Iranian Journal of Basic Medical Sciences
ijbms.mums.ac.ir
Immunomodulatory activities of gemifloxacin in mice
Muhammad Umair
1, Aqeel Javeed
1*, Aamir Ghafoor
2, Muhammad Ashraf
11 Department of Pharmacology and Toxicology, University of Veterinary and Animal Sciences, Lahore, Pakistan 2 University Diagnostics Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan
A R T I C L E I N F O A B S T R A C T
Article type: Original article
Objective(s): Gemifloxacin is a broad spectrum antibiotic and has shown excellent coverage against a wide variety of microorganisms. In this study, an attempt was made to evaluate the immunomodulatory potential of gemifloxacin in male swiss albino mice in vivo.
Materials and Methods: Three doses of gemifloxacin 25 mg/kg, 50 mg/kg and 75 mg/kg were used intraperitoneally (IP) for the evaluation of immune responses in mice. Delayed type hypersensitivity (DTH), heamagglutination assay, jerne hemolytic plaque formation assay and cyclophosphamide induced neutropenia assay were performed to evaluate the effect of gemifloxacin on immune responses.
Results: DTH assay has shown the significant immune suppressant potential of gemifloxacin at 25 mg/kg dose and 75mg/kg dose. Total leukocyte count (TLC) has shown decrease in leukocyte count (P<0.05) in drug treatment groups before cyclophosphamide administration and significant decrease (P<0.001) in leukocyte count after cyclophosphamide administration as compared to negative control group. Differential leukocyte count (DLC) has shown significant decrease (P<0.001) in percentage count of lymphocytes in 75 mg/kg treatment group in leukopenic mice while increase (P<0.01) in monocytes percentage in 50 mg/kg treatment group in leukopenic mice and increase in neutrophil percentage count (P<0.05) in all treatment groups was observed after cyclophosphamide administration. Humoral immune response is shown to be suppressed in dose dependent manner by both heamagglutination titre values (P<0.001) and jerne hemolytic plaque formation assay (P<0.001). Conclusion: The results of this work clearly demonstrate that gemifloxacin has significant immunomodulatory potential.
Article history: Received: Dec 212, 2015 Accepted: Jun 30, 2016
Keywords: Gemifloxacin Immunomodulatory Immune response Cell mediated Humoral
►
Please cite this article as:Umair M, Javeed A, Ghafoor A, Ashraf M. Immunomodulatory activities of gemifloxacin in mice. Iran J Basic Med Sci 2016; 19:985-992.
Introduction
Gemifloxacin is considered as a respiratory fluoroquinolone and its pharmacokinetics as well as pharmacodynamics profile favor its use in the conditions of community acquired pneumonia and infectious bronchitis (1, 2). Gemifloxacin like other quinolones is effective against both Gram-positive and Gram negative microorganisms. In Gram-positive microorganisms, topoisomerase IV is the enzyme which is preferentially inhibited by this drug while in Gram-negative microorganisms, DNA gyrase is the target enzyme for its antibacterial activity (3). A study also supports the fact that gemifloxacin has increased activity against both DNA gyrase and
topoisomerase IV of Streptococcus pneumoniea (4).
Gemifloxacin has also been found to be more potent and efficacious against S. pneumoniea as compared to
moxifloxacin, gatifloxacin, ciprofloxacin and
levofloxacin (5). The structure of gemifloxacin shows bicyclic aromatic core and nitrogen atom at position 8. Gemifloxacin demonstrates an N-1 cyclopropyl
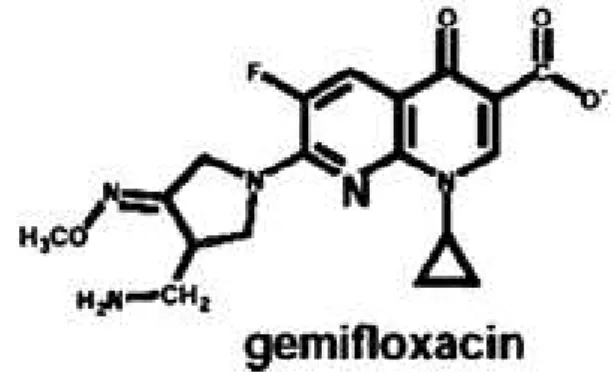
moiety in its structure which imparts increased antibacterial activity to this drug against anaerobes and Gram-positive microorganisms. The structure of this drug also indicates that halide ion is absent at position number 8, which confers reduced chances of photosensitivity as compared to some other members of fluoroquinolones (6) (Figure 1).
Figure 1. Structure of gemifloxacin
The structure of gemifloxacin is indicating the presence of cyclopropyl moiety at position number 1, fluoride at position number 6 and absence of halide at position number 8.
The presence of cyclopropyl moiety at position 1 in gemifloxcin gives us an idea that the drug may have immunomodulatory potential as literature supports that those fluoroquinolones which possess cyclopropyl moiety at position 1 increased the level of G-CSF which leads to increased heamatopoesis and exaggerated immune response (7). This drug has also shown the potential to suppress the release of
cytokine (IL- , IL- , IL-6, IL- , TNF ) from the
monocytes isolated from healthy volunteers (8). Immunomodulation by fluoroquinolones has also been reviewed in some studies and the data clearly demonstrated that different members of fluoro-quinolone class exhibit altered effect on various
immune cells (9). So the in vitro studies are not
adequate to elaborate the effects of the drugs in vivo. Therefore, we made an attempt to evaluate the effect of gemifloxacin on immune responses in vivo in male albino mice.
Materials and Methods
Animals
Male swiss albino mice were used for this research project. Animals were purchased from Department of Theriogenology UVAS, Lahore and kept under standard conditions of temperature and humidity. Animals having weight of 25-30 g and age of 5-6 weeks were used for this study. They were provided adequate environment for proper sleep wake cycle of 12 hr. Standard pellet diet and adequate water was provided
to the animals on regular basis.
Chemicals
Gemifloxacin was gifted by CCl Pharmaceuticals (Pakistan) and acetone was bought from Riedel-de Haen AG, (USA) and dinitrochlorobenzene (DNCB) from A.B Enterprises Maharashtra (India), Cyclo-phosphamide powder for injection (cyclomide) was purchased from Pharmedic Laboratory Pvt. Ltd Lahore (Pakistan) and water for injection from Elixir Laboratories Pvt. Ltd. Lahore (Pakistan). Ether solvent was bought from Den Norsken Eterfabrikk Oslo (Norway), and phosphate buffer saline (PBS) from Oxoid Limited Basing Stoke Hampshire (England).
Antigen
Sheep RBCs were used as antigen for evaluation
of humoral immune responses. Sheep RBCs were obtained from sheep available at animal house UVAS, Lahore. Blood from the sheep was withdrawn under aseptic conditions and RBCs were washed with PBS three times and these washed RBCs were used as antigen for the tests.
DNCB was used as antigen for the assessment of DTH response in mice. DNCB was dissolved in acetone and 2% suspension was made. This suspension served as antigen for DTH test.
Experimental design
This project was designed to evaluate the effect of gemifloxacin on immune responses in mice. Cell mediated immune responses were evaluated by DTH assay while humoral immune responses was evaluated by heamagglutination assay and jerne hemolytic plaque formation assay.
In each test 25 mice were used and were randomly divided into five groups (group 1, group 2, group 3, group 4 and group 5), each group having five mice. Group 1 in every test was used as the control group which received only the water for injection intraperitoneally. Gemifloxacin at the dose of 25 mg/kg, 50 mg/kg and 75 mg/kg body weight was administered intraperitoneally in equal volume (0.1 ml) in group 2, 3 and group 4 respectively. Group 5 was injected with cyclophosphamide at the dose of 150 mg/kg after every 6 days. Group 5 served as positive control in this project.
Cyclophosphamide induced neutropenia assay was performed to analyze the effect of gemifloxacin
on TLC and DLC before cyclophosphamide
administration and in leukopenic mice. In the cyclophosphamide induced neutropenia assay, four groups were made and every group had 5 mice. Among them group 2, 3 and 4 received gemifloxacin at the dose of 25 mg/kg, 50 mg/kg and 75 mg/kg in equal volume (0.1 ml) respectively while group 1 receive (0.1 ml) water for injection. All these groups were given cyclophosphamide subcutaneously at the
dose of 200 mg/kg on 10th day of the trial.
All the protocols for ethical handling of animal mentioned in the institutional guidelines of ethical handling of animals were strictly observed during the whole experiment.
DTH assay
In this test, two sides of mice were used for application of DNCB solution. Left side was used for sensitizing dose while right side was used for
challenging dose. On 2nd day of the trial, hair was
removed by surgical scissors from left side of mice
and 0.1ml DNCB solution was applied. On 8th day of
trial, right side of the animal was shaved and 0.2 ml DNCB was applied. Change in thickness of skin was measured after 24 hr, 48 hr and 72 hr of application of challenging dose. Results were analyzed and level of significance was determined in comparison to negative control group (10).
Cyclophosphamide induced neutropenia assay
In this assay, on 10th day of trial, blood was
heparinized vacutainer tubes. TLC and DLC were performed on the blood collected from these mice. Cyclophosphamide at the dose of 200 mg/kg was
injected in all mice subcutaneously. On 13th day of
the trial blood was collected by cardiac puncture and percentage reduction in TLC and DLC was calculated. The results were analyzed and the level of significance was determined between control group and gemifloxacin treated groups (11).
Haemagglutination assay
The method described by Fulzele et al with slight modification was used for performing heamagglu-tination assay (12). In this assay gemifloxacin was administered in treatment groups for 28 days.
Animals were injected with 0.5×109 sheep RBCs
intraperitonealy on 14th day of the trial and booster
dose was injected on 21st day. On 28th day of trial
blood was withdrawn from the animal by means of cardiac puncture. Blood was collected in eppendroff tubes which were placed in vertical position. After 30 min blood was clotted and serum appeared on the top of clotted blood. In order to obtain clear serum, eppendroff tubes containing the clotted blood along with serum were subjected to centrifugation at 1000
RPM for 5 min at 4 °C. Supernatants were separated
which contained the serum of mice having antibodies produced against SRBCs. 96 well round bottom plates were used for performing HA titre. 50 microliter of PBS was added to each well and 50 µl of the serum sample of mice was added to the first well of the column of the plate. Now 50 µl of liquid was taken from first well and added to next well. Same
procedure was repeated upto 11th well. From 11th
well 50 µl was discarded so that every well contained equal volume of the liquid. Last well served as positive control where no serum was added. Now 50 µl of 1% suspension of SRBCs was added to each well. Same procedure was repeated for different mice serum samples. The plates were placed for 2 hr, the highest value of serum showing heamagglutina-tion was considered as heamagglutinaheamagglutina-tion titre value. The results were compared between control group and groups treated with gemifloxacin.
Jerne hemolytic plaque formation assay
The protocol adopted previously with slight modification was used for performing this assay (13). On day 2, all the animals were immunized with 5% washed SRBCs. On day 6 animals were euthanized by high dose of ether solvent. Single cell suspension of spleenocytes was made. Spleen cells were counted under microscope and its count was
adjusted upto 1×107 per ml. Now 50 µl of
spleenocytes, 50 µl of diluted (1:2.5) guinea pig
complement, 50 µl of 10% SRBCs were taken in an
eppendroff tube. 50 µl of this sample was taken on the slit formed between two glass slides. These glass
slides were incubated for 1 hr at 37 °C. After 1 hr, the
plaques were observed under microscope at 40X. The final results were compared between drug treated groups and control groups.
Statistical analysis
Results were analyzed by mean±SEM and level of significance was determined by one way ANOVA and Duncans post-test. Differences were compared with negative control group and were considered significant for *P<0.05, **P<0.01 and ***P<0.001.
Results
Gemifloxacin showed decrease in skin thickness in 25 mg/kg treatment and 75 mg/kg treatment.
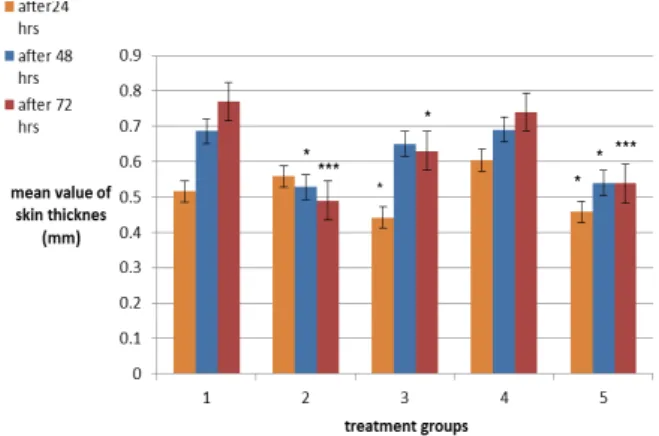
In this assay the effect of three doses of gemifloxacin (25 mg/kg, 50 mg/kg and 75 mg/kg) and cyclophosphamide 150 mg/kg/7days on the skin thickness of mice was determined by comparing with the skin thickness of mice in negative control group (water for injection treated group) after 24 hr, 48 hr and 72 hr of challenge dose of DNCB. Results are indicating that the drug is suppressing (P<0.05) the increase in skin thickness after 24 hr in gemifloxacin treatment groups 25 mg/kg and 75 mg/kg. Decrease in skin thickness (P<0.05) is also observed after 48 hr in 75 mg/kg tretment group. After 72 hr, decrease in skin thickness is observed in 25 mg/kg (P<0.05) treatment group and 75 mg/kg (P<0.001) treatment group (Figure2).
Treatment group 1, 2, 3, 4 and 5 are representing negative control group, positive control group, gemifloxacin 25 mg/kg, gemifloxacin 50 mg/kg and gemifloxacin 75 mg/kg respectively. Skin thickness after challenge with DNCB after 24 hr, 48 hr and 72 hr is measured and mean values ± SEM are expressed in this figure. Values are ***P<0.001,
**P<0.01, * P<0.05 for n=5 as compared to group 1.
Results are
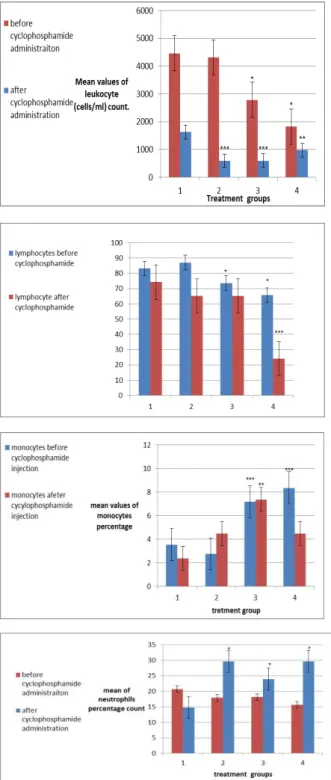
Figure 3. (A) Average count of leukocytes (cells /ml) before and after cyclophosphamide administration
(B) Mean values of percentage of lymphocytes before and after cyclophososphamide administration
(C) Mean values of monocytes percentage count before and after cyclophoshphamide administration
(D)Neutrophils percentage count before and after cyclophospha-mide administration
indicating that after 24 hr, group 2, 3 and 5 is showing significant immune suppression (P<0.05). After 48 hr of challenge, significant (P<0.05) decrease in skin
thickness is found only in group 2 and group 5. After 72 hr of challenge, group 5 and group 2 are having significant decrease in skin thickness (P<0.001), while group 3 is having significant difference (P<0.05) after 72 hr of challenge.
Cyclophosphamide induced neutropenia showed highly significant effect on TLC and DLC before and after cyclophosphamide administration
In cyclophosphamide induced neutropenia assay, TLC and DLC were performed before
cyclophos-pamide adminsitration on 10th day of the trial and
after cyclophosphamide administration on 13th day
of the trial. TLC values have shown significant decrease (P<0.05) in leukocyte count in 50 mg/kg and 75 mg/kg treatment group before cyclophos-phamide administration. Among the treatment groups after cyclophosphamide administration, significant (P<0.001) decrease in TLC was found in 25 mg/kg treatment group and 50 mg/kg treatment group while significant decrease (P<0.01) in TLC was observed in 75 mg/kg treatment groups (Figure 3(A)).
Negative control group is represented by 1, gemifloxacin treatment 25 mg/kg is represented by 2, gemifloxacin treatment 50 mg/kg is represented by 3 while gemifloxacin treatment 75 mg/kg is represented by 4. TLC was performed before and after cyclophosphamide administration. Mean values of leukocytes (cell/ml) ±SEM are represented in this figure. Results are compared between negative control treatment group and gemifloxacin treatment groups. Significant (***P<0.001) decrease in leukocyte count is
found in group 2 and group 3 while significant (**P<0.01) decrease is observed in gemifloxacin
treatment 75 mg/kg. Significant (P<0.05) decrease in leukocyte mean value is observed in treatment group 3 and 4 before cyclophosphamide administration.
Percentage mean value of lymphocytes shows decrease (P<0.05) in lymphocytes percentage count before cyclophosphamide administration in 50 mg/kg treatment group and 75 mg/kg treatment group. Significant (P<0.001) decrease in mean percentage value of lymphocyte was observed in 75 mg/kg treatment after cyclophosphamide administration (Figure 3(B)).
In this figure, treatment 1 is representing negative control group, while group 2, 3 and 4 are representing gemifloxacin treatment 25 mg/kg, 50 mg/kg and 75 mg/kg respectively. DLC was performed before
cyclophosphamide administration on 10th day and after
cyclophosphamide administration on 13th day of trial.
This figure is indicating mean percentage value of lymphocytes ±SEM and results are compared with negative control group before cyclophosphamide
administration and after cyclophosphamide
administration. Results are indicating that the drug is
Figure 4. Mean values of HA titre among treatment groups and control group in mice
percentage in group 4 in comparison to lymphocyte percentage after cyclophosphamide administration in negative control group. Significant decrease (P<0.05) in lymphocyte percentage before cyclophosphamide administration is observed in group 3 and group 4.
Percentage value of monocyte indicate significant (P<0.001) increase in monocyte count before cyclophosphamide administration in 50 mg/kg treatment group and 75 mg/kg treatment group. Significant (P<0.01) increase in monocytes was observed in 50 mg/kg treatment group after cyclophosphamide administration (Figure 3(C)).
Treatment group 1, 2, 3 and 4 represent negative control group, gemifloxacin 25 mg/kg, gemifloxacin 50 mg/kg and gemifloxacin 75 mg/kg respectively. DLC was performed before cyclophoshamide
administration on 10th day and after
cyclophospha-mide administration on 13th day. Mean values of
monocytes ± SEM are shown in this figure. Results are compared between negative control group and drug treatment groups. Significant (P<0.001) increase in monocyte percentage count is found in group 3 and 4 before cyclophosphamide administra-tion. While in group 3, significant (P<0.01) increase in monocyte percentage is found after cyclophospha-mide administration.
Percentage count of neutrophil has shown significant increase (P<0.05) in neutrophil percentage after cyclophospamide administraiton while no significant change in neutrophil count has been observed in treatment groups before cyclophospha-mide administration (Figure 3(D)).
Treatment group 1, 2, 3 and 4 are representing negative control group, gemifloxacin 25 mg/kg, gemifloxacin 50 mg/kg and gemifloxacin 75 mg/kg respectively. DLC was performed before
cyclophos-phamide administration on 10th day of trial and after
cyclophosphamide administration on 13th day of
trial. Mean values of neutrophil percentage count ±SEM are shown in this figure. The results obtained by comparing results between negative control group and treatment group before and after cyclophosphamide administration. The figure is indicating the drug treatment group have significant
(P>0.05) increase in neutrophils percentage after cyclophosphamide administration.
Dose dependant decrease in HA titre value in gemifloxacin treatment groups has been observed
Humoral immune responses were evaluated by
using SRBCs as antigen. 0.5×109 SRBCs were injected
IP on 14th day and 21st day of trial. Circulating
antibodies were measured on 28th day of trial by
collecting blood of mice via cardiac puncture. Serum was separated and HA titre was calculated by using SRBCs and serum sample. Dose dependant decrease in HA titre value has been observed in gemifloxacin treatment groups as compared to negative control group. This decrease in titre value is significant (P<0.01) for treatment group 25 mg/kg and 50 mg/kg while this decrase is significant (P<0.001) in 75 mg/kg treatment group (Figure 4).
Treatment groups 1, 2, 3, 4 and 5 are representing negative control group, positive control group, gemifloxacin 25 mg/kg treatment, gemifloxa-cin 50 mg/kg treatment and gemifloxagemifloxa-cin 75 mg/kg treatment respectively. After 28 days, circulating antibodies raised against SRBCs were evaluated by using SRBCs and HA was performed. Mean values of HA titre± SEM is shown in this figure. The results are compared between negative control group and treatment group. Significant (P<0.001) decrease in HA titre value is found for group 5 and group 2 while significant (P<0.01) decrease is found in treatment groups 3 and 4.
Dose dependant decrease in hemolytic plaque formation has been observed in gemifloxacin treatment groups
Hemolytic plaque formation assay was performed against SRBCs and variation in number of plasma cells collected from spleenocytes in drug treatment group and negative control group was measured. Dose dependant decrease in antibody producing plasma cell was observed in drug treatment groups as compared to negative control group. Treatment group 25 mg/kg has shown significant (P<0.01) decrease in number of plaques formed while treatment group 50 mg/kg and 75 mg/kg has shown significant decrease (P<0.001) in number of plaques formed (Figure 5).
Treatment 1, 2, 3, 4 and 5 are representing the negative control group, gemifloxacin treatment 25
mg/kg, gemifloxacin treatment 50 mg/kg,
gemifloxacin treatment 75 mg/kg and cyclophospha- mide treatment group respectively. Single cell suspension of spleenocytes was formed and cultured with guinea pig complement and SRBCs. After one hour incubation slides were observed under 40X and plaques were counted. In this figure mean values of plaques formed ± SEM are shown. The results are compared with group 1 and figure shows group 2 is having significant decrease (P<0.01) while group 3, 4 and 5 has significant decrease (P<0.001) in mean values of plaques formed as compared to negative control group.
Discussion
This project was designed to evaluate the effect of gemifloxacin on cellular and humoral immune responses in mice. The effect of gemifloxacin on cellular immune response was evaluated by DTH in which DNCB was used as antigen. DNCB forms dinitrophenyl complex which serves as antigen and provokes an immune response (10). Therefore, we used DNCB as irritant which could produce the delayed type hypersensitivity in the mice. In this assay, it was found that gemifloxacin is suppressing immune response after 24 hr of challenge in 25 mg/kg treatment and 75 mg/kg treatment groups. After 48 hr, decrease in skin thickness was observed in 75 mg/kg treatment group. After 72 hr decrease in skin thickness was observed in 25 mg/kg treatment and very significant decrease in skin thickness was found in treatment group 75 mg/kg. This result is indicating that at 25 mg/kg treatment and 75 mg/kg treatment the drug is suppressing immune system. At 50 mg/kg treatment, no significant increase or decrease in skin thickness was observed. Decreased skin thickness is due to decrease in leukocyte infiltration which may be caused by a wide range of mechanism at the site where antigen complex is formed (14). No significant increase or decrease in skin thickness at 50 mg/kg treatment group, is suggesting that the drug is having dual effect on cell mediated immune response. That is the drug is suppressing as well as potentiating the immune system. The overall
preclinical effect of immunosuppression or
immunopotentiation is decided on the basis of doses used in the animal.
Cyclophosphamide has the ability to interfere with DNA replication and it also causes leukopenia, the properties of this drug which make it an anticancer as well as immunosuppressive drug (15).
Cyclophosphamide induced neutropenia was
designed to evaluate the effect of gemifloxacin on leukopenic mice. Cyclophosphamide also decreases the production of lymphocytes which are mainly
involved in the adaptive immune responses (16). Keeping this perspective, cyclophosphamide was used to induce leukopenia in mice and effect of gemifloxacin on TLC and DLC in leukopenic mice was evaluated. TLC and DLC were performed before and after the subcutaneous administration of cyclo-phosphamide. Significant decrease (P<0.001) in leukocytes count in 50 mg/kg treatment and 75 mg/kg treatment groups indicates the ability of gemifloxacin to suppress the immune status of the leukopenic mice while significant decrease (P<0.05) in leukocyte count before cyclopho-sphamide administration in 50 mg/kg treatment group and 75 mg/kg treatment group is indicating that gemifloxacin is also suppressing the immunity in the mice with normal immune status. The results of DLC indicate that the drug is significantly (P<0.05) increasing the percentage count of nuetrophils in leukopenic mice. Significant (P<0.001) increase in monocyte count before cyclophosphamide adminis-tration at 50 mg/kg dose and 75 mg/kg dose and significant increase (P<0.01) in monocytes count in leukopenic mice at 50 mg/kg treatment was observed. Decrease in monocytes count at 75 mg/kg treatment group to the level where no significant difference from negative control group is observed,
indicates the direct cytotoxicity caused by
gemifloxacin at 75 mg/kg on monocytes in leukopenic mice. Neutrophils and monocytes are main players of innate immune system and the results of this project are depicting clearly that the innate immune response is strengthened by gemifloxacin in mice with normal immune system as well as mice with weakened immune status. Neutrophils and macrophages are derived from myeloid progenitor cells. Increase in the count of both the neutrophils and monocytes indicate that gemifloxacin may have directly stimulating effect on myeloid progenitor cells. The result of this project is slightly similar with the work done by others (17) where increase in myeloid progenitor cells was observed in response to two other fluoroquinolones,
Cipro-floxacin and moxifloxacin. In case of
lymphocytes, significant decrease in lymphocyte count before cyclophosphamide administration at 50 mg/kg treatment group and 75 mg/kg treatment group indicates the ability of gemifloxacin to
suppress adaptive immune response while
significant (P<0.001) decrease in lymphocytes in leukopenic mice at 75 mg/kg treatment group suggests that gemifloxacin is having very high suppression on adaptive immune responses at 75 mg/kg treatment on leukopenic mice.
antibodies against the specific epitope of the antigen used for provoking immune response. Sheep RBCs were used in this project as antigen and when animal is given this antigen via peritoneal route, antibodies are produced in the serum of the mice. Antibodies are produced by B cells and specifically the antibodies are produced by plasma cells. When antigen is processed by APCs, T helper cells sense the antigen on the surface of APCs and give the message to B cells which upon activation divide and form plasma cell and memory cell. HA titre is the direct measure to determine the level of antibodies formed in the serum of mice. The antibodies formed inside the serum of mice had the ability to bind with the sheep RBCs. Binding of antibody with sheep RBCs lead to agglutination in the round bottom well plate. This observation gives a positive result. While those cases when antibody is not produced, SRBCs settle in the bottom in form of bead which is read as negative result. The dose dependent decrease in antibody titre is observed in this work indicating the ability of gemifloxacin to suppress the humoral immune responses in mice.
Hemolytic plaque formation assay was performed to evaluate the effect of gemifloxacin on plasma cells count. Plasma cells are antibody secreting cells and can be collected from spleen. So, spleenocytes were cultured along with guinea pig serum and sheep RBCs. guinea pig serum had the complement which has very good hemolytic activity so, when antibodies formed in the mice serum and SRBCs were incubated along with guinea pig serum, the antigen antibody complex and guinea pig complement were fixed together. Guinea pig complement lyses the SRBCs which bind with antibody present on the surface of plasma cell and a clear hemolytic zone was observed under microscope. Due to the same hemolytic zone, the test is named as Jerne hemolytic plaque
formation assay. Significant dose dependent
decrease in number of plaques formed was observed among the treatment groups of gemifloxacin. This result is in accordance with the previous results of HA titre evaluation and lymphocytes count where the decrease in HA titre and decreased percentage of lymphocyte count was observed.
Results of this project suggest that gemifloxacin affects the both innate and adaptive immune responses. Decrease in skin thickness, decrease in leukocytes count, decrease in lymphocyte count, decrease in HA titre value and decrease in hemolytic plaque formation assay indicate the suppressive effect of gemifloxacin on immune responses while increase in neutrophil count and monocyte percentage count is depicting the immuno-potentiating property of gemifloxacin on immune responses in mice.
The well-established antibacterial potential of this drug may be an added advantage in those
conditions where immune suppression is induced by immunosuppressive drugs e.g. in organ transplan-tation or autoimmune diseases and those conditions where immunopotentiation is desired e.g. in cancer. These are the conditions where patient becomes prone to infections and an antibacterial drug may serve dual benefit, i.e. in immunomodulation and antibacterial activity. So we can conclude that although the drug is potentiating innate immune
response and suppressing adaptive immune
responses, both the components of immune system cannot be completely separated from each other (18, 19). So there is a need to further evaluate the effect of this drug on various disease models like cancer, organ transplantation and autoimmune diseases etc. where in addition to antibacterial activity, immune-modulation is desired.
Conclusion
Our study clearly demonstrates that gemifloxacin has the potential to affect both innate and adaptive components of immune responses. The drug has shown dual effect on the both these system, i.e. it potentiates innate system while it suppresses adaptive immune system. The immunomodulating property of this drug should be tested further in various disease models of cancer, autoimmune disorders, organ transplantation etc. in which immunomodulation is desired.
Acknowledgment
This research project was conducted in
Department of Pharmacology and Toxicology, UVAS, Lahore, and University Diagnostic Laboratory, UVAS, Lahore. The financial support to this project was provided by Department of Pharmacology and Toxicology, UVAS, Lahore.
Conflict of interest
The authors have no conflict of interest.
References
1. Wispelwey B, Schafer KR. Fluoroquinolones in the management of community-acquired pneumonia in primary care. Expert Rev Anti Infect Ther 2010; 8:1259-1271.
2. Albertson TE, Dean NS, El Solh AA, Gotfried MH, Kaplan C, Niederman MS. Fluoroquinolones in the
management of community‐acquired pneumonia. Int
J Clin Pract 2010; 64:378-388.
3. Bearden DT, Danziger LH. Mechanism of action of and resistance to quinolones. Pharmacotherapy 2001; 21:224S-232S.
4. Heaton VJ, Ambler JE, Fisher LM. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an
in vivo target preference for gyrase, and enhanced
stabilization of cleavable complexes in vitro.
5. Yague G, Morris JE, Pan XS, Gould KA, Fisher LM.
Cleavable-complex formation by wild-type and
quinolone-resistant Streptococcus pneumoniae type II topoisomerases mediated by gemifloxacin and other fluoroquinolones. Antimicrob Agents Chemother 2002; 46:413-419.
6. Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquino-lones. Clin Infect Dis 2003; 37:1210-1215.
7. Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis 2003; 3:359-371. 8. Araujo F, Slifer T, Li S, Kuver A, Fong L, Remington J. Gemifloxacin inhibits cytokine secretion by lipopolysaccharide stimulated human monocytes at
the post‐transcriptional level. Clin Microbiol Infect
2004; 10:213-219.
9. Dalhoff A. Immunomodulatory activities of fluoroquinolones. Infection 2005; 33:55-70.
10. Sajid MS, Iqbal Z, Muhammad G, Sandhu MA, Khan
MN, Saqib M, et al. Effect of ivermectin on the cellular
and humoral immune responses of rabbits. Life Sci 2007; 80:1966-1970.
11. Thomas L, Asad M, Hrishikeshavan HJ, Chandrakala GK. Effect of centchroman on cellular and humoral immunity. Indian J Physiol Pharmacol 2007; 51:387-394.
12. Fulzele S, Satturwar P, Joshi S, Dorle A. Study of
the immunomodulatory activity of Haridradi ghrita in rats. Indian J Pharmacol 2003; 35:51-54.
13. Cho WCS, Leung KN. In vitro and in vivo
immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol 2007; 113:132-141.
14. Black CAP. Delayed type hypersensitivity: Current theories with a historic perspective. Dermatol Online J 1999; 5:7.
15. Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy 1997; 17:146S-154S.
16. Winkelstein A. Mechanisms of immunosuppre-ssion: effects of cyclophosphamide on cellular immunity. Blood 1973; 41:273-284.
17. Shalit I, Kletter Y, Halperin D, Waldman D,
Vasserman E, Nagler A, et al. Immunomodulatory effects
of moxifloxacin in comparison to ciprofloxacin and
G‐CSF in a murine model of cyclophospha mide‐ induced
leukopenia†. Eur J Haematol ; : -296.
18. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327:291-295.