w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Effect
of
exogenous
phytohormones
treatment
on
glycyrrhizic
acid
accumulation
and
preliminary
exploration
of
the
chemical
control
network
based
on
glycyrrhizic
acid
in
root
of
Glycyrrhiza
uralensis
Yan-peng
Li
a,
Chun-xia
Yu
b,
Jing
Qiao
a,
Yi-mei
Zang
a,
Yu
Xiang
a,
Guang-xi
Ren
a,
Li
Wang
a,
Xin-yue
Zhang
a,
Chun-sheng
Liu
a,∗aSchoolofChinesePharmacy,BeijingUniversityofChineseMedicine,Beijing,China
bHenanProvinceforDrugEvaluationCertificationCenter,Zhengzhou,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19August2015 Accepted23February2016 Availableonline19May2016
Keywords:
Auxin
Chemicalnetwork Gibberellin Glycyrrhizicacid Methyljasmonate
a
b
s
t
r
a
c
t
One-year-oldGlycyrrhizauralensisFisch.exDC,Fabaceae,wastreatedwiththreeexogenous
phytohor-monesinJuneandJuly,namelygibberellin,auxin(indole-3-aceticacid),methyljasmonateatdifferent
concentrations.Controlplantsweretreatedwithwater.Rootsofcontrolsandhormones-treatedG.
uralen-sisplantswereharvestedatdifferenttimes,andthecontentsofsevenmainchemicalcomponentswere
determined.RootglycyrrhizicacidcontentofplantstreatedinJuneincreasedsignificantlycompared
withcontrols,andthedifferencewassignificant.AsforplantstreatedinJuly,rootglycyrrhizicacid
contentincreasedinwhichsprayedwithappropriateconcentrationsofhormones,buttheeffectsof
hormonesweremoreevidentinplantstreatedinJunecoincidedwiththevigorousgrowthperiodthan
thosetreatedinJuly.Gibberellinat40mg/landauxinat40mg/lappliedinthetwotreatmentperiods
significantlypromotedtheaccumulationofglycyrrhizicacidinG.uralensisroot.Treatmentwithmethyl
jasmonateat100and25mg/linJuneandJuly,respectively,alsoincreasedglycyrrhizicacidcontent
signif-icantly.Thedeterminationofmajoractivecompositionsindicatedthatliquiritin,isoliquiritin,isoliquiritin
apiosideandliquiritinapiosidecontentswerepositivelyrelatedtoglycyrrhizicacidcontent.Thestudy
preliminarilyfoundphytohormonesandthemainchemicalcomponentsassociatedwithglycyrrhizic
acidcontent,andthesediscoveriescouldprovideabasisforestablishingachemicalcontrolnetwork
withglycyrrhizicacidasthecore,confirmingthesecondaryproductmetabolicpathwaysinthenetwork
andcompletelyuncoveringsynthesismechanismunderlyingglycyrrhizicacid-combinedfunctionalgene
polymorphism.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
GlycyrrhizauralensisFisch.exDC,Fabaceae,isknowntobethe
‘king’oftraditionalChinesemedicine.Thisplantisthemost com-monlyusedmedicinalmaterialand is animportant additivein cosmetic,health product and tobaccoindustry. A highdemand
forG.uralensisisreportedeveryyear.CultivationofG.uralensis
hasbecomethemainstreambecauseofthelackingwildresources. However,awidespreadproblemhasbeenreportedregardingG.
∗ Correspondingauthor.
E-mail:maxliucs@263.net(C.-s.Liu).
uralensisqualityintermsofthesubstandardcontentofglycyrrhizic
acid. Therefore, improvingthe quality ofcultivated G. uralensis hasbecomeafocusofresearchinthefieldofChinesemedicine resources.
Glycyrrhizicacid(1),atriterpenoidsaponinscomponents,isthe mainbioactivecomponentwithanti-viral,anti-inflammatory, anti-tumorandothermajorpharmacologicalactivitiesinG.uralensis root(ZhangandYe,2009).Asanothermajoreffectivecomponents
inG.uralensisroot,flavonoidshavesignificantanti-tumour(Zhang
andYe,2009;Lietal.,2012), anti-oxidantactivities(Zhangand Ye,2009;Caietal.,2004),andthemostrepresentedflavonoidsare liquiritin(2),isoliquiritin(3),liquiritigenin(4),isoliquiritigenin(5), liquiritinapioside(6),andisoliquiritinapioside(7)(Zhangetal., 2013).
http://dx.doi.org/10.1016/j.bjp.2016.02.009
O
H O
H
H
CO2H
O HO2C HO
HO
O O HO2C HO
HO
OH
1
O HO
O
O
2
R=Glu3
R=H4
R=Glu-ApioseHO
O
O
5
R=Glu6
R=H7
R=Glu-ApioseO CO2HOH
OH
O O
O
OH OH HO
R R
S
Currently, various chemical and physical factors affect the medicinalplantgrowthandsecondarymetaboliteproductionhad beenresearchedwidely.Moisture(Lietal.,2011),light(Houetal., 2010; Afreenet al.,2005), salt (Wanet al.,2011), mineral ele-ments(Yinetal.,2014;Wangetal.,2010;LiuandWang,2009)and otherinducedfactorshavebeenstudiedinrelationtoG.uralensis growthandaccumulationofglycyrrhizicacidthroughfield culti-vation,invitrocultureandhairyrootculture.However,studieson theregulatoryeffectsofphytohormonesonG.uralensisonlyfocus intheaspectofplantgrowth,andin-depthresearchintheaspect ofthesecondarymetabolismislacking.
Alargenumberofstudieshavesuggestedthatphytohormones serve acrucial functionin alteringplantgrowthand secondary metabolism.Gibberellin(GA)isawidespreadandwidelystudied phytohormonethat couldeffectively regulate plantgrowthand formationofsecondarymetabolites.Zhangetal.(2005)reported that GA3 can induce the transformation of artemisinic acidto
artemisininandstimulateartemisininbiosynthesis.
Auxin(indole-3-acetic acid,IAA)is aphytohormonethathas closerelationshipandsimilareffectstoGA.Studieshaveshownthat IAAtreatmentcanstimulategrowthinhairyrootculture,and pro-ducedifferenteffectsonsecondarymetabolitesindifferentplants (Rhodesetal.,1994;Arrooetal.,1995).However,relativelyfew studieshave focused onIAA’sregulation onthemetabolism of medicinalplants.
As growth regulator that widely exists in plants, methyl jasmonate(MeJa)caninducechemicaldefencesthatsimulate bio-logical stress, which is an exogenous inducer on induction of secondarymetabolismintheplants,plantcellsandcalli(Qianetal., 2004;Yuetal.,2002;Zhaoetal.,2001;Bulgakovetal.,2002). Exoge-nousMeJaincreasedthecontentofginsenosidesinPanaxginseng cell(Luetal.,2001)andadventitiousrootscultivation(Yuetal., 2002),andenhancedphenolic acidcontentinSalviamiltiorrhiza hairyroot(Xiaoetal.,2009).
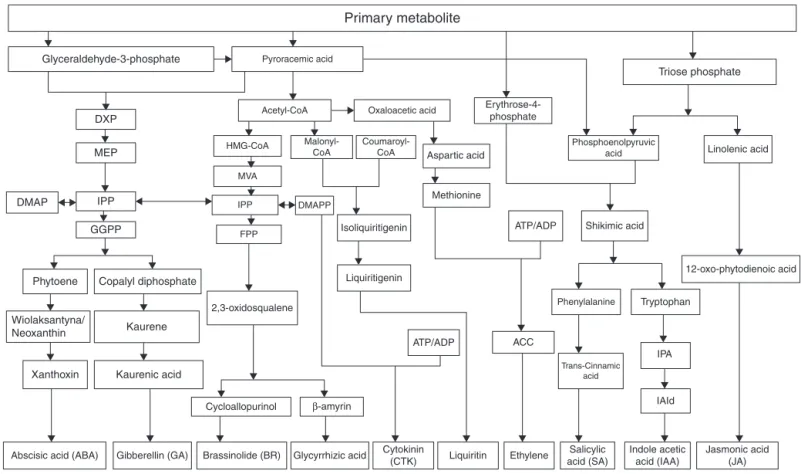
Productionandmetabolismofeachproductintheplantarenot isolated,andtheseprocessesshouldformaninterrelated interac-tionnetworkinwhichmultiplemetabolicpathwaysinterconnect bynodes.Researchershavefoundaninterplaybetweenthe end-productofdifferentmetabolicpathwaysinmanyplants.A theoret-icalmetabolicnetworkdiagramthatcorrelateswiththecontentof glycyrrhizicacid(1)inG.uralensisroothasbeendepictedbasedon acombinationofresearchandliterature(Fig.1).Theoriginalview, whichfocusesonterpenoidmetabolicpathway,hasbeenamplified tocoverallkindsofsecondarymetabolitebiosynthesispathways.
Thecurrent article aimstostudy rootglycyrrhizic acid
con-tentofG.uralensisaftertreatmentswiththreekindsofexogenous
hormones,thecorrelationbetweenmajorendogenous chemical componentsandglycyrrhizicacidcontent,thenpreliminarilyfind phytohormonesandmainchemicalcomponentsassociatedwith glycyrrhizicacidcontent,whichcouldlayasolidfoundationfor definingconstitutionofchemicalcomponentsandmetabolic path-waysinthecontrolnetwork basedonglycyrrhizicacid,thereby completelyexplainingtheunderlyingsynthesismechanismof gly-cyrrhizicacidcombiningfunctionalgenepolymorphism.
Materialsandmethods
Plantmaterials
One-year-old liquorice plant collected from Jingtai, Gansu Province,Chinawereculturedinplastic potsinMay2014filled with sandy loam soil (with identical composition and weight in each pot) in BeijingUniversity of ChineseMedicine medical plantgarden.EverypotcontainedeightG.uralensisplants,which weresubsequentlytreatedwithexogenoushormones.Theseplants weremanagedinparallelaccordingtotheconventional cultiva-tionmethod.Thevoucherspecimen(No.GU-0010)ofthesample, whichwasidentifiedasGlycyrrhizauralensisFisch.exDC,Fabaceae, byprofessorChun-shengLiuintheBeijingUniversityofChinese Medicine,waspreservedinBeijingUniversityofChinesemedicine specimenroom.
Exogenoushormonetreatmenttothesamplecollection
GA3(BioDee),IAA(Bioway)andMeJa(Sigma)atl5,25,40and
100mg/lsolutions,respectively,wereusedasexogenoushormone treatments.G.uralensisplantswereseparatedintotwo batches. Leaveswere sprayedwithprepared hormonesolutions in mid-to-late June and July.Control plants were sprayed withwater. Exogenoushormonesweresprayedeveryotherday(threetimes intotal)andmarkedwhenallwereleavesmoistandliquidwas hangingontheleaftips.Eachconcentrationofthreehormoneswas
usedon16potsofG.uralensisinrandomisedblockarrangement.
Thepotsweremanagedinparallelaccordingtotheconventional cultivationapproach.
ThefirstbatchofG.uralensis(treatedinJune)washarvestedfive timeson10July,20July,20August,20Septemberand20 Octo-ber.ThesecondbatchofG.uralensisplants(treatedinJuly)was harvestedthriceon15August,15Septemberand15October.At eachsamplingperiod,twopots(aboutsixteenplants)ofG.uralensis plantsbelongingtodifferenttreatmentgroups(different concen-trationofhormonetreatmentsandcontrolplants)wereharvested asonesample.Taproots(10cmbelowtherhizome)ofthemwere cutforcontentanalysis.
DeterminationofthesevenmaincomponentscontentofG.
ularensisroot
Chemicalsandmaterials
Primary metabolite
Glyceraldehyde-3-phosphate
DXP
MEP
DMAP IPP
GGPP
Phytoene
Wiolaksantyna/ Neoxanthin
Xanthoxin Kaurenic acid
Kaurene
2,3-oxidosqualene
Abscisic acid (ABA) Gibberellin (GA) Brassinolide (BR) Glycyrrhizic acid Cycloallopurinol β-amyrin
Cytokinin
(CTK) Liquiritin Ethylene
Salicylic acid (SA)
Indole acetic acid (IAA)
Jasmonic acid (JA) IAId
IPA Tryptophan
12-oxo-phytodienoic acid
Phenylalanine
ACC Methionine
Liquiritigenin Isoliquiritigenin
Aspartic acid
Coumaroyl-CoA Oxaloacetic acid
FPP
IPP DMAPP MVA
HMG-CoA Acetyl-CoA Pyroracemic acid
Malonyl-CoA
ATP/ADP
Erythrose-4-phosphate
Triose phosphate
Shikimic acid
Linolenic acid Phosphoenolpyruvic
acid
ATP/ADP
Trans-Cinnamic acid
Copalyl diphosphate
Fig.1. TheoreticallychemicalcontrolnetworkbasedonglycyrrhizicacidinGlycyrrhizauralensisroot.
gradientpump,onlinedegasser,andDAD detector.Glycyrrhizic acid(1) reference substance was purchased from the National InstitutefortheControlof PharmaceuticalandBiological Prod-ucts(BatchNo. 110731-201317). Referencesubstances, namely liquiritin(2),isoliquiritin(3),liquiritigenin(4),isoliquiritigenin(5) (DingGuoChangSheng),liquiritinapioside(6),isoliquiritinapioside (7)(Yuanmu),HPLC-gradeacetonitrile(Fisher),analyticallypure phosphoricacid,andultrapurewater.
Apparatusandconditions
GradientelutionwasachievedusingaAgilentTC-C18column (250mm×4.6mm, 5m), as follows: 0min, acetonitrile–0.05% phosphoric acid (20:80); 8min, acetonitrile–0.05% phosphoric acid(20:80);30min,acetonitrile–0.05%phosphoricacid(38:62); 42min,acetonitrile–0.05%phosphoricacid(50:50).Thedetected wavelengthswereasfollows: 237nm(0–15min,liquiritin apio-side,liquiritin),365nm(15–23min,isoliquiritinapioside, isoliquir-itin), 237nm (23–30min, liquiritigenin), 370nm (30–37min, isoliquiritigenin),237nm(37–42min,glycyrrhizicacid).The col-umntemperatureandflowrateweresetat30◦Cand1ml/min,
respectively.Intheabovementionedchromatographyconditions, theoreticalplatenumberwasmorethan5000forglycyrrhizicacid andthedegreeofseparationbetweenadjacentchromatographic peakwasgreaterthan1.5.
The appropriate seven reference substances were measured accuratelyasstandardstocksolution,themethodconsistsofadding methanolto0.2440mg/mlliquiritinapioside,0.7500mg/ml liquir-itin,0.2280mg/mlisoliquiritinapioside,0.3300mg/mlisoliquiritin, 0.2820mg/ml liquiritigenin, 0.3240mg/ml isoliquiritigenin and 1.0280mg/ml glycyrrhizic acid. The seven above mentioned standardstocksolutionsweremeasuredaccuratelyas1.00,1.00, 0.40,0.60,0.20,0.08,0.80ml,respectively,andweresubsequently transferredin10mlvolumetricflask.Methanolwasaddedtoscale toproduce a mixedreference solution permillilitre containing
24.40gliquiritinapioside,75.00gliquiritin,9.12g isoliquir-itinapioside,19.80gisoliquiritin,5.64gliquiritigenin,2.59g isoliquiritigeninand246.72gglycyrrhizicacid.
Approximately0.2gofG.uralensisrootsamplepowder (pass-ing60mesh)wasmeasuredaccuratelyandplacedinsideacovered conicalflask.About50ml70%ethanolwasadded.Theplugwas subsequentlyclosed,andthesampleswereweighed.After30min ofultrasonic treatmentandcooling,thesampleswereweighed again.Complementweightloss,filteringandcollectingsubsequent filtratepassingthrougha0.45mmilliporefilter,namelytest sam-plesinthepresentstudy.
Each hormone-treatedG. uralensis root sample wasused as test sample based on the abovementioned method. Moreover, 10lsampleswereinjectedatthesetHPLCconditions.Thus,the contentsofthesevenmaincomponentswerecalculatedbyusing theexternalstandardmethod.
Methodinspectionofcontentdetermination
Differentconcentrationsofthemixedreferencesolutionwere accuratelymeasuredat10lrespectivelyandinjectedintoHPLC. Injectionvolumewastheabscissa,andthepeakareawasthe ordi-nate,plottingstandardcurveandcalculatingregressionequation (Table1).
G.uralensisrootsamplepowdersat0.2gwereusedtomakethe
testsolutionbasedontheabovemethodoftestsamplepreparation. Moreover,thetestsamplewascontinuouslyinjectedsixtimesat thesetHPLCconditions.ThepeakareaRSD(n=6)ofliquiritin apio-side, liquiritin, isoliquiritin apioside, isoliquiritin, liquiritigenin, isoliquiritigeninandglycyrrhizicacidwere1.2%,1.8%,1.6%,1.1%, 1.0%,1.1%,0.8%,respectively.Thesevaluesindicateagoodprecision oftheapparatus.
Table1
Regressionequation,correlationcoefficientandlinearityrangeofthesevenmaincomponentsinGlycyrrhizauralensisroot.
Component Regressionequation r Linearityrange(g/ml)
Liquiritinapioside(6) Y=13.905X+0.8565 0.9997 2.44–24.4
Liquiritin(2) Y=16.152X+1.9249 0.9998 7.5–75
Isoliquiritinapioside(7) Y=30.667X−0.3979 0.9998 0.912–9.12
Isoliquiritin(3) Y=35.502X−0.8873 0.9997 1.98–19.8
Liquiritigenin(4) Y=34.028X−0.3297 0.9997 0.564–5.64
Isoliquiritigenin(5) Y=69.708X−0.2655 0.9997 0.2592–2.592
Glycyrrhizicacid(1) Y=5.9346X−4.7191 0.9999 24.672–246.72
atthesetHPLCconditions.ThepeakareaRSDofliquiritin apio-side, liquiritin, isoliquiritin apioside, isoliquiritin, liquiritigenin, isoliquiritigeninandglycyrrhizicacidwere1.9%,1.5%,1.8%,1.1%, 0.6%,0.9%,1.2%,respectively,whichalsoindicatesrepeatabilityof themethod.
Thesametestsolutionwasinjectedafterthesamplewasplaced atroomtemperaturefor0, 2,4,8,12, 24h. ThepeakareaRSD ofliquiritinapioside,liquiritin,isoliquiritinapioside,isoliquiritin, liquiritigenin, isoliquiritigenin and glycyrrhizic acid were 1.4%, 1.5%,1.2%,0.8%,0.4%,0.4%and0.4%.Thesevaluesindicatethatthe sevenmaincomponentsinthetestsolutionwerestablefor24h.
Sixcopiesofcontent-knownsamplepowderswereindividually andaccuratelymeasuredtobeabout0.2g.Subsequently,standard stocksolutionsofliquiritinapioside,liquiritin,isoliquiritin apio-side,isoliquiritin,liquiritigenin,isoliquiritigeninandglycyrrhizic acidwereprecisely addedatthefollowing respectivevolumes: 0.51,0.78,0.25,0.30,0.14,0.07and1.44ml.Thetestsolutionwas madebasedontheabovemethodoftestsamplepreparationand wasinjectedundersetHPLCconditions.Afterwards,therecovery ratewascalculated.Theaveragerecoveryrate(n=6)oftheseven abovementionedcomponentswere103.3%,98.2%,101.5%,98.1%, 99.6%,103.2%,98.8%andtheRSDwere1.9%,1.0%,2.9%,1.5%,0.6%, 0.5%,and0.6%.
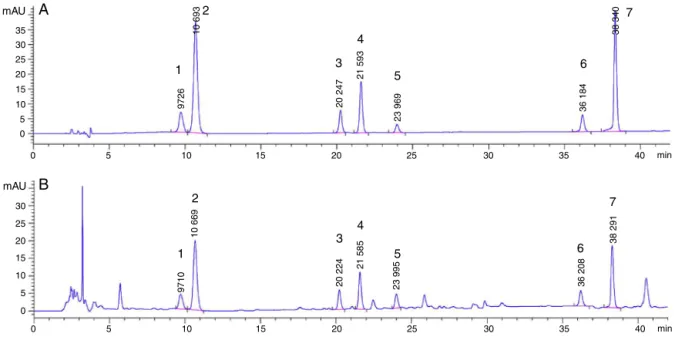
Thechromatogramofreferencesubstanceandsample(Fig.2) indicatedthatthepeakshapeandseparationweregood.
Statisticalanalyses
StatisticalanalyseswereperformedusingtheSPSS17.0 statisti-calpackage.Thedifferencebetweenmeanvaluesofeachtreatment wasdeterminedbyDuncan’smultiplerangetestandwas consid-eredsignificantat p<0.05. Correlation among componentswas analysed usingPearson bivariatecorrelation.Figures were con-structedusingExcel2007.
Results
InfluenceonglycyrrhizicacidaccumulationinG.uralensisrootof
exogenoushormonetreatment.
EffectofexogenousGA3treatmentonglycyrrhizicacidcontent
Rootglycyrrhizicacid(1)contentofG.uralensisplantstreated withdifferentconcentrationsofGA3inJuneshowedobvious
differ-ences(Fig.3).GlycyrrhizicacidcontentsofmostG.uralensistreated withGA3werehighercomparedwithcontrolplantsineach
samp-lingperiod.GlycyrrhizicacidcontentsofG.uralensistreatedinJuly alsohaddifferences,butwerenotasobviousasthosetreatedin June.TwobatchesofG.uralensisthatweresubjectedtodifferent GA3treatmentswerecomparedforglycyrrhizicacidcontents.GA3
at40mg/lgreatlyimprovedglycyrrhizicacidcontentinalleight samplingperiods,andthedifferencebetweentreatedplantsand controlswassignificant.Thecontentsincreasedby22.73%,12.42%, 34.73%,69.02%,22.34%,14.66%,36.43%and12.09%,respectively. Therefore,40mg/lexogenousGA3couldsignificantlypromote
gly-cyrrhizicacidaccumulationin one-year-oldG. uralensisroot.In
addition, GA3 treatmentin June which was a vigorous growth
periodofplantwasappropriate.
EffectofexogenousIAAtreatmentonglycyrrhizicacidcontent
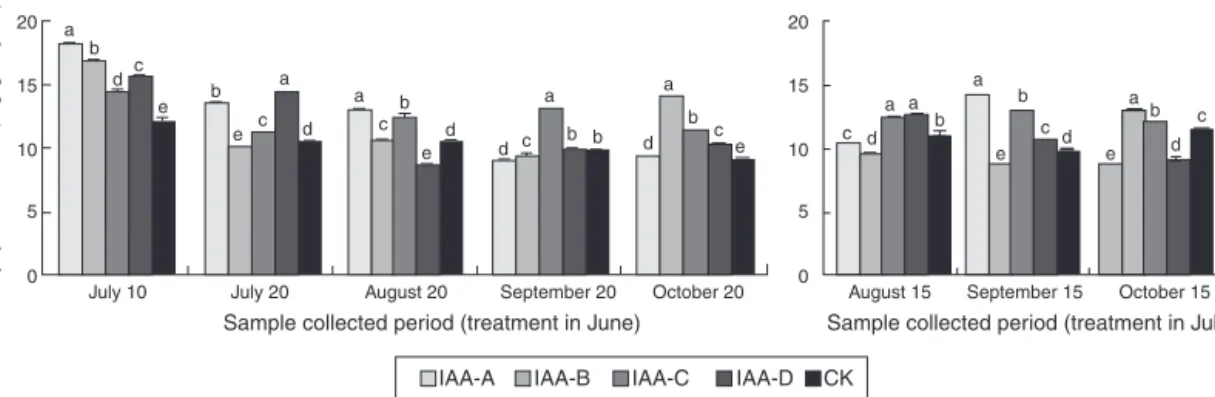
SimilartoGA3,rootglycyrrhizicacidcontentsofG.uralensis
root treated with differentconcentrations of IAA in June were obviously different (Fig. 4). Glycyrrhizic acid contents of most IAA-treatedplantswerehigherthancontrolplantsineach samp-lingperiod.Rootglycyrrhizic acidcontentsofG.uralensisplants treatedinJulyalsoshoweddifferencescomparedwiththecontrol, butthedifferenceswerenotasobviousasthoseofplantstreated in June. Root glycyrrhizic acidcontents of plants treated with IAA at 40mg/l improved and showed a significant difference compared with controls in the eight sampling periods of two batches.Theincrease in contentfortheeight samplingperiods were18.40%,6.20%, 18.93%,33.86%, 24.20%,13.03%, 30.03%and 4.23%,buttheincreasewaslessthanthatobservedinGA3-treated
plants. Hence,40mg/l exogenous IAA couldobviously promote glycyrrhizic acidaccumulationin one-year-oldG. uralensisroot, andglycyrrhizicacidaccumulationwashigherwhenexogenous hormonetreatmentswereconductedinJunethaninJuly.
EffectofexogenousMeJatreatmentonglycyrrhizicacidcontent
Ingeneral,rootglycyrrhizicacidcontentofG.uralensistreated withdifferentconcentrationsofMeJainJuneobviouslyincreased comparedtocontrolsineachperiod,buttreatedinJulywasnot thatobvious(Fig.5).MeJaat100mg/lappliedinJuneobviously increasedglycyrrhizicacidcontentinthefivesamplingperiods,and thedifferencesweresignificant.Thepercentagesofincreasewere 21.36%,32.79%,11.65%,46.40%and37.84%.InplantstreatedinJuly, MeJaat25mg/lobviouslyinfluencedglycyrrhizicacidcontent,and thepercentagesofincreaseincontentwere23.11%,63.00%and 1.46%inthethreesamplingperiods.Therefore,exogenousMeJaat 100mg/land25mg/ltreatmentsinmid-to-lateofJuneandJuly, respectivelycouldsignificantlypromoteglycyrrhizicacid accumu-lationinone-year-oldG.uralensisroot.Inaddition,similartoGA3
andIAAtreatments,MeJaapplicationinJunewasmoreappropriate.
Correlationbetweenthemaincomponentsandglycyrrhizicacid
contentinG.uralensisroot
Correlationanalyses(Table2)indicatedthatliquiritin(2) con-tent had significant positive correlation with glycyrrhizic acid (1)contentat 0.01 level (r=0.799), which wasstatistically sig-nificantbecauseofp=0.000<0.01. Isoliquiritin(3), r=0.725and p=0.000<0.01, was significantly and positively correlated with glycyrrhizicacidat0.01level.Isoliquiritinapioside(7)hada pos-itiveandstatisticallysignificantcorrelationwithglycyrrhizicacid contentat0.01level,r=0.418,p=0.000<0.01.Inaddition, liquir-itinapioside(6)andglycyrrhizicacidcontentswerealsopositively andstatisticallysignificantlycorrelatedat0.05level,r=0.222and p=0.023<0.05.Therefore,themaincomponentsthatwere
quanti-fiedinG.uralensisrootwerepositivelycorrelatedwithglycyrrhizic
mAU
35 30 25
20 15
10
5 0
0 5
2
3 4
5 6
7
1 1
2
3 4
5
6 7
10 15 20 25 30 35 40 min
0 5 10 15 20 25 30 35 40 min
mAU
30
25
20
15
10
5
0
A
B
9710
9726
10 693
20 247
21 593
23 969
36 184
38 340
10 669
20 224
21 585
23 995 36 208
38 291
Fig.2.HPLCchromatogramofthemixedreferencesubstance(A)andsample(B).(1)Liquiritinapioside,(2)liquiritin,(3)isoliquiritinapioside,(4)isoliquiritin,(5)liquiritigenin, (6)isoliquiritigeninand(7)glycyrrhizicacid.
20
15
10
5
0
20
15
10
5
0
Glycyrrhizic acid (mg
·g
1dr
y wt)
c a
July 10 July 20
Sample collected period (treatment in June) Sample collected period (treatment in July)
August 20 September 20 October 20 August 15 September 15 October 15 b
d d d
d c a
a b
b
c e
b c a
d e
b d c
a
e
a
e
a b
b
c c b d ba
c d d c
c
GA-A GA-B GA-C GA-D CK
Fig.3.TheeffectofGA3treatmentonglycyrrhizicacidcontentinG.uralensisroot(GA-A,GA-B,GA-CandGA-Dstandfor15,25,40and100mg/lGA3treatment,respectively. Differentlettersfollowedbymean±standarderrorofthreereplicatesindicatedsignificantdifferencesatp<0.05).
Discussion
Currently, wide-ranging studies on MeJa’s influence on G.
uralensishavebeenconducted.Shabaniet al.(2009)foundthat
MeJa treatment increased glycyrrhizic acid content in
65-day-old in vitro plantlet roots of G. uralensis, but restrained root
growth. These results were consistent with our study results. Besides the influence of MeJa on glycyrrhizic acid (1), Yang
etal.(2008)indicatedMeJatreatmentimprovedtotalflavonoids production insuspension culturecells of Glycyrrhizainflata but restrainedcellsgrowth.Hayashietal.(2003)reportedthatMeJa promotedsoyasaponinaccumulationbecauseitisconnectedwith a keyenzyme activitythat couldimprovebiosynthesis or gene expressionquantity.Intheendogenouschemicalcomponents
net-work of G. uralensis, many studies confirmed that liquiritin, a
majorflavonoidcomponent,wassignificantlypositivelycorrelated
a b
d b b
a c
c c
a
d b a
d e
d
d
a b b
b a
a
a b
c c c c
e d c e
e d e
a
e d
d
July 10 July 20
Sample collected period (treatment in June) Sample collected period (treatment in July)
August 20 September 20 October 20 August 15 September 15 October 15
ME-A ME-B ME-C ME-D CK
18
15
0 3 6 9 12
18
15
0 3 6 9 12
Glycyrrhizic acid (mg
·g
1dr
y wt)
July 10 July 20
Sample collected period (treatment in June) Sample collected period (treatment in July)
August 20 September 20 October 20 August 15 September 15 October 15
IAA-A IAA-B IAA-C IAA-D CK
20 a b
dc
c d
b b
b b b d d d
a
a a a
c
c c c
c c
a a a
a
b b
b
e e
e e
d
d d
e e 15
10
5
0
20
15
10
5
0
Glycyrrhizic acid (mg
·g
1dr
y wt)
Fig.5. TheeffectofMeJatreatmentonglycyrrhizicacidcontentinG.uralensisroot(MeJa-A,MeJa-B,MeJa-CandMeJa-Dstandfor15,25,40and100mg/lMeJatreatments, respectively.Differentlettersfollowedbymean±standarderrorofthreereplicatesindicatedsignificantdifferencesatp<0.05).
with glycyrrhizic acid (Guo et al., 2014), which was consis-tentwiththeresultsobtainedinthepresent study.Otherthan secondarymetaboliteslikephytohormonesandmedicinal compo-nents,primarymetabolitesofmedicinalplantswerealsoessential ‘sources’inthechemicalnetwork.Many researchershave stud-iedphysicochemicalproperties andfunctional characteristicsof polysaccharideinG.uralensis(WanandCheng,2009),andreported that starch contenthad a positive correlation withglycyrrhizic acid(Liu etal.,2009).Allthesedatalaida solid foundationfor establishingachemicalregulationnetworkbasedonglycyrrhizic acid.
Chemicalregulation,one ofthemaincontroltechnologiesof cropsandmedicinalplantsintherecentyears,couldadjustplant growthand metabolism by changingthe endogenous hormone system.Thisstudyinvestigatedtheeffectsofdifferenthormone treatments(GA3,IAAandMeJa),timesofapplication,
concentra-tionandsamplecollectionperiodonrootglycyrrhizicacidcontent. Phytohormones,appropriate concentration and treatmenttime, whichcouldsignificantlyaffectglycyrrhizicacidcontentwere pri-marily defined,providing a significant guidefor producing and cultivatingmedicinalplants.Changesofglycyrrhizicacidcontent showedsomeregularitiesunderdifferenthormonetreatmentsand sampling times. Parallel conventional cultivation and hormone treatmentsaswellascontent determinationofgroupsampling weremaximumreducederror,therebyensuringtheaccuracyand reliability of experimental results. Results from different time nodesshowedthewholetrendwasthat mostglycyrrhizic acid contentinG.uralensisrootatfirstsamplingtimeafterhormone treatmentobviouslyincreasedthenevidentlydeclined,afterwhich content increased again and tended to steady. Finally, a slight dropincontentwasobserved.Thepossiblereasonforthis phe-nomenonwasthatthehormonetreatmentstimulatedtheplant, therebyinducingstressresponseandmetabolisingmoresecondary products.Afterwards,alargeamountofhormoneswasabsorbed, therebyacceleratingmetabolismorthemobilisationofreservesin plantsandleadingtothedecreaseinhormonecontent.Then,a rela-tivelybalancedstateisreachedthroughautogenousregulation,and agreatincreaseofglycyrrhizicacidcontentatthisperiodconfirmed thatphytohormonescouldpromoteaccumulationofglycyrrhizic
acid. As growth period progressed, during which metabolism shiftedtodormancyperiod,synthesisrateofsecondarymetabolites inplantalsograduallydecreased.Resultsfromdifferent concen-trationsofexogenoushormonesindicatedtoo-lowconcentrations weakly affected glycyrrhizic acid content and showed shorter maintenancetimethantheoptimumconcentration.Too-high con-centrations of hormoneslikely inhibited growth.The optimum concentrationandtreatmenttimevariedaccordingtoplantspecies. Thechemicalcontrolnetworkbasedonglycyrrhizicacidinrootof
G.uralensisshouldincludeGA3,IAA,MeJaandliquiritin,
isoliquir-itin,isoliquiritinapiosideandliquiritinapioside.Besidesthethree kindsofhormonesstudied,ABA,cytokinin(CTK),brassinolide(BR) havealsobeeninvestigatedinpreliminaryexperiments,andresults indicatedappropriateconcentrationofABAandCTKcould signif-icantlyimprovedglycyrrhizicacidcontent.However,theeffects ofdifferenthormonetreatmentsappliedatdifferenttimesonG.
uralensis,endogenoushormoneslevelsafterexogenoushormones
absorbedbyleavesandtransitedtorootandontheglycyrrhizicacid anabolismmechanismneedtobestudiedfurtherfromthe perspec-tivesofplantphysiology,phytohormonessyntheticpathwayand actionmechanismandbiosyntheticpathways.
Plantisanorganicunity,andmetabolicpathwaysarenot iso-lated.Becauseofthecloserelationshipamongvariousmetabolic pathways,thechangeinactivityofonemetabolicpathwaycould obviouslyaffecttheothermetabolicpathways,therebyresultingin thefinalcontent.Researchershavereportedthatimprovementin thefunctionalgeneexpressionquantityofonemetabolicpathway couldenhancetheothers’,therebyincreasingthecontentofthe endproduct.Moreinterestingly,improvedfunctionalgene expres-sionquantityofonemetabolicpathwaycouldalsoreducethatof otherpathways, therebyleading tothedecreasein thecontent oftheend product.Regulatory mechanismsofglycyrrhizic acid theoreticalcorenetworkcouldbecategorisedintotwokinds,as follows:eachbranchpathwayinthenetworkenjoysacommon substrate,andchangeofdifferentbranchmetabolicfluxescould controlglycyrrhizicacidcontent.Endogenouschemicalcomponent contenttransformationinG.uralensis,especiallywhenthe concen-trationofaphytohormoneismaintainedatahighlevelforalong timebecauseofgenepolymorphism,thusinfluencingbiosynthesis
Table2
CorrelationamongthemaincomponentsandglycyrrhizicacidcontentinGlycyrrhizauralensisroot.
Glycyrrhizicacid(1) Liquiritin(2) Isoliquiritin(3) Isoliquiritinapioside(7) Liquiritinapioside(6) Liquiritigenin(4) Isoliquiritigenin(5)
Pearsoncorrelation significance(bilateral)
0.799a 0.725a 0.418a 0.222b 0.095 −0.045
0.000 0.000 0.000 0.023 0.338 0.654
n 104 104 104 104 104 104
enzymeactivityofglycyrrhizicacidandregulatingglycyrrhizicacid content.Studyingfunctionalgenepolymorphisminthecore con-trolnetworkofactivecomponentscontentcouldentirelyuncover geneticmechanismsunderlyingthechangesintheactive compo-nentconcentrationinmedicinalplants.
Authors’contribution
YLandCY,asjointfirstauthors,designedthestudyandwrote the manuscript under the guidance of CL. YL, JQ and YZ per-formedtheexperiments.LWandXZwasinchargeofculturingG.
uralensisplants.YXandGRwereresponsibleforstatistical
analy-sis.Allauthorscontributedextensivelytotheworkpresentedand approvedthismanuscript.
Conflictsofinterest
Theauthordeclaresnoconflictsofinterest.
Acknowledgments
ThisworkwassupportedbytheNationalNaturalScience Foun-dationofChina(81373909).WewishtothankSijiaWang(HuQiao Pharmaceuticalco.EngineeringResearchCenter,Anhui,China)for thehelpinmethodestablishment.Wealsowouldliketosincerely thankalltheothermembersinthelabandHenanProvinceforDrug EvaluationCertificationCenterfortheirgeneroushelpwithplant cultivationandcriticalreviewofthismanuscript.
References
Afreen,F.,Zobayed,S.M.A.,Kozai,T.,2005.SpectralqualityandUV-Bstressstimulate glycyrrhizinconcentrationofGlycyrrhizauralensisinhydroponicandpotsystem. PlantPhysiol.Biochem.43,1074–1081.
Arroo,R.,Develi,A.,Meijers,H.,Westerlo,E.,Kemp,A.,Croes,A.,Wullems,G.,1995. Effectsofexogenousauxinonrootmorphologyandsecondarymetabolismin Tagetespatulahairyrootscultures.Physiol.Plant93,233–240.
Bulgakov,Tchernoded,V.P.,Mischenko,G.K.,Glazunov,N.P.,2002.Effectofsalicylic acid,methyljasmonate,ethephonandcantharidinonanthraquinoneproduction byRubiacordifoliacallusculturestransformedwiththerolBandrolCgenes.J. Biotechnol.97,213–221.
Cai,Y.Z.,Luo,Q.,Sun,M.,Corke,H.,2004.Antioxidantactivityandphenolic com-poundsof112traditionalChinesemedicinalplantsassociatedwithanticancer. LifeSci.74,2157–2184.
Guo,Z.Z.,Wu,Y.L.,Wang,R.F.,Wang,W.Q.,Liu,Y.,Zhang,X.Q.,Gao,S.R.,Zhang, Y.,Wei,S.L.,2014.Distributionpatternsofthecontentsoffiveactive com-ponentsintaprootandstolonofGlycyrrhizauralensis.Biol.Pharm.Bull.37, 1253–1258.
Hayashi,H.,Huang,P.Y.,Inoue,K.,2003.Up-regulationofsoyasaponinbiosynthesis bymethyljasmonateinculturedcellsofglycyrrhizaglabra.PlantCellPhysiol. 44,404–411.
Hou,J.L.,Li,W.D.,Zheng,Q.Y.,Wang,W.Q.,Xiao,B.,Xing,D.,2010.Effectoflow lightintensityongrowthandaccumulationofsecondarymetabolitesinroots ofGlycyrrhizauralensisFisch.Biochem.Syst.Ecol.38,160–168.
Liu,C.L.,Wang,W.Q.,2009.Theinfluenceoftheexogenouscalciumonthe accu-mulationofglycyrrhizininthecultivatedGlycyrrhizauralensisFisch.Chin.Arch. Trad.Chin.Med.27,2281–2283.
Liu,C.S.,Wang,X.Y.,Liu,R.Q.,Nan,B.,Zhang,Y.J.,2009.Researchoncorrelationof starchandglycyrrhizicacidcontentinGlycyrrhizauralensisFisch.Chin.J.Chin. Mater.Med.34,2831–2832.
Li,W.D.,Hou,J.L.,Wang,W.Q.,Tang,X.M.,Liu,C.L.,Xing,D.,2011.Effectofwater deficitonbiomassproductionandaccumulationofsecondarymetabolitesin rootsofGlycyrrhizauralensis.Russ.J.PlantPhysiol.58,538–542.
Li,W.,Song,X.B.,Zhang,L.J.,Li,W.,Yu,H.S.,An,J.,2012.Advancesinresearch onchemicalconstituentsofRadixGlycyrrhizae.J. LiaoningUniv.TCM14, 40–43.
LShabani,L.,Ehsanpour,A.A.,Asghari,G.,Emami,J.,2009.Glycyrrhizinproduction byinvitroculturedGlycyrrhizaglabraelicitedbymethyljasmonateandsalicylic acid.Russ.J.PlantPhysiol.56,621–626.
Lu,M.,Wong,H.,Teng,W.,2001.Effectsofelicitationontheproductionofsaponin incellcultureofPanaxginseng.PlantCellRep.20,674–677.
Qian,Z.G.,Zhao,Z.J.,Xu,Y.F.,Qian,X.H.,Zhong,J.J.,2004.Novelchemically synthe-sizedhydroxyl-containingjasmonatesaspowerfulinducingsignalsforplant secondarymetabolism.Biotechnol.Bioeng.86,809–816.
Rhodes,M.J.C.,Parr,A.J.,Giuletti,A.,Aird,E.L.H.,1994.Influenceofexogenous hor-monesonthegrowthandsecondarymetaboliteformationintransformedroot cultures.PlantCellTiss.Organ.Cult.38,143–151.
Wan,C.Y.,Wang,D.,Hou,J.L.,Wang,W.Q.,Chen,H.J.,Liu,F.B.,2011.Effectofsalt stressondifferentcomponentsofGlycyrrhizauralensis.Chin.Trad.Herb.Drugs 42,2312–2316.
Wan,F.C.,Cheng,A.W.,2009.PolysaccharideisolatedfromGlycyrrhizauralensisFisch inducesintracellularenzymeactivityofmacrophages.Mediterr.J.Nutr.Metab. 1,165–169.
Wang,D.,Wan,C.Y.,Hou,J.L.,Wang,W.Q.,Li,W.D.,Wei,S.L.,2010.Effectof differ-entconcentrationsofMoonyieldandaccumulationofactiveconstituentsof GlycyrrhizauralensisFisch.Agric.Sci.Technol.11,17–20,+49.
Xiao,Y.,Gao,S.H.,Di,P.,Chen,J.F.,Chen,W.H.,Zhang,L.,2009.Methyljasmonate dramaticallyenhancestheaccumulationofphenolicacidsinSalviamiltiorrhiza hairyrootcultures.Physiol.Plant137,1–9.
Yang,Y.,Zheng,H.,He,F.,Ji,J.X.,Yu,L.J.,2008.Theeffectsofmethyljasmonateon theflavonoidssynthesisincellsuspensioncultureofGlycyrrhizainflate (Legu-minosae).ActaBot.Yunnanica30,586–592.
Yin,S.S.,Zhang,Y.,Gao,W.Y.,Wang,J.,Man,S.L.,Liu,H.,2014.Effectsofnitrogen sourceandphosphateconcentrationonbiomassandmetabolitesaccumulation inadventitiousrootcultureofGlycyrrhizauralensisFisch.ActaPhysiol.Plant36, 915–921.
Zhang,Y.S.,Ye,H.C.,Liu,B.Y.,Wang,H.,Li,G.F.,2005.ExogenousGA3and flower-inginducetheconversionofartemisinicacidtoartemisinininArtemisiaannua plants.Russ.J.PlantPhysiol.52,58–62.
Yu,K.W.,Gao,W.,Hahn,E.J.,Paek,K.Y.,2002.Jasmonicacidimprovesginsenoside accumulationinadventitiousrootcultureofPanaxginsengCAMeyer.Biochem. Eng.J.11,211–215.
Zhang,Q.,Ye,M.,2009.ChemicalanalysisoftheChineseherbalmedicineGan-Cao (licorice).J.Chromatogr.A1216,1954–1969.
Zhang,Y.B.,Xu,W.,Yang,X.W.,Yang,X.B.,Wang,W.Q.,Liu,Y.M.,2013.Simultaneous determinationofnineconstituentsintherootsandrhizomesofGlycyrrhiza uralensisfromdifferentproducingareasbyRP-HPLC.Chin.J.Pharm.Anal.33, 214–229.