0103 - 5053 $6.00+0.00
Article
* e-mail: rlsernaglia@uem.br
Oxidative Dimerization of Ru(III)-EDTA Complex on the Surface of Functionalized Silica Gel
Lúcia Codognoto, Patrícia Graça Zanichelli and Rosana Lázara Sernaglia*
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020 - 900 Maringá - PR, Brazil
Este trabalho descreve um estudo da reação de ancoramento de H[Ru(III)Cl2(H2EDTA)].4.5 H2O na superfície da sílica gel funcionalizada (SF) com o composto [3-(2-aminoetil)aminopropil]trimetoxissilano (AEATS), gerando por meio de ligação amida o composto dimérico (EDTA)2Ru2(IV,IV) na superfície da sílica modificada. A oxidação da água por esse complexo imobilizado na superfície da sílica foi observada pela formação de O2 e decréscimo do pH, originando a sílica modificada SF-AEATS/(EDTA)2Ru2(III1/2,III1/2). Este dímero ancorado foi caracterizado por espectroscopia eletrônica e vibracional e também por espectroscopia de EPR. O comportamento eletroquímico de um eletrodo de pasta de carbono modificado com a sílica SF-AEATS/(EDTA)2Ru2(III1/2,III1/2) também foi estudado.
The present paper deals with the reaction of oxidative dimerization of the H[Ru(III)Cl2(H2EDTA)].4.5 H2O complex on the surface of silica gel functionalized (SF) with [3-(2-aminoethyl)aminopropyl]trimethoxysilane (AEATS), generating the dimeric (EDTA)2Ru2(IV,IV) complex immobilized on the surface of this silica through the coordination with amide bonds. The oxidation of water by the anchored dimeric complex proceeded rapidly and was observed by the evolution of O2 and the decrease in pH, yielding the SF-AEATS/(EDTA)2Ru2(III1/2,III1/2). This anchored dimer was characterized by electronic, vibrational and EPR spectroscopies. The electrochemical behavior of a modified carbon paste electrode prepared with SF-AEATS/ (EDTA)2Ru2(III1/2,III1/2) was also studied.
Keywords: Ru(III)-EDTA complex dimerization, modified silica gel, silica immobilized
ruthenium edta dimer
Introduction
The synthesis and characterization of electroactive mono and polymetallic complexes of ruthenium (III)/(II) attached to solid supports have been the subject of extensive studies. These materials can be applied in areas such as catalysis and electroanalytical chemistry and, particularly as electron-transfer mediators in molecular devices and multielectron transfer catalysis.1-15
Our particular interest is focused on modified carbon paste electrodes made with functionalized silica gel with Ru(III)/(II)-EDTA complexes covalently immobilized on its surface.1-3, 15-17 The synthetic route to support
Ru(III)-EDTA complex on the surface of silica gel functionalized with [3-(2-aminoethyl)aminopropyl]trimethoxysilane (AEATS) is similar to the one described by Anson et al.18
for the ethylenediaminetetraacetate complexes of ruthenium (II) and (III). The ruthenium complex was
attached to graphite electrodes by amide bonds formed by the condensation of an uncoordinated acetate group in Ru(III)-EDTA with amine groups introduced into the graphite surface by a plasma etching procedure.2
In this paper, amine groups are introduced onto the silica surface by silanization reaction with [3-(2-aminoethyl)aminopropyl]trimethoxysilane, yielding SF-AEATS. The reaction of this support with H[Ru(III)Cl2(H2EDTA)].4.5H2O in the presence of dicyclohexylcarbodiimide (DCHC) in N,N-dimethylformamide (DMF) is presented.2
This reaction leads to the dimeric complex (EDTA)2Ru2(IV,IV) attachments on the surface of the SF-AEATS silica.
Experimental
was used throughout the work; RuCl3.xH2O was used as the starting material for the synthesis of the ruthenium complex.
The dicloro(dihydrogen ethylenediaminetetra-acetate)ruthenate(III), H[Ru(III)Cl2(H2EDTA)].4.5H2O acid complex was prepared and characterized following procedures previously described.20-22 The product, obtained
in the form of yellow crystals, was readily soluble in water, ethanol and N,N’-dimethylformamide. (Found: C, 22.04; H, 4.10; N, 4.99, Anal Calc. for: C, 22.06; H, 4.44; N, 5.14%). Elemental analyses were performed in the Microanalytical Laboratory of the University of São Paulo. The IR spectrum of the product was in agreement with the one reported by Mukaida.21
Silanization reaction
The silica gel-60 (SPA) (Aldrich, 70-230 mesh) with specific surface area of 506 ± 6 m2g-1 was activated by
onto a platinum grid (total area, 0.2 cm2). The studies in
the electrochemical cell were performed under argon atmosphere.
The electrochemical behavior of such electrodes was analyzed by cyclic voltammetry and the ionic strength (0.80 mol L-1) was controlled with sodium trifluoracetate
(NaCF3COO), at 25.0 ± 0.5 °C. The potentials were reported to Ag/AgCl (satured KCl) electrode, double junction Orion model 90-02 (E° = 0.197 V vs. SHE, at 25°C). A platinum wire was used as an auxiliary electrode and the modified carbon paste electrode (CPE) was used as a working electrode.
The comproportionation constant (Kc)27 was calculated
by equation 3:
(3)
where E0 = midpoint potential, n = number of electrons, F
= Faraday constant. When n1 = n2 = 1, Kc = exp('E0 / 25.69) at 298 K, with 'E0 given in mV.
Infrared spectra
The infrared spectrum of the self-supported disk of the material was obtained without any dilution of the immobilized complexes with KBr. The equipment used was an FT-IR Bome Hertmann & Braum, model MB100 spectrophotometer with sample disk containing approximately 10 mg cm-2.
Electron paramagnetic resonance spectra
Low temperature electron paramagnetic resonance (EPR) spectra were measured using a Bruker ESP-300E spectrometer operating at X-band frequencies at 77 K. The EPR spectra were obtained at the Instituto de Química de São Carlos, USP - São Carlos.
Thermogravimetric Analysis (TGA)
TGA curves were obtained in a Shimadzu (TGA-50) thermogravimetric analyser, at a heating rate of 20 °C min-1,
in the range of 25 to 1000 °C and under N2 atmosphere flowing at 10 mL min-1.
Results and Discussion
The immobilization1-3 of H[Ru(III)Cl
2(H2EDTA)]
complex on functionalized silica gel SF-AEATS was achieved by the condensation of the uncoordinated acetate
groups of Ru(III)-EDTA with amine groups (silane en) of SF-AEATS. Approximately 2.0x10-4 (mol g-1) mol of Ru
were immobilized per g of silica, implying that 23% of the nitrogen sites were bonded to the metal centers. A decrease in the surface area of the silica with the functionalization of (silane en) groups was observed. The specific area of the silica decreased from 506 ± 6 m2 g-1 to 337 ± 6 m2g-1,
probably due to the blocking of the pores with the coupling of (silane en) groups on its surface.12 The value of (1.5 ±
0.1)x1018 M-2 was obtained for the surface bonded (silane
en) groups density. Based on equation 2, the estimated value for the average distance between two AEATS groups was 8.1 ± 0.3 Å. This distance could be considered an estimate of the minimum average distance necessary for two metal centers immobilized by amide bonds undergoing oxidative dimerization reactions on the surface of the SF-AEATS or vice-versa.
The specific area of the solid after anchoring the metal complex decreases to 320 ± 6 m2g-1.
Besides the immobilization of the ruthenium complex, metal centers in high oxidation states were observed, probably due to the formation of (EDTA)2Ru2(IV,IV) dimer. Upon contact of SF-AEATS/(EDTA)2Ru2(IV,IV) with water, a copious gas evolution (O2) and an increase in the solution hydrogen ion concentration (3 pH units, for 20 mg of solid) were observed. A dimeric complex was produced according to the reaction :
2H2O + 4((EDTA)2Ru2(IV,IV)) O2+
4((EDTA)2Ru2(III1/2,III1/2)) + 4H+
on the surface of the AEATS silica, yielding the SF-AEATS/(EDTA)2Ru2(III1/2,III1/2).
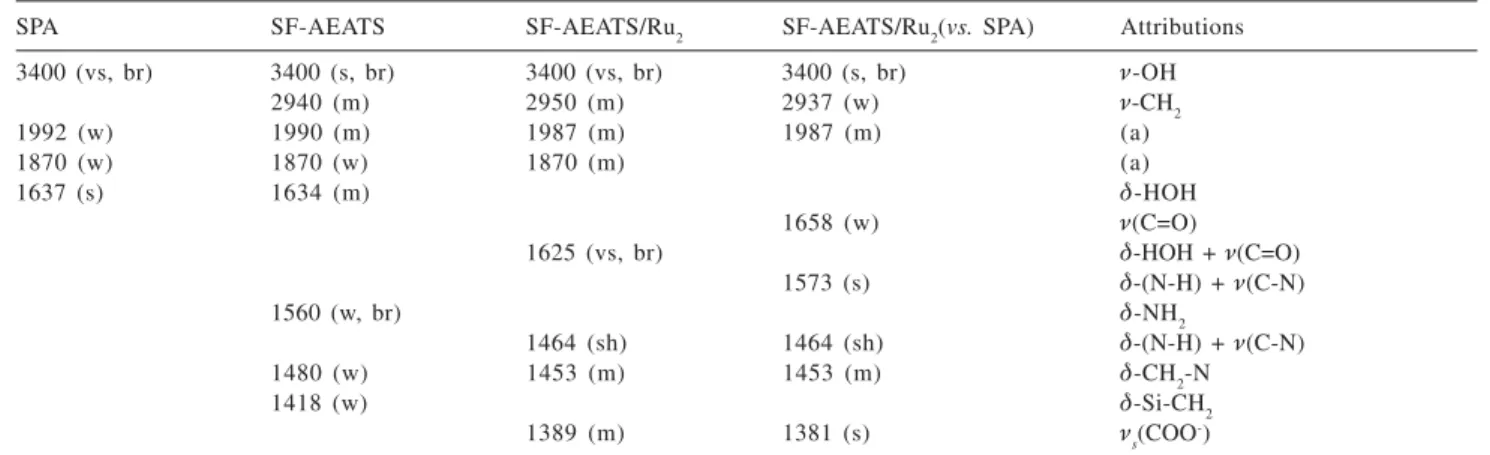
The infrared spectrum of the SF-AEATS/ (EDTA)2Ru2(III1/2,III1/2) was an important evidence for amide bond formation in the immobilization of the complex by covalent Ru-N (amide) bonds. Table 1 shows the main infrared frequencies observed in the spectrum of the anchored complex. The spectrum shows the three characteristic bands of amide bonds, i.e., 1658 cm-1 (Q (C=O)), 1573 cm-1 and a shoulder at 1464 cm-1 (Q(C-N) + G
(N-H) in the plane).28
The electron paramagnetic resonance spectrum of the SF-AEATS/(EDTA)2Ru2(III1/2,III1/2) sample was recorded at 77 K, and is shown in Figure 1.
1 indicates that the unpaired electron is substantially delocalized in the bridging ligand.29
The thermoanalytical data obtained by TGA analysis of silicas SPA, SF-AEATS and SF-AEATS/(EDTA)2Ru2(III1/2, III1/2) indicate a similar thermal behavior in an N2 atmosphere. The mass loss occurs in two steps: up to 400.0 ± 0.5 K, which is attributed to the loss of H2O and between 453.0 ± 0.5 and 813.0 ± 0.5 K, attributed to the loss of the organofuntional groups.
The SF-AEATS/(EDTA)2Ru2(III1/2,III1/2) showed 21.8 % of total mass loss whereas SF-AEATS presented a loss of 16.2 %. This difference indicates the loss of weighed groups of SF-AEATS/(EDTA)2Ru2(III1/2,III1/2) surface, confirming the presence of the dimeric complex of Ru(III)-EDTA adsorbed in that modified material.
The electronic spectrum of the SF-AEATS silica modified with the reduced ruthenium dimeric complex, Ru2(III1/2,III1/2), (Figure 2) showed the presence of a band at
Omax = 940 ± 10 nm, with bandwidth value at half intensity
of'Q1/2 = 6152 cm-1. This band was tentatively attributed
as being due to the Ru(III)-Ru(IV) intervalence transition (IT) of the Ru(III)-Ru(IV) dimer anchored on the SF-AEATS silica surface by comparison with the similar compound described in the literature (IT, Omax at 632 nm).18 Two more
absorption bands were observed in the electronic spectrum for the supported complex: Omax at 370 nm and at 470 nm (insert in Figure 2).
The electrochemical studies performed with a modified carbon paste electrode prepared with SF-AEATS/ (EDTA)2Ru2(III1/2, III1/2) are also consistent with the presence of the Ru(III1/2,III1/2) dimeric complex, on the SF-AEATS silica surface.
Figure 3 shows the cyclic voltammograms of this modified carbon paste electrode (P = 0.8 mol L-1 in
NaCF3COO, to 25.0 ± 0.5 °C) at various scan rates, in the potential range of 0.700 to - 0.700 V (vs. Ag/AgCl). The electroactive species presents two midpoint potentials, i.e.,
Table 1. Infrared absorption frequencies of silica SPA, SF-AEATS and SF-AEATS/(EDTA)2Ru2(III1/2, III1/2)
SPA SF-AEATS SF-AEATS/Ru2 SF-AEATS/Ru2(vs. SPA) Attributions
3400 (vs, br) 3400 (s, br) 3400 (vs, br) 3400 (s, br) Q-OH
2940 (m) 2950 (m) 2937 (w) Q-CH2
1992 (w) 1990 (m) 1987 (m) 1987 (m) (a)
1870 (w) 1870 (w) 1870 (m) (a)
1637 (s) 1634 (m) G-HOH
1658 (w) Q(C=O)
1625 (vs, br) G-HOH + Q(C=O)
1573 (s) G-(N-H) + Q(C-N)
1560 (w, br) G-NH2
1464 (sh) 1464 (sh) G-(N-H) + Q(C-N)
1480 (w) 1453 (m) 1453 (m) G-CH2-N
1418 (w) G-Si-CH2
1389 (m) 1381 (s) Qs(COO
-)
(a) Silica skeleton combinations (reference 28) ; (s) strong; (w) weak; (br) broad; (sh) shoulder; (vs) very strong; (vw) very weak.
Figure 1. Electron Paramagnetic Resonance (EPR) spectrum of SF-AEATS/(EDTA)2Ru2(III1/2,III1/2).
E1/2 = - 0.215 ± 0.10 V and E1/2 = 0.090 ± 0.10 V. A plot of peak current (PA)versus scan rate (V s-1) in the two processes is linear up to 50 mV s-1.
The electrochemical behavior of this electroative anchored species is similar to the one described for the Ru(III1/2)-Ru(III1/2) dimer complex characterized by Baar and Anson:18
From the electrochemical data and assuming the formation of the symmetric binuclear complex, the comproportionation constant (Kc) for reaction:
was estimated as Kc = 1x105, suggesting a considerable
coupling between the metallic centers.
The anchored dimer exhibits pH-dependent E1/2 values at low pH for the two electrochemical processes. From Pourbaix plots (pH vs. E1/2, Figure 4) the pKa values of 3.3 ± 0.2 for the (EDTA)2Ru2(III1/2,III1/2) anchored dimer and 3.5 ± 0.2 for the (EDTA)2Ru2(III,III) species were calculated. These pKas values were assigned to the single uncoordinated carboxylate group of the EDTA ligant also present in the dimer complex.
10. O’Shea, T. J.; Leech, D.; Smyth, R. M.; Vos, J. G.; Talanta
1992,39, 443.
11. Toma, H. E.; Matsumoto, F. M.; Cipriano, C.; J. Electroanal.
Chem.1993,346, 261.
12. Neiva, S. M. C.; Santos, J. A.V.; Moreira, J. C.; Gushikem, Y.; Vargas, H.; Franco, D. W.; Langmuir1993,9, 2982. 13. Peixoto, C. R. M.; Kubota, L. T.; Gushikem, Y.; Anal. Proc.
1995,32, 503.
14. Gushikem, Y.; Peixoto, C. R. M.; Rodrigues-Filho,U. P.; Kubota, L. T.; Stadler, E.; J. Colloid Interface Sci.1996,184, 236. 15. Lazarin, A. M.; Sernaglia, R. L.; Quim. Nova 1999,22, 342.
16. Toma, H. E.; Sernaglia, R. L.; Talanta1993,40, 515. 17. Toma, H. E.; Sernaglia, R. L.; Talanta1995,42, 1867.
18. Baar, R. B.; Anson, F. C.; J. Eletroanal. Chem. 1985,187, 265.
19. Perrin, D.; Armarego, W. L. F.; Perrin, D. R.; Purification of
Laboratory Chemicals, Pergamon Press Ltd.: Oxford, 1980.
20. Yoshino, Y.; Uehiro, T.; Saito, M.; Bull. Chem. Soc. Jpn.1979,
52, 160.
21. Mukaida, M.; Okuno, H.; Ishimori, T.; Nippon Kagaku Sasshi
1965,86, 589.
22. Matsubara, T.; Creutz, C.; Inorg. Chem.1979,18, 1956.
23. Burggraf, L. W.; Kendall, D. S.; Leyden, D. E.; Pern, F.; J.
Anal. Chim. Acta1981,129, 19.
24. Skopenko, V. V.; Trofimchuk, A. K.; Kaminskii, V. P.; Soviet
Prog. Chem.1982,48, 14.
25. Loon, J. C. V.; Selected Methods of Trace Metal Analysis
Biological and Environmental Samples, John Wiley & Sons:
New York, 1985.
26. Iler, R. K.; The Chemistry of Silica, John Wiley & Sons: New
York, 1977.
27. Denofre, S.; Gushikem, Y.; Castro, S. C.; Kawano, Y.; J. Chem.
Soc. Faraday Trans.1993,89, 1057.
28. Colthup, N. B.; Daly, L. H.; Wiberley, S. E.; Introduction to
Infrared and Raman Spectroscopy, 4th ed., Acad. Press, Inc.:
New York, 1969.
29. Matsumoto, K.; Matsumoto, T.; Kawano, M.; Ohnuki, H.; Shichi, Y.; Nishide, T.; Sato, T.; J. Am. Chem. Soc.1996,118,
3609.