ISSN 0104-6632 Printed in Brazil
www.abeq.org.br/bjche
Vol. 33, No. 01, pp. 235 - 241, January - March, 2016 dx.doi.org/10.1590/0104-6632.20160331s00003451
*To whom correspondence should be addressed
This is an extended version of the manuscript presented at the
Brazilian Journal
of Chemical
Engineering
MOLECULAR VALIDATED MODEL FOR
ADSORPTION OF PROTONATED DYE ON LDH
B. M. Braga, D. V. Gonçalves, M. A. G. Paiva and S. M. P. Lucena
*Departamento de Engenharia Química, GPSA, Universidade Federal do Ceará, Campus do Pici s/n Bl. 709, CEP: 60440-554, Fortaleza - CE, Brasil.
Phone: + 55 3366 9611; Fax: + 55 3366 9601
*
E-mail: lucena@ufc.br; smlucena@uol.com.br
(Submitted: April 14, 2014 ; Revised: January 18, 2015 ; Accepted: April 29, 2015)
Abstract - Hydrotalcite-like compounds are anionic clays of scientific and technological interest for their use as ion exchange materials, catalysts and modified electrodes. Surface phenomenon are important for all these applications. Although conventional analytical methods have enabled progress in understanding the behavior of anionic clays in solution, an evaluation at the atomic scale of the dynamics of their ionic interactions has never been performed. Molecular simulation has become an extremely useful tool to provide this perspective. Our purpose is to validate a simplified model for the adsorption of 5-benzoyl-4-hydroxy-2-methoxy-benzenesulfonic acid (MBSA), a prototype molecule of anionic dyes, onto a hydrotalcite surface. Monte Carlo simulations were performed in the canonical ensemble with MBSA ions and a pore model of hydrotalcite using UFF and ClayFF force fields. The proposed molecular model has allowed us to reproduce experimental data of atomic force microscopy. Influences of protonation during the adsorption process are also presented.
Keywords: Adsorption; Hydrotalcite; Dye; Molecular simulation.
INTRODUCTION
Layered double hydroxides (LDH) are an im-portant class of natural compounds and also easily obtained by synthesis. They have a permanent posi-tive charge on their surface. In addition, LDHs can exchange anions that lie between the layers. For these characteristics, these compounds are of great scientific and technological interest and are used as ion exchange materials (Dutta et al., 1991), catalysts (Reichle et al., 1986) and modified electrodes (Itaya
et al., 1987). LDHs with magnesium and aluminum atoms on their layers are known as hydrotalcite and the framework of the layers are similar to brucite
(Mg(OH)2). We can imagine a starting structure,
electrically neutral, being composed by one brucite layer (Figure 1a).
As magnesium is isomorphically replaced by
Al3+, a permanent positive charge is created that is balanced by intercalation species (Figure 1b). In hydrotalcite, CO32- and water molecules are the
inter-calation species in the interlayer space.
The permanent positive charge is responsible for the extraordinary LDH performance in the adsorp-tion of anionic dyes (Zhu et al., 2005; Abdelkader et al. 2011; Boudiaf et al. 2012; Aguiar et al., 2013). To be able to understand and predict the behavior of LDH’s for anionic dyes adsorption, it is important to analyze on an atomic-scale, the surface phenomena that result in the macroscopic properties. Experi-mental techniques are limited for clarifying these phenomena.
C ap en ti d d en w 2 m it ou A so ti ch so p F (b fr (O dx si ad m ex p b m v m g n
Cl- ions and pplied the M nsemble to m ion of CO2 a
rotalcite. Th ominantly b nce is reveal with variation -methoxy-be model, ideal f ts small size
us media the At pH > 2, M
olution. Incr ion rich in t hanges in pH orption occu
H on the sur
Figure 1: Bru b). Green - M rom Serwick
Original color ver x.doi.org/10.1590/0 Thus, our imulation mo dequately de model MBSA xperience in ose, Monte le were perf model of hyd alidated with microscopy (
iven to the ation on the
water interc Monte Carlo m
measure diffu at high temp he LDH/dye by electrostat
led by chang ns in pH. MB enzenesulfon
for studies o and well-kno e MBSA mo MBSA1- is t easing the pH
the MBSA
2-H affect the urs. However rface of LDH
(
( ucite layer (a Mg; Pink - A ka et al., 2004
rsion of this figur 104-6632.20160331
main goal odel of the sy escribe the ad A in LDH sur n describing t Carlo simul formed with drotalcite por
h experimen (Cai et al.,
aspects relat e adsorption. calated. Kim method usin usivity value peratures (> system is i tic interactio ges in the am BSA (5-benz nic acid) is of molecular
own ionic be olecule has tw
the predomin H (pH >8) p
anion. We surface of L r, in this stud H will not be
(a)
(b)
a) and hydrot l and Black -4).
re is available at 1s00003451
is to valida ystem LDH/D dsorption of rface, to get f these system lations in can
MBSA ions re. The simu ntal data from
1994). Spec ted to the im . To the bes
m et al. (20 ng the canoni es and interca
200 °C) in influenced p ons. This inf mount adsorb zoyl-4-hydro
an anionic d simulation d havior. In aq wo ionic form
nant species produces a so also know t LDH where dy, the effects
considered.
talcite structu - CO32-(adap the journal
web-ate a molecu Dye, which c the anionic d first insight a ms. For this p nonical ense s in a propo ulated data w m atomic fo cial attention mpact of pro t of our kno
07) ical ala- hy- pre- flu-bed xy-dye due que-ms. s in olu-that ad-s of ures pted -site: ular can dye and pur- em-sed were orce n is oto- ow-led per LD tai (B cry 22 ato res tal wa Mg ing lay the Al du CO lay po sho Fig Re der (Or dx.d and ten bet are Re LD 10 of new thr dro
dge, no such rformed.
DH Model
The model ned by refi ellotto et a
ystallizes in .77 Å). The oms was mu sulting in a s llographic un as replaced b g:Al. The in g enough CO yers. For eac ere should b long with CO uced in the p
O32- (Wang e
yer space w sitioning of owed in Figu
gure 2: Mod ed – O; Pink –
rs – CO32-. iginal color versi doi.org/10.1590/010
After that, d the inner s nded to simu
tween inner e showed in F
The pore si eichle et al. ( DH pore volu
0 Å. The hy the most st w species to rough ion-ex otalcite takes
h previous th
METHOD
was based inement of
l., 1996). T space group unit cell co ultiplied by t supercell con nit cells (10
by aluminum nterlayer spac O32- ions to n
ch two alum be one CO32
O32- ions, wa
proportion of
et al., 2001) was minimize
the layer fix ure 2.
del of the stac – Al; Green –
ion of this figure 04-6632.20160331s
the c directi space betwe ulate a LDH layers. The Figure 3. ize of 72 Å (1986). They ume was con ydrotalcite w able structur o intercalatin xchange. In th place exclusi
heoretical st
DOLOGY
on a crystal powder X-r The hydrotal p R3m (a= b ontaining on ten in a and ntained a tota x 10 x1). Th m following
ce was desig neutralize th minum atom
in the int ater molecul f four water . The energy ed while m xed. The who
cked LDH un – Mg; Gray a
is available at th 00003451
ion was mult en the two l average size results of th
was based in y estimate th ncentrated be with carbona
res of LDH, ng in the int his case, ads ively by surfa
tudy had bee
l structure o ray diffractio
lcite unit ce = 3.04 Å, b ly magnesiu d b direction al of 100 cry he magnesiu a 3:1 ratio gned introdu he overcharge ms in the lay
terlayer spac les were intr molecules p y of the inte aintaining th ole supercell
nit. White – H and Red cyli
he journal web-si
tiplied by tw layers was e e pore of 72 hese last step
n the study hat most of th
F G
(O
M
at za g th at H m (+ th fi te
E
b ou fo at T tr ti C st u so
th G 4
Figure 3: Sim Green – Mg.
Original color ver
MBSA Mode
MBSA mo tom model. T ation, the hy roup was rem hat, removin
tom of the b Hydronium i model of hy +1). The MB he Steepest D ield COMPA ential functio
4 2 4
2 b
t i i
t
i i
cross
E k
k E
The total e ond lengths ut-of-plane b or example:
tom, two an The nonbond rostatic (elec ions. All con COMPASS v
teps are cruc noptimized m orption equil
The partia he Charge E Goddard, 199 . Both the en
Brazilia mulation cell w
rsion of this figur
el
olecules wer To represent ydrogen atom moved to giv ng the hydrog benzene ring ions were r ydrogen atom BSA anion en
Descent algor ASS (Sun, 1 on is given as
0
( )
(1 cos )
i
i
s terms
b b i
E
energy consi (b), angles bending ang
between tw ngles with a ded energies c) and van d nstant values
validation p cial for obta molecules fr librium. al charges us Equilibration 91) and are sh
nergy minim
Molecular V
an Journal of Che with 72 Å bet
re is available at t
re represente t the differen m attached ve the anion gen attached g gives the represented b ms with a p nergies are m rithm with th 1998). The C
s:
4 2
0 0
(
( a
i i
i
elec VD
k θ θ k χ χ
E E
ist of a sum o (a), torsion gles (o) and
o bonds wit common bo were repres der Waals (V
can be found paper (Sun, aining reliabl
requently did
sed was obta n methodolog hown in Tab mization and c
Validated Model
emical Engineerin tween layers
the journal web-s
ed by an ato nt states of io
to the sulfo n MBSA1-; af d to the oxyg
anion MBSA by a spheri positive cha minimized us he generic fo COMPASS
0 2 0
)
) i
DW
θ
of functions angles (t) a cross terms th one comm ond and so o sented by el VDW) inter d in the origi 1998). Th le results, sin d not reach
ained based gy (Rappe a ble 1 and Fig
charge distrib
for Adsorption of
ng Vol. 33, No. 0 to represent t
site: dx.doi.org/10.
om– oni-onic
fter gen A2-. ical arge sing orce
po-(1)
for and (as mon on). lec- rac-inal
ose nce
ad-on and gure
bu-tio Ce
Ta for
A
Fig typ
(Or dx.d
f Protonated Dye
01, pp. 235 - 241 the hydrotalc
1590/0104-6632.20
on were impl erius2 from A
able 1: Char r atom refer
Atom Ch MBSA
C1 -0.19 C2 0.04 C3 -0.12 C4 0.04 C5 0.05 C6 -0.12 C7 0.31 C8 0.05 C9 -0.12 C10 -0.12 C11 -0.12 C12 -0.12 C13 -0.12 C14 0.00
O1 -0.70 O2 -0.70 O3 -0.70
gure 4: M
pes. White –
iginal color versi doi.org/10.1590/010
e on LDH
, January - Marc ite pore. Whi
0160331s00003451
lemented in Accelrys.
rges of the M rence).
harges (e)
A1- MBSA
2-98 -0.221 42 0.019 27 -0.15 42 -0.023
2 0.029 27 -0.15 5 0.292 2 0.029 27 -0.15 27 -0.15 27 -0.15 27 -0.15 27 -0.15 01 -0.022
06 -0.729 06 -0.729 06 -0.729
Model ofMB H; Red – O;
ion of this figure 04-6632.20160331s
ch, 2016 ite – H; Red –
the commer
MBSA ions
Atom MB
H1 0. H2 0. H3 0. H4 0. H5 0. H6 0.
H7 0
H8 0. H9 0. H10 0. H11 0.
S 1.
O4 -0. O5 -0. O6 -0.
SA1-molecul ; Gray – C; Y
is available at th 00003451
2
– O; Pink – A
rcial packag
(see Figure
Charges (e)
BSA1- MBSA
.127 0.104 .127 0.104 .127 0.104 .127 0.104 .127 0.104 .127 0.104 0.41 - .127 0.104 .053 0.03 .053 0.03 .053 0.03
.318 1.295
.419 -0.442 .452 -0.305 .202 -0.225
le with ato Yellow – S.
he journal web-si
237
Al;
es
e 4
2-4 4 4 4 4 4
4
2
om
Forcefield
The system LDH/dye was described using the LJ potential for repulsion-dispersion forces plus Cou-lombic contributions between point charges:
4. . 12 6
i j ij
ij
σij σij q q U rij ε
rij rij r (2)
where εij (kcal/mol) represents the depth of the
po-tential well, σij (Å) is the finite distance at which the
energy of interaction is zero and rij (Å) is the
dis-tance between the molecular centers i and j. In the second term, qi and qj are point charges separated by
the distance rij. The cross LJ terms were obtained
using the usual arithmetic and geometric combina-tion rules (Lorentz-Berthelot).
For the interactions between MBSA molecules and the LDH framework, we used the ClayFF force field (Cygan et al., 2004). This force field has been developed especially for clay minerals.
The interaction between MBSA molecules are computed based in the UFF force field (Rappe et al., 1992). Table 2 presents the parameter’s values for each atomic species (r0 = σ / 1.1224).
Table 2: Forcefield parameters.
Atom r0 (Å) εij (kcal/mol) Charges (C)
Magnesiuma 5.909 1.3298.10-6 1.36
Aluminiuma 4.7943 9.0298.10-7 1.575
Hydrogena - - 0.425
Oxygena 3.5532 0.1554 -0.95
Nitrogenb 3.660 0.069 c Carbonb 3.851 0.105 c Oxygenb 3.500 0.060 c Sulfurb 4.035 0.274 c Hydrogenb 2.886 0.044 c
a
Cygan et al., 2004 (LDH); b
UFF-Rappe et al., 1992 (MBSA); c
See Table 1 and Figure 4
Computational Details
The electrostatic potential was calculated by Ewald method. The Ewald method accuracy was set for an energy tolerance of 0.001 kcal/mol within a cut-off of 15.2 Å. This means that larger cut-offs will give energy variation in the third decimal place as done in a similar study (Lima et al, 2015). All LJ potential had also a 15.2 Å truncation. We also did tests with increasing LJ cut-offs and obtained similar results. After 1.5 x 106 equilibration steps, more 1.0 x 106 steps were used to obtain the average values of
the thermodynamic properties and the most stable configuration.
The simulations in the canonical ensemble (NVT) were performed with a variable number of MBSA anions until the surface was saturated. The number of molecules at saturation will be compared with that obtained experimentally by Cai et al. (1994) for
MBSA1-. The molecular simulation algorithm of
Cerius2 (Accelrys) was used to obtain the NVT equi-librium configurations. During the Monte Carlo simu-lations, the MBSA molecules and LDH framework were considered rigid. While this approach is ac-ceptable for LDH, it is not suitable for dye mole-cules. However, MBSA is a special case because its structure consists basically of two aromatic rings with reduced mobility. Furthermore, the molecule has previously been minimized and we did not ex-pect significant conformation changes.
RESULTS AND DISCUSSION
Model Validation
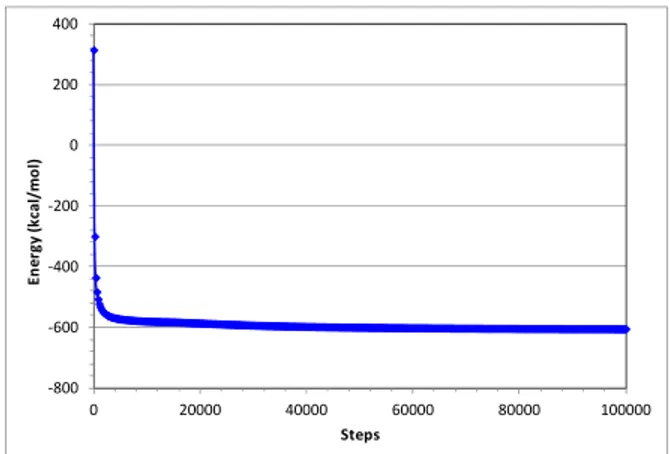
In order to compare simulated and experimental data (Cai et al., 1994), an increased number of MBSA molecules were introduced in the simulation cell until saturation. During simulation, MBSA1- mole-cules are translated and rotated inside the simulation cell until equilibrium is reached (Figure 5).
Figure 5: The evolution of the energy in the system
LDH/MBSA-1 at 298 K. The most stable
configura-tion occurred after about 30,000 steps.
At equilibrium, the molecules of MBSA1-
as-sumed a perpendicular position with the LDH sur-face. This same orientation of the anions with respect to the hydrotalcite surface was verified experimen-tally by Cai et al. (1994) for MBSA1- adsorption onto
‐800
‐600
‐400
‐200 0 200 400
0 20000 40000 60000 80000 100000
En
e
rg
y
(k
ca
l/
mo
l)
hy co M th w ch su F ti (O dx p m fa ti 1 to T m su ex th to Im M in v cu T a ydrotalcite. opy to gener MBSA anions he sulfonic which has a harges. The urface that is
Figure 6: Sid ion of MBSA
Original color ver x.doi.org/10.1590/0
In the sim acking of 1 mately 12.2 m
ace was full ion capacity
994) that res o 10.5 mole Thus, in the e molecules. C
urface in the xperimental he models fo owards the p
mpact of Pr
The intera MBSA anions
n Table 3. A iew, the cha ules should i
Table 3: Inte nd LDH. Number of MBSA anions 1 2 4 6 8 10 Brazilia The authors rate impressi s. This speci acid group a superior
sulfonic acid s positively c
de and top vi A1- at the end
rsion of this figur 104-6632.20160331
mulation, the molecule/59 molecules wh
ly saturated. was 2.41 x sults in 1 mo ecules at ou
experiment, onsidering t e simulation,
data was e or the system rotonation st
rotonation
ction energy s and each L Analyzing fro arge increase increase adso
raction ener
MBSA-1 Int
Energy (kc -585.7 -595.2 -597.2 -505.9 -465.7 -416.4 Molecular V
an Journal of Che used atomic ive nanoscal ific position of the MB concentratio d group mov charged (Figu
iew of the eq d of the simul
re is available at 1s00003451
e MBSA1- an 9.5 Å2, result
hen the simu The experi x 10-10 mol/c olecule/69.1 ur simulation the LDH ad that we are u
some discre expected. Ha m LDH/MBS
tudy.
y of an increa LDH pore sur om an electr
e on MBSA2
orption. rgy between eraction cal/mol) MB En 77 22 25 95 74 43 Validated Model emical Engineerin c force micr e images of is associated BSA1- molec on of negat ves towards ure 6).
quilibrium po lation.
the journal
web-nions exhibi ting in appro ulation cell s imental adso cm2 (Cai et
Å2 (equival n cell surfac dsorbs 13% l
using a pref epancy from aving valida SA1-, we mov
asing number rface are sho rostatic point
until 8 mo
n MBSA anio
BSA-2 Interacti
nergy (kcal/mo -1026.77 -1121.70 -981.47 -784.59 -582.83 -399.20
for Adsorption of
ng Vol. 33, No. 0 ros-the d to cule tive the osi--site: it a oxi- sur- orp-al., lent ce). less fect the ated ved r of own t of ole-ons on ol) 56 wa tio tio ani inc par all MB and int pu and res Ta an Nu H+ mo the the sor act ter bec po on ser sur the Ou MB cul mo sur MB tiv cul sys is a the
f Protonated Dye
01, pp. 235 - 241 Experiment % decrease ant verify if on reduction. on cell to st
ions and pro creases, the rtial (-1), and l protonated BSA2-, we o d 2:1 for M teraction ene uted between d one MBSA sults (Table 4
able 4: Inter nd H+ cations
umber of
+
cations In
Ener
1
2
-4
-6
-8
-10
-To MBSA
1-olecule and i e energy of i erefore, in th rbed on the tion involves raction betw
comes equiv When the c re surface an ns (10 or 20) rved that, wh rface, 38% o e interlayer s ur simulation BSA2- adsor les at exper olecules) was
When MBS rrounds the BSA2- anion vely charged les that adso stem more en adsorbed by e H+ from
e on LDH
, January - Marc tally, the ext in adsorptio our model c The species tudy MBSA otons. In aqu
deprotonati d finally com
hydrogen obtain Hcation
MBSA1- and M
ergy (= Etotal
n an increasi A anion mo 4). raction ener s. MBSA 1-nteraction rgy (kcal/mol) -78.77 -129.76 -161.63 -204.07 -261.61 -324.66
-, the energy ionized catio interaction w he equilibriu
surface. For s 12 or more een the mol valent to the s
omplete syst nd the corre is simulated hile all MBS of the MBSA space of the p ns always sh rbed, even w rimental satu s changed. SA is first p
MBSA1- ani n there are t d LDH surfa orb on the s nergetically s
the surface the first pr
ch, 2016 tra protonatio
on (Cai et a
could also p s introduced A’s protonati ueous soluti ion of MBS mplete (-2). A
came from
n: MBSAanion
MBSA2- resp - ∑Eeach comp
ing number olecule show gy between Number of H+ cations En 2 4 8 12 16 20 of interactio ons are alwa with the surf um, the mole r MBSA-2, w e cations, the lecule and io surface energ tem with 10 M
sponding qu d in the LDH SA1-molecule A2- molecule pore as show howed a smal when the num
uration (appr
protonated, o ion to stabil two H+ catio ace attracts surface, mak stable. Thus,
it will attract rotonation. B
2
on results in
al. 1994). W predict adsor
in the simul ion consist on, as the p SA is initial Assuming th MBSA1- an
n ratios of 1
pectively. Th
ponent alone) com
of H+ cation wed interestin MBSA anio MBSA 2-Interaction nergy (kcal/mo -177.69 -268.99 -365.88 -505.68 -661.85 -849.54
on between th ays lower tha face (Table 3 ecules are a when the inte
e energy of i onized cation gy.
MBSA anion uantity of cat H pore, we o
es were on th es remained wn in Figure ller amount mber of mol roximately 1
one H+ catio ize it. For th ons. The pos
M st M
F M
(O dx
p
et
m th h
3 H re p C to ad h 4 re
MBSA2- anio trong compe MBSA2- that r
Figure 7: Equ MBSA2-.
Original color ver x.doi.org/10.1590/0
Evidences henomena co
t al. (2007) methods a fun
he anions ar exafluoropho
The MBS 8% is less th However, th
epresents a f rotonation in Changes in th
o 10.5 may dsorption red ave found a 5 to 30 mV espectively.
on produces etition from H
remains on t
uilibrium pos
rsion of this figur 104-6632.20160331
of a simila ould be foun . The autho nction showin
round 1-n-b osphate catio SA2- theoreti
han the expe ese are ver first attempt n adsorption he LDH surf
y also con duction. Xu a variation o
when the p
s a second H+ cations, so the surface de
sition of (a) M
re is available at 1s00003451
ar anion-catio nd in the stud ors deduced
ng the spatia butyl-1,3-met
ons.
cal adsorptio erimental de ry promisin t to quantify n of this cla face when th ntributed to
et al. (2008 of LDH zeta pH changes f
H+, it suff o the amount
ecreases.
MBSA1- and
the journal
web-on stabilizat dy of Bharga
from quant al distribution thylimidazoli
on decrease ecrease of 56 ng results a y the impact
ass of system he pH increa
the MBS 8), for examp
potential fr from 6.5 to
fers t of
(b)
-site:
tion ava tum n of ium
of 6%.
and t of
ms. ases SA
2-ple, rom 10,
hav LD dro hav eva occ in spe hy wi
sup
Ab
Ag
Be
Bh
Bo
Ca
Cy
Du
Through sim ve validated DH through
otalcite and t ve obtained aluate the i curs in real s
the amount ecies MBSA ydrogen catio th the surfac
A
The author pport receive
bdelkader, E study of ph pension by Technol., 16 guiar, J. E., B P. D. S., No Silva Jr. J. dyes from a Al layered theoretical 2316 (2013 ellotto, M., Reexaminat try. J. Phys. hargava, B. tential mod liquid [bmim 114516 (20 oudiaf, H. Z. methyl ora calcined an hydroxides (2012). ai, H., Hillier
Ward, M. D adsorption.
ygan, R. T., L lar models phases and field. J. Phy utta, P. K., P
CONCLU
mulations on d an adsorpti
its position the amount a evidence tha mpact of in systems as th t adsorbed o A2- is related t ons with MB ce for global
ACKNOWL
rs wish to a ed from CAP
REFERE
., Nadjia, L. henazin neutr
paper sludg 69, 231-238 Bezerra, B. T ogueira, R. E I., Adsorptio aqueous solu double hyd study. Separ ).
Rebours, B. tion of Hyd Chem., 100 L., Balasubr del for atom m][PF6]. J. P
07).
, Boutahala, ange from a nd calcined (LDHs). Ch
r, A. C., Fra D., Nanosca
Science, 266 Liang, J. -J., K
of hydroxid the develop ys. Chem., 10
Puri, M., A
USIONS
n the NVT ion model fo n on the su adsorbed at s at the model ncreasing pro he pH varies of the more to the strong BSA molecul minimum en
LEDGMENT
acknowledge PES and CNP
ENCES
, Ahmed, B ral red from ge. J. Chem.
(2011). T. C., Braga, E. F. Q., Luc on of anioni ution on non droxide: Exp r. Sci. Techn
., Clause, O drotalcite Cry 0, 8527-8534
ramanian, S. mistic simula Phys. Chem.,
M., Arab, L aqueous sol MgNiAl la hem. Eng. J.,
anklin, K. R. ale imaging 6, 1551-1555 Kalinichev, A e, oxyhydrox pment of a 08, 1255-126
nion exchan
ensemble, w or MBSA1- o urface of h saturation. W
is also able otonation th . The decrea e deprotonate g interaction
les, competin nergy.
T
e the financi Pq.
., Degradatio m aqueous su
Eng. Proces
, B. M., Lim cena, S. M. P
c and cation n-calcined M perimental an nol., 48, 230
O., Elka E., ystal Chemi (1994). ., Refined p ations of ion , 127, 11451
L., Removal lution by u ayered doub , 187, 142-14
, Nunn, C. C of molecul 5 (1994). A. G., Molec
xide, and cla general for 6 (2004). nge in lithiu
we on hy-We
to hat ase ed of ng
ial
on
us-ss.
ma, P., nic
Mg-nd
7-A
is- po-nic
0-of
un-ble 49
C., lar
cu-ay rce
Molecular Validated Model for Adsorption of Protonated Dye on LDH 241
Brazilian Journal of Chemical Engineering Vol. 33, No. 01, pp. 235 - 241, January - March, 2016 aluminate hydroxides. J. Phys. Chem., 93,
376-381 (1989).
Itaya, K., Chang, H. C., Uchida, I., Anion-exchange clay (hydrotalcite-like compounds) modified elec-trodes. Inorg. Chem., 26, 624-626 (1987).
Kim, N., Harale, A., Tsotsis, T. T., Sahimia, M., Atomistic simulation of nanoporous layered double hydroxide materials and their properties. II. Adsorption and diffusion. J. Chem. Phys., 127, 214701-214713 (2007).
Lima, A. E. O., Gomes, A. A. M., Lucena, S. M. P.,
Theoretical study of CO2:N2 adsorption in
faujasite impregnated with monoethanolamine. Braz. J. Chem. Eng., 32(3), 663-669 (2015). Rappe, A. K., Casewit, C. J., Colwell, K. S., Goddard,
W. A., Skiff, W. M., UFF, a full periodic-table force-field for molecular mechanics and molecu-lar dynamics simulations. Journal of the Ameri-can Chemical Society, 114, 10024-10035 (1992). Rappe, A. K., Goddard, W. A., Charge equilibration
for molecular dynamics simulations. J. Phys. Chem., 95, 3358-3363 (1991).
Reichle, W. T., Kang, S. Y., Everhardt, D. S., The
nature of the thermal decomposition of a catalyti-cally active anionic clay mineral. Catalysis Jour-nal, 101, 352-359 (1986).
Serwicka, E. M., Bahranowski, K., Environmental catalysis by tailored materials derived from lay-ered minerals. Catalysis Today, 90, 85-92 (2004). Sun, H., COMPASS: An ab initio force-field opti-mized for condensed-phase applications-overview with details on alkane and benzene compounds. J. Phys. Chem., B, 102, 7338-7364 (1998).
Wang, J., Kalinichev, A. G., Kirkpatrick, J., Hou, X., Molecular modeling of the structure and ener-getics of hydrotalcite hydration. Chem. Mater., 13, 145-150 (2001).
Xu, Z. P., Jin, Y., Liu, S., Hao, Z. P., Lu, G. Q., Sur-face charging of layered double hydroxides dur-ing dynamic interactions of anions at the inter-faces. Journal of Colloid and Interface Science, 326, 522-529 (2008).