more activating surface than naked PLA NC under our experimental conditions. It is worth pointing out that the only di!erence between these formulations is the pres-ence of lecithin and oil in the NC composition, which obviously changes the surface characteristics. Indeed, PLA-POLOX NC without lecithin showed a strong
ac-tivation, indicating that lecithin plays a role in reducing C3 cleavage. At the same time, we observed a signi"cant decrease of C3 activation on PLA-PEG 20 kDa NC compared with PLA-PEG 20 kDa NS, although the dis-tance between PEG chains on the surface is twice as great in NC as in NS (Figs. 6G and H). PEG 5 kDa is not very e!ective at reducing complement activation at the NC surface even at smallDvalues (Figs. 6I and J). The same observation holds for NC prepared from PLGA-PEG and PCL-PEG copolymers with a PEG chain of 5 kDa (Table 2).
3.7. Uptake and localization in J774A1 cells
Fig. 6. Crossed immunoelectrophoresis of C3 antigens in human serum diluted 1/4 in VBS>after 60 min incubation with NP suspensions at constant surface area (500 cm). (A) Serum/VBS>; (B) serum/EDTA 10 mM1 : 3 v/v; (C) serum/VBS>/zymosan; (D) naked-PLA NS 143 nm; (E) naked-PLA NC 206 nm; (F) PLA NC without lecithin 252 nm; (G) PLA-PEG 20 30% NS 128 nm,D"2.0 nm; (H) PLA-PEG 20 30% NC, 241 nm,D"3.82 nm; (I) PLA-PEG 5 10% NC 224 nm,D"3.48 nm; (J) PLA-PEG 5 30% NC 255 nm,D"2.0 nm.
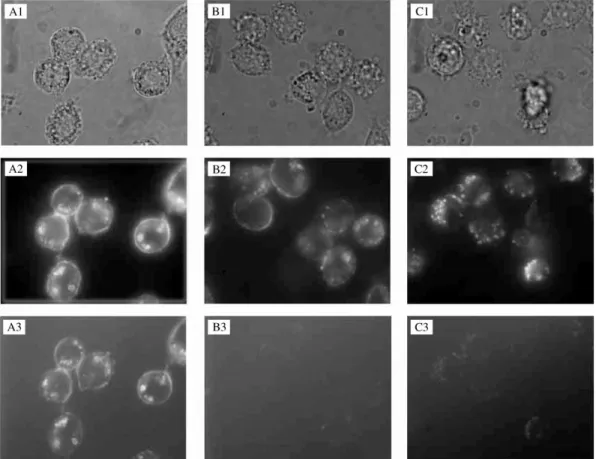
#uorescent technique gives a semi-quantitative indica-tion of the #uorescence intensity di!erence between the three formulations (Figs. 7A3, B3 and C3). A very low intensity was observed for PEG and PLA-POLOX NC. Reducing the incubation temperature from 373C to 43C led to a lower#uorescence association for all NC formu-lations, with dramatically reduced #uorescence inside cells consistent with a passive binding of NC to the J774A1 cell membrane (results not shown). These
obser-vations con"rm our previous quantitative studies [13], summarized in Table 3.
4. Discussion
Fig. 7. Phase-contrast (1),#uorescent (2) and confocal-like (3) microphotographs of living J774A1 cells exposed to DiD-labeled NC. Cells were treated with naked PLA NC (A), PLA-PEG 45}20 KDa NC (30% of PEG) (B) and PLA-POLOX NC (C) at 300g of polymer/ml, at 373C and 43C for 4 h. Pictures were immediately taken after NC were removed from the medium using the same conditions of illumination exposure and were printed without modi"cations, to allow direct comparison. Three cover slips were observed for each formulation, representative areas are shown.
Table 3
In#uence ofDand chain length on the cellular uptake by J774A1 Nanocapsule formulation and
polymeric blend composition
PEG % w/w DPEG-PEG (nm)
calculated
Cell-associated#uorescence (ng DiD/10cell)
Naked PLA 0 * 313
PLA-PEG 45}5 10 4.27 122
PLA-PEG 45}5/2}5 1 : 2 30 2.24 89
PLA-PEG 45}20/PLA 1 : 2 10 7.82 83
PLA-PEG 45}20 30 4.49 24
Lecithin at 0.75% w/v.
w/w of total polymer concentration (6 mg/ml).
300g/ml (4200 ng DiD/ml) of NC suspension was added to the culture medium for 4 h. Measured as described in Ref. [13].
reduction of thepotential as a result of the presence at the surface of the PEG layer that shifts the plane of shear to the outer boundary of the layer has been observed [8,22,23]. The surface charge of NC as re#ected by their potential is drastically reduced at higher PEG densities only at low soy lecithin content (0.3% w/v). The longer chain length (20 kDa) had a more marked e!ect in reduc-ing the electro-kinetic mobility of these particles than had PEG 5 kDa. Thispotential decrease could be due
(compared with NS) would demand a higher chain length and density of PEG (more bound water in the surround-ing medium) to obtain the optimal colloidal steric stabil-ization.
The pro"le ofpotential of PEG NC as a function of pH is very similar to that of lecithin (PL) vesicles (Fig. 1). Lecithin was used in NC preparations whatever the poly-mer, PLA or PLA-PEG. Indeed, in previous work it was shown that lecithin has a major role in determining surface properties because minor components of lecithin are able to impart a strong negative charge to the inter-face [20]. Thus, this result could explain in part the absence of the`PEG e!ectaon the NC surface charge at the higher lecithin content. However, after reducing the lecithin content the`PEG e!ectacan be observed more clearly.
The presence of ionizable groups, mainly phosphates and choline in the phospholipids used to stabilize NC (lecithin), confers hydrophilic properties to the interface of these systems, as observed in hydrophobic interaction chromatography experiments. The presence of polyethy-lene oxide chains at the surface, adsorbed or covalently grafted, is expected to rend these colloids even less hydro-phobic (Fig. 2). It is interesting to note that some prep-arations are composed of di!erent populations of particles of similar size but with di!erent hydrophobici-ties, of varying densihydrophobici-ties, in accordance with the gradient density results shown in Fig. 3. These studies con"rm the presence of PL vesicles in some NC preparations. Lecithin (PL) vesicles and PLA-PEG 45}20 30% NC are the least hydrophobic formulations. The latter is the only formulation for which it is possible to a$rm that the hydrophilicity is mainly due to the PEG coating layer because PLA-PEG 45}20 30% NC is the most homo-geneous formulation, as shown in Fig. 3, in which the presence of contaminant PL vesicles could not be detec-ted. The preparation of PLA-PEG NC without lecithin yielded aggregated particles under our preparation con-ditions, regardless of their PEG content, which prevented their surface analysis. For all the other formulations, soy lecithin remains the principal hydrophilic component at the NC surface and masks the e!ect of di!erent PEG chains. This could be due to the large volume of the hydrophilic head group of the lecithin phospholipids that could be placed at electrical double layer around the NC surface. It was previously shown that when 0.75% w/v of lecithin was used in the NC formulation a large contami-nation with phospholipids vesicles occurred but this was reduced by reducing the lecithin content to 0.15% in NC [20].
Particle size and potential measurements can be measured easily and changes in these parameters can give information about protein absorption onto particles. Generally, the adsorption pattern can be di!erent in serum when compared with plasma due to depletion of some proteins. However, the complement system remains
functional in serum and was chosen for our experiments. The dramatic change in thepotential value of the naked NC after incubation indicates that a layer of protein was adsorbed at the NC surface, in contrast to the reduced e!ect onpotential and size for PLA-PEG NC (Fig. 5). The presence of a PEG layer at the NC surface reduces the charge-induced and hydrophobic binding of plasma, as discussed by other authors [25]. The analysis of the NC morphology by freeze-fracture electron microscopy shows indirectly but clearly that the nature of PLA-PEG and naked-PLA NC surfaces are di!erent, in#uencing even the physical freeze-fracture properties (Fig. 4).
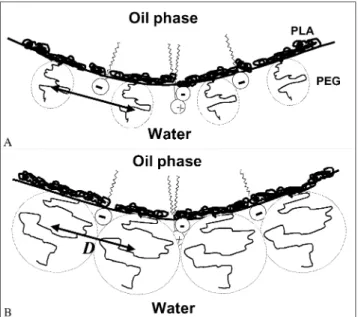
Fig. 8. A schematic and hypothetical representation of NC surface showing the PEG hydrated cloud shield over charged groups located underneath it and PLA blocks at the oil}water interface at the same surface distance (D). In (A) PEG 5 kDa and in (B) PEG 20 kDa chains. As the PLA blocks are insoluble in both phases they acquire the most energetically favorable`side-onaorientation at the oil}water interface [28].
proposed in Fig. 8, based on the PLA-PEG molecular chain arrangements at the oil}water interface as pre-viously described [28]. It is widely expected that the preferential distribution of PEG layers at surfaces would change as the area per group decreases, from a` mush-rooma in the dilute, unhindered state at low surface concentration, to an extended `brusha at high surface concentration, when interactions between adjacent head groups force each chain to extend further from the sur-face to which the PEG is attached [29], especially for higher PEG molecular masses.
It is noteworthy that for the same amount of polymer the NC surface is six to ten times greater than that of NS leading to lower concentration of PEG chains at the NC surface. The crossed immunoelectrophoresis results show that at equivalent surface area, reduced complement ac-tivation is obtained for NC compared to NS systems for both naked PLA and PLA-PEG particles. However, the values of D for nanospheres were lower than for NC (Figs. 6G and H). These di!erences between NC and nanospheres seems to be due to the presence of lecithin in NC formulations. Furthermore, the increasing comp-lement activation with the PLA-POLOX formulation without lecithin provides additional evidence in this sense (Fig. 6F). Some preliminary results concerning complement consumption by PLA-POLOX NC were obtained using the CH50 method [7]. They indicated that a PLA-POLOX NC suspension/serum/VBS> at 2 : 1 : 1 ratio was able to induce 27% consumption of
CH50 units at 5500 cm. This value is very low compared to PLA-F68 nanospheres consumption, estimated in a previous work as being greater than 100% for the same surface area [7]. This method did not allow a precise comparison between NC formulations because of the interference of NC turbidity when estimating hemolysis spectrophotometrically. However, this last result also indicates that lecithin probably play a role in reducing C3 activation when placed at the NC surface.
In our study, the PEG 5 kDa chains at higher density are more e!ective in reducing C3 cleavage than at lower density (Figs. 6I and J). Furthermore, PEG 20 kDa chains were also more e!ective in preventing C3 cleavage than PEG 5 kDa chains even at a lower PEG density. PEG 20 kDa chains might be more#exible than PEG 5 kDa chains at almost the same surface distance (D), thus reducing the accessibility to C3 fragments. Long hydrophilic chains could also exert steric e!ects of sur-face hindrance, thus shielding lecithin charge and further reducing C3 deposition, as well as the interaction with proteins in general as shown in Fig. 5.
To summarize, in this work two major e!ects were observed. The"rst is the e!ect of lecithin in NC surface and the second is the e!ect of PEG chains providing a steric stabilization. They seem to be additive in reduc-ing complement activation of NC compared to NS. Fur-thermore, our results suggest the limit value (D&2.2 nm) previously predicted for the NS system for protein rejec-tion might to be higher for NC systems [4,7,8]. It could be speculated that the kind of polymeric carrier, NC or NS, would determine the response of complement system as a function of the other factors such as surface morpho-logy and chemical topomorpho-logy of its structure, as already observed for nanospheres [3].
The fragments C3band iC3bgenerated by comp-lement activation provide speci"c recognition by type CR1 and CR3 receptors, respectively, on macrophages. Thus a reduced activation of complement system af-forded by the PEG layer around the NC surface would be expected to reduce interactions with phagocytic cells and prevents direct contact with cell membrane. The results obtained in this study using #uorescence con-"rmed the conclusions of our recent paper [13], which is the "rst report using PLA-PEG diblock polymers to obtain surface-modi"ed NC. NC containing PEG on their surface show reduced interaction with cells and di!erent mechanisms of uptake. Table 3 illustrates the quantitative reduction of #uorescence association with macrophages as a function of PEG distance and chain length at the NC surface. The PLA-PEG 45}20 kDa NC with 30% of PEG yielded the lowest level of interaction (13 times less than naked-PLA NC) and were therefore used in the microscopic study.
little probe bound to the surface. It has been reported that poloxamer in micellar form enters the cell by# uid-phase endocytosis in bovine brain microvessel endo-thelial cells [30]. Since #uid-phase endocytosis in J774 macrophages usually involves vesicles of 30}300 nm dia-meter and does not require binding in the cell surface as a"rst step to internalization [31,32] it is possible that PLA-POLOX NC are taken up by this mechanism. In-deed, in Fig. 7C2, the vesicles inside cells are smaller than those in Fig. 7A2, indicating that di!erent endocytic processes are taking place. This phenomenon merits fur-ther studies.
Taken together, the results suggest that the low level of protein binding in PLA-PEG 45}20 30% NC is likely to be due to the steric barrier surrounding these particles which prevents protein adsorption and reduces interac-tion with macrophages.
5. Conclusion
The in vivo behavior of surface-modi"ed NC could be a!ected by many aspects of their formulation. The NC obtained by nanoprecipitation process are more or less heterogeneous depending on the polymers and the con-centration of surfactants used. The PEG NC obtained with PLA-PEG 20 kDa copolymer are the most homo-geneous according to density studies. It is necessary to consider the results of several di!erent techniques (HIC, potential, electronic microscopy) in order to have a clear picture of the nature of the NC surface. The techniques of crossed immunoelectrophoresis and# uor-escent microscopy were able to demonstrate that the di!erences between PEG modi"ed NC preparations are closely correlated with our previous results obtained in vitro with J774A1 cells [13]. All of our preparations activate complement C3 protein during 1 h of incubation. However, PLA-PEG NC seems to activate complement to a lesser extent than PLA-PEG nanospheres, although direct comparison is di$cult because the NC surface is very di!erent in nature from that of NS. More detailed studies are needed to understand the role of the phos-pholipids (lecithin) in the interactions with complement. PLA-PEG NC with higher PEG chain lengths (20 kDa) and densities showed a good correlation between some of their surface physicochemical aspects and biological in-teractions in vitro. These`Stealthaproperties need to be con"rmed by a study of the behavior of these NC in vivo; the results of such experiments will be the subject of a forthcoming paper.
Acknowledgements
The authors thank the Brazilian National Council of Scienti"c and Technological Development (CNPq)-Brazil
personal"nancial support to the"rst author. This work was supported by CNRS, France. The authors would like to thank Dr. O. Seksek and F. Robinet for their help with #uorescence microscopy experiment and Dr. C. Passirani for her skilful help in demonstrating the crossed im-munoelectrophoresis technique.
References
[1] Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv Drug Del Rev 1995;16:195}214.
[2] AlleHmann E, Gravel P, Leroux JC, Balant L, Gurny R. Kinetics of blood component adsorption on poly(D,L-lactic acid) nanopar-ticles: evidence of complement C3 component involvement. J Biomed Mater Res 1997;37:229}34.
[3] Luck M, Paulke B-R, SchroKder W, Blunk T, MuKller RH. Analysis of plasma protein adsorption on polymeric nanoparticles with di!erent surface characteristics. J Biomed Mater Res 1998;39:478}85. [4] Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnish
S, Blunk T, MuKller R. Stealth corona-core nanoparticles surface modi"ed by polyethylene glycol (PEG): in#uences of the corona (PEG chain length and surface density) and of the core composi-tion on phagocytic uptake and plasma protein adsorpcomposi-tion. Collo-ids Surf B: Biointerfaces 2000;18:301}13.
[5] Kazatchkine MD, Carreno MP. Activation of complement sys-tem at the interface between blood and arti"cial surfaces. Bio-materials 1988;9:30}5.
[6] Carstensen H, MuKller BW, MuKller RH. Adsorption of ethoxylated surfactants on nanoparticles. I. Characterization by hydrophobic interaction chromatography. Int J Pharm 1991;67:29}37. [7] Vittaz M, Bazile D, Spenlehauer G, Verrecchia T, Veillard M,
Puisieux F, Labarre D. E!ect of PEO density on long-circulating PLA-PEO nanoparticles which are very low complement ac-tivators. Biomaterials 1996;17:1575}81.
[8] Gref R, DombA, Quellec P, Blunk T, MuKller RH, Verbavatz JM, Langer R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Del Rev 1995;16:215}33.
[9] Peracchia MT, Vauthier C, Passirani C, Couvreur P, Labarre D. Complement consumption by poly(ethylene glycol) in di!erent conformations chemically coupled to poly(isobutyl 2-cyanoac-rylate) nanoparticles. Life Sci 1997;61:749}61.
[10] Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta 1992;1113:171}93.
[11] Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nano-spheres. Science 1994;263:1600}3.
[12] Verrecchia T, Spenlehauer G, Bazile DV, Murry-Brelier A, Ar-chimbaud Y, Veillard M. Non-stealth (poly(lactic/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as in-jectable drug carriers. J Control Rel 1995;36:49}61.
[13] Mosqueira VCF, Legrand P, Gref R, Heurtault B, Appel M, Barratt G. Interactions between a macrophage cell line (J774A1) and surface-modi"ed poly(D,L-lactide) nanocapsules bearing poly(ethylene glycol). J Drug Target 1999;7:65}78.
[14] Mosqueira VCF, Legrand P, Gref R, Barratt G. In vitro release kinetic studies of PEG-modi"ed nanocapsules and nanospheres loaded with a lipophilic drug: halofantrine base. Proc Int Symp Control Rel Bioact Mater 1999;26:1074}5.
[15] Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition follow-ing solvent displacement. Int J Pharm 1989;55:R1}4.
[17] Passirani C, Barratt G, Devissaguet J-P, Labarre D. Interactions of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate) with the complement system. Life Sci 1998;62:775}85.
[18] Pick E, Keisari Y. Superoxide anion and hydrogen peroxide pro-duction by chemically elicited peritoneal macrophages-inpro-duction by multiple nonphagocytic stimuli. Cell Immunol 1981;59:301}18. [19] Washington C. Stability of lipid emulsions for drug delivery. Adv
Drug Del Rev 1996;20:131}45.
[20] Mosqueira VCF, Legrand P, Pinto-Alphandary H, Puisieux F, Barratt G. Poly(D,L-lactide) nanocapsules prepared by a solvent displacement process: in#uence of the composition on physicochemical and structural properties. J Pharm Sci 2000; 89:614}26.
[21] MuKller RH, Wallis KH, TroKster SD, Kreuter J. In vitro character-ization of poly(methyl-methacrylate) nanoparticles and correla-tion to their in-vivo fate. J Control Rel 1992;20:237}46. [22] Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AGA,
Taylor DC, Irving MP, Purkiss SC, Tadros TF, Davis SS, Illum L. Surface modi"cation of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)-poly(ethylene glycol) copolymers. Pharm Res 1994;11:1800}8.
[23] Jansen J, Song X, Brooks DE. Interfacial thickness of liposomes containing poly(ethylene glycol)-cholesterol from electrophoresis. Biophys J 1996;70:313}20.
[24] Bazile D, Prud'Homme C, Bassoulet M-T, Marlard M, Spen-lehauer G, Veillard M. Stealth Me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci 1995;84:493}8.
[25] Leroux JC, AlleHmann E, De Jaeghere F, Doelker E, Gurny R. Biodegradable nanoparticles-from sustained release formulations to improve site speci"c drug delivery. J Control Rel 1996; 39:339}50.
[26] Semple SC, Chonn A, Cullis PR. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clear-ance behavior in-vivo. Adv Drug Del Rev 1998;32:3}17. [27] Chonn A, Cullis PR, Devine DV. The role of surface charge in the
activation of the classical and alternative pathways of comp-lement by liposomes. J Immunol 1991;146:4234}41.
[28] Gref R, Babak V, Bouillot P, Lukina I, Bodorev M, Dellacherie E. Interfacial and emulsion stabilizing properties of amphiphilic water-soluble poly(ethylene glycol)-poly(lactic acid) copolymers for the fabrication of biocompatible nanoparticles. Colloids Surf A: Physicochem Eng Aspects 1998;14:413}20.
[29] Buscall R, Ottewill RH. The stability of polymer latices. In: Buscall R, Corner T, Stagema JF, editors. Polymer colloids. London: Elsevier Applied Science publishers, 1986. p. 141}217.
[30] Batrakova E, Han HY, Miller DW, Kabanov AV. E!ects of Pluronic P85 unimers and micelles on drug permeability in polar-ized BBMEC and Caco-2 cells. Pharm Res 1998;15:1525}32. [31] Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis
and the recycling of plasma membrane. J Cell Biol 1983; 96:1}27.