w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Phytochemical
composition
and
chronic
hypoglycemic
effect
of
Rhizophora
mangle
cortex
on
STZ-NA-induced
diabetic
rats
Adolfo
Andrade-Cetto
a,∗,
Sonia
M.
Escandón-Rivera
a,
Gerado
Mata
Torres-Valle
a,
Leovigildo
Quijano
baLaboratoriodeEtnofarmacología,FacultaddeCiencias,UniversidadNacionalAutónomadeMéxico,México,DF,Mexico
bInstitutodeQuímica,UniversidadNacionalAutónomadeMéxico,México,DF,Mexico
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5May2017 Accepted25September2017 Availableonline2November2017
Keywords:
Type2diabetes Redmangrove Hypoglycemic Hypolipidemic
a
b
s
t
r
a
c
t
Type2diabetesisamajorhealthprobleminMexico,asitisinothercountries,isachroniccondition thatdevelopswhenthebodycannotproduceenoughinsulinorcannotuseitappropriately.Bothinsulin deficiencyandinsulinresistanceleadtohighbloodglucoselevels.InMexico,peoplewithdiabetesare knowntousethedecoctionofredmangrove(RhizophoramangleL.,Rhizophoraceae)barktocontrolblood glucoselevels.Therefore,inthisstudy,wesoughttoinvestigatethechronichypoglycemicand hypolipi-demiceffectsofR.mangle;wealsoelucidatesomeofthemajorphytochemicalcompoundsofR.mangle. Toanalyzethehypoglycemicandhypolipidemiceffects,weusedratswith streptozotocin–nicotinamide-inducedhyperglycemia;theratswereclassifiedintofourgroups(sixratseach),basedonthetreatment given,asfollows:group1,non-hyperglycemiccontrol;group2,hyperglycemiccontrol;group3, gliben-clamide(5mg/kgbodyweight);andgroup4,Rhizophoraethanol–waterextract(90mg/kg).Theextract orglibenclamidewasorallyadministered,dissolvedin1.5mlofphysiologicalNaCl-solution,twiceaday (inthemorningandintheevening)overaperiodof42days.Themethanolicextractwasusedtoelucidate themaincompoundspresentinR.mangleviaconventionalphytochemicalmethods,suchasTLC,HLPC, UPLC–DAD–MS,andNMR.Thefollowingcompoundsweredetected:cinchonainsIaandIb, catechin-3-O-rhamnopyranoside,epicatechin,lyoniside,andnudiposide.ThedailyadministrationofRhizophora
ethanol–waterextract,similartothetraditionalusagetocontroltype2diabetes,wasshowntoexert chronichypoglycemicandhypolipidemiceffects.Thiseffectmaybeassociatedwhittheconstituentsin theextract.ThesefindingssuggestthatR.mangleanditsconstituentscouldbepotentiallyusedtotreat type2diabetes.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Diabetesisachronicconditionthatoccurswhenthebody can-notproduceenoughinsulinorcannotuseitappropriately.Intype2 diabetes(T2D),thebodycanproduceinsulinbutbecomesresistant toit,causingineffectivenessofinsulin.Consequently,insulin lev-elsmaybecomeinsufficient,andthusinsulinresistanceandinsulin deficiencyresultinhighbloodglucoselevels(IDF,2015)and(ADA, 2015).IndividualswithT2Dsufferfrominsulinresistanceand usu-allyrelative,ratherthanabsolute,insulindeficiency.However,at leastinitially,andoftenthroughouttheirlifetime,theseindividuals maynotrequireinsulintreatmenttosurvive.
∗ Correspondingauthor.
E-mail:aac@ciencias.unam.mx(A.Andrade-Cetto).
In2015,theIDFestimatedthat415millionpeopleareliving withdiabetesworldwide;Mexicostandssixthamongthetopten countries,with11.5millionpeople(IDF,2015).Diabetes-associated complications,suchascardiovascular disease,blindness,kidney failure,andlower-limbamputation,areamajorcauseofdisability, lowqualityoflife,andprematuredeath.
AmongtheWorldHealthOrganizationlistofessentialdrugs usedforthetreatmentofdiabetes,metformin(abiguanide)and gli-clazide(asulfonylurea)arewell-establishedmedicationsandthey shouldbeavailableandeasilyaccessible(accordingtoneed),toall patientswithT2D(IDF,2015).Itisofsignificancethatmetformin wasoriginally isolated from theFrench lilac(Galega officinalis)
(Witters,2001).
PlantshavebeenusedformedicinalpurposesinMexicosince pre-Hispanictimes.ThehighprevalenceofT2DamongMexicans, associatedtovulnerableeconomicstability,andthefactthatpeople trusttheeffectivenessofmedicinalplantshaveledtotheincreased useofplantstotreatT2D.Thesefactorshavemadeitessentialto
https://doi.org/10.1016/j.bjp.2017.09.007
studythepharmacologicalandphytochemicalpropertiesofplants withhypoglycemicpropertiesinMexico.
RhizophoramangleL.,Rhizophoraceae(Andrade-Cettoand
Hein-rich,2005),traditionallyknownas“mangrove”or“redmangrove,”
iswidelyusedforthetreatmentofdiabetesinMexico.Itisa 25-m-talltreethatgrowsinmangrovesanddistributedalongthePacific andGulfCoastsofMexico.Ithasatall,straighttrunkwithabundant roots,aroundtreetopwithsympodialbranching,bitter-redwood, andcortex(PenningtonandSarukhán,1998).
The anti-hyperglycemic effect of the plant was previously reportedbyAlarcon-Aguilaraetal.(1998),theytesttheeffectof 28 plants in rabbits under a glucose tolerancetest, the results obtainedfromthevarianceanalysisshowedthatR.mangle signifi-cantlydecreasedthehyperglycemicpeakby16.1%.
Inapreviousstudyperformedbyourgroup,the ethnobotan-ical relevance and the acute hypoglycemic effect of R. mangle
werereported(Andrade-CettoandMares,2012),inthatstudywe confirmthat thedoseof 90mg/kg hasthebetterhypoglycemic effect, this dose is thetraditional used dose multiplied by 10. In the present study, we aimed to examine thechronic hypo-glycemiceffectoftheethanolicextractof thebarkofR.mangle
in streptozotocin–nicotinamide (STZ-NA)-induced diabetic rats; we also evaluated the lipid profile and glycated hemoglobin after chronicadministration. In addition, we sought to charac-terizethemajorphytochemicalcompoundspresentintheplant cortex.
Materialsandmethods
Plantextracts
Basedontheresultsofthepreviousstudyinwhichthewater and ethanol–water extracts (EW) were tested (Andrade-Cetto
andMares,2012),weselectedtheethanol–waterextractwhich
is similartothe traditional usedinfusion and presented better activity(Fig.1).New botanicalsamplesofRhizophoramangleL., Rhizophoraceae, werecollected withthe help of informants in Manialtepec,OaxacaMexico,theoriginalplantwasdepositedat theIMSS, Herbarium in MexicoCity withthe voucher number IMMSM15816.Theextracttobeusedinpharmacologicaltestswas preparedaspreviouslydescribed;inbrief;a 50gsampleofthe plantmaterialwasaddedto500mlofanethanol–watermixture (1:1),itwasthenheatedat40◦Cfor4hbeforebeingfilteredfor threetimes.Thiswasfollowedbyeliminationofthesolventunder reducedpressure ina Büchi rotaryevaporator.The yieldofthe extractethanol–water(1:1)was14.75g.
Forthephytochemicalidentificationofthemaincompoundsof thecortex;themethanolicextract(ME)waspreparedusing200g ofplantmaterialthroughSoxhletextraction.Defattingwith hex-ane(24h)followed bymethanol (MeOH)extraction(48h), and theresultingextractevaporatedunderreducedpressureuntilit reacheddrynessproducing15gofME.
Compoundsisolation
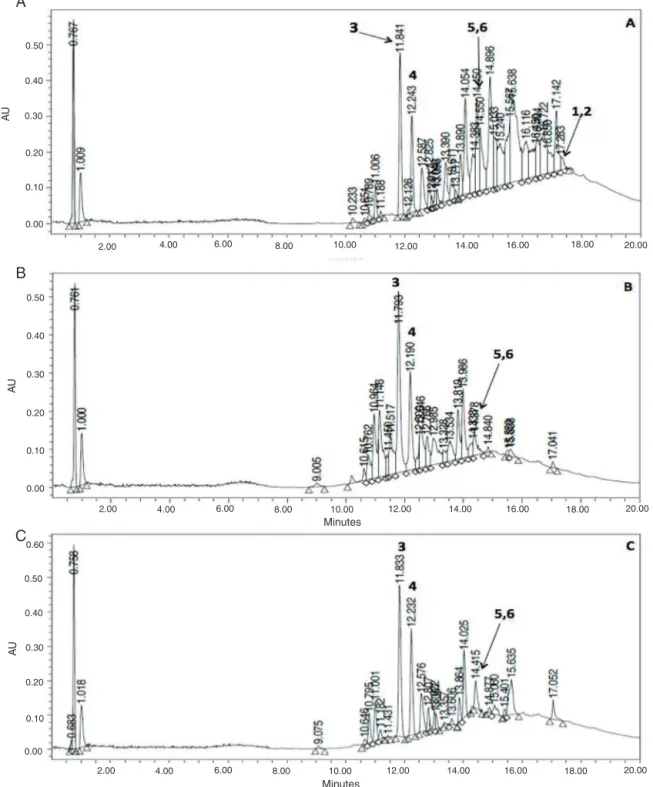
HPLC–DAD–MSanalysis was performed to confirm that the ethanol–water extract used in thepharmacological testing has a similarphytochemical profilethanthe water and methanolic extracts(Fig.1),butthelastonewasmoreaccessibleforthe isola-tionprocess.
AsampleofME(3g)wasdissolvedinMeOHandpartitioned withhexane toyield a hexane soluble fraction(HSF; 50mg), a MeOH-solublefraction(MSF, 2.90g), and a red precipitate(RP; 28mg).
The MSF was subjected tocolumn chromatography (CC) on 360gofsilicagel(70–230mesh,Merck)startingwithhexane100% (400ml),increasingthepolaritywithEtOAcusingamixtureof hex-ane/EtOAcaseluent,until100%(500ml),andsubsequentlywith MeOHuntil100%(500ml).Thisprocessledtofourteenprimary fractions(MSF1–MSF14).FractionMSF8(400mg)wassubjected tosilicagelCCelutedwithEtOAc/MeOH(10:0–0:10),thisprocess ledtofivesubfractions(MSF8.1–MSF8.5).Preparativethinlayer chromatography (TLC)(Macherey&Nagel,0.25mm)of fraction MSF8.2(20mg)usingEtOAc/MeOH/H2O,7:2:1,aseluentresulted intheisolationofamixtureof1and2(10mg).PreparativeTLC (EtOAc/MeOH/H2O,7:2:1)offractionMSF8.3(80mg)resultedin theisolationof3(23.7mg)and4(13mg).FSM8.4wasresolvedby HPLC(Nucleosil250×10mmi.d.,5m,C18,Macherey&Nagel); usingagradientofMeCN/H2Ostartingwith20/80to70/30during 17min(3ml/min;250and280nmUV-det.)toobtain10mgofa mixtureof5and6withanRt10.5min.
AnefficientmethodbasedonHPLC–DAD–MStechnique was usedforidentifyingtheisolatedcompoundsfromthe methano-licextractcorrespondingto(1–6)inthewaterandethanol–water extract.ThecomponentswereseparatedonaKinetexHPLC/UPLC XB-C18 column (50×2.1mm i.d., 2.6m) at 25◦C. The mobile phaseconsistedofawatergradient(containing0.1%FA)(A)and acetonitrile(B).Thefollowinggradientelutionprogramwasused: 1%Bduring0.5min,1–35%B0.5–15min,35–100%B15–18min, 100–1%B18–20minataflowrateof0.2mlmin−1,theinjection volumenwas3l.Majoritycompoundsofthetradicional decoc-tionandtheethanol–waterextractwereidentifiedandareshown inFig.1.
Generalexperimentalprocedures
NMR spectraincludingHSQC,HMBC,COSY,andTOCSYwere recordedinaVarianInovaspectrometerat500(1H)and125MHz (13C) or a JEOL-ECA at 300 (1H) and 75MHz (13C); chemi-cal shifts were recorded as ı values. HRESIMS were recorded on a Thermo Scientific LTQ Orbitrap XL hybrid FTMS (Fourier transform mass spectrometer). Data were collected in both positive and negative ionization modes via a liquid chromato-graphic/autosampler system that consisted of an Acquity UPLC system.ProfileHPLC–DAD–MSwereperformedusinganAgilent 1200InfinitysystemequippedwithaG1312-95006Binarypump, G1329-90012 Autosampler, controlled by Agilent ChemStation software,coupledtoaWatersdiodearraydetector(DAD)anda Squire6000BrukerESI-MSinnegativemodeionpolarity.
Analyticaland preparativeHPLCanalyseswereperformedin anAgilent1260InfinitysystemequippedwithaG1311B Quater-narypump,G1367EAutosampler,G1315CDADVL+andcontrolled byAgilentChemStationsoftware.Foranalyticaland semiprepara-tiveHPLC,Macherey-Nagel(NucleosilC18,250×4.6mmi.d.,5m), Macherey-Nagel(NucleosilC18,250×10mmi.d.,5m)columns, respectively,wereused.Columnchromatography(CC)wascarried outonsilicagel(70–230mesh,Merck).Thin-layer chromatogra-phyanalysiswascarriedoutonsilicagel60F254plates(Macherey &Nagel)usingcericsulfate(10%)solutioninH2SO4ascolorreagent.
Experimentalanimals
Eight-week-oldWistarratsweighing200–250gwereobtained fromtheBioteriumoftheScienceSchool,UNAM,andwere accli-matedwithfreeaccesstofoodandwaterforatleastoneweek inanair-conditionedroom(25◦Cwith55%humidity)ona12h light–dark cycle prior to performing theexperiments. The ani-malswerehandledaccordingtotheNationalInstituteofHealth,
USA(CommitteefortheUpdateoftheGuidefortheCareandUse
A
B
C
0.50
0.40
0.30
0.20
0.10
0.00
0.50
0.40
0.30
0.20
0.10
0.00
0.60
0.50
0.40
0.30
0.20
0.10
0.00
2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00
2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00
AU
AU
AU
Minutes
2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00
Minutes
Fig.1.HPLC–DADprofilecomparisonofthemethanolic,ethanol–waterandwaterextracts.(A)Methanolicextract,(B)waterextract,(C)ethanol–waterextract.
as described by Masiello et al. (1998). In brief, the rats were fastedovernight and injected intraperitoneallywith 150mg/kg nicotinamide(NA)(Sigma,N3376)15minbeforeanintravenous injectionof65mg/kgstreptozotocin(STZ)incitratebuffer(Sigma, S0130).Diabetes was identified by polydipsia, polyuria and by measuringnon-fastingplasmaglucoselevels48hafterinjection. Animalswhichdidnotdevelopmorethan250mg/dlglucoselevels wererejected.
The hyperglycemic animals wereclassified into four groups (1–4) each of them with six rats. Group 1 as normal control received1.5ml ofphysiologicalNaCl-solution(vehicle),group2
as hyperglycemic control received also 1.5ml of physiological NaCl-solution,group3wasgivena standard oralhypoglycemic agent, glibenclamide (5mg/kg bodyweight (bw)), in the same vehicle, while group 4 received Rm-EW (90mg/kg bw) dis-solved in 1.5ml of physiological NaCl-solution. The extract or the hypoglycemic agent was orally administered twice a day (in the morning and in the evening) over a period of 42 days. All groups were fed Purina Rodent Laboratory Chow 5001.
fortheUpdateoftheGuidefortheCareandUseofLaboratory
Ani-mals(2015).Allmethodsusedinthisstudywereapprovedbythe
InternalCouncilofthe“FacultaddeCiencias”oftheUniversidad NacionalAutónomadeMéxico.Glucosemonitoringwasperformed weeklyand analyzedwithglucose teststripsand a glucometer Accutrend® plus.Glycatedhemoglobin(HbA1c)wasanalyzedin aDCAVantage®Siemens,equipment.Thelipidprofile(HDLTGand cholesterol)weremeasuredwithCardioCheck®andstripsPanels® PTS.VLDLwascalculatedusingthefollowingVLDL=0.2×TG.Both HbA1candlipidprofilesweremeasuredondays0,14,28and42 aftertheinitiationofadministrationoftreatments.
Statisticalmethods
Thedatawerestatisticallyanalyzedbyunpairedt-testwiththe helpofthesoftwareGraphPadPrism.Theplasmaglucoselevels wereexpressedasthemean(S.E.M.).
Resultsanddiscussion
Ethnobotany
TraditionaluseofR.mangle cortexforthetreatmentoftype 2diabeteswasconfirmedbythetraditionalhealerswhoassisted duringtheplantcollectioninManialtepec,Oaxaca,Mexico.Forthis purpose,aroughapproximationof20gofplantcortexareboiled in500mlofwaterfor15min,oncethedecoctioniscold,itis con-sumedthroughoutthedayasthesocalled“aguadeuso”.
Compoundidentification
CinchonainsIaandIb(1and2)
Compounds 1 and 2 were identified by their 1H and 13C NMRspectraldata,included2Dexperiments(COSY,HSQC,HMBC, NOESY),andmassspectraldata,whichallowedtheidentification ofcompounds1and2asamixture.
Themassspectrum(ESI-MSnegativeionmode),showedonly onepseudomolecularionatm/z451.66[M−H]−indicatinga molec-ularformula C24H20O9 for both compounds 1and 2, whilethe 1Hand13CNMRspectrashowedduplicatedsignals.The1HNMR
spectrum (CD3OD, 300MHz) showed pairs of signals at ıH(1/2)
4.81/4.87(brs,H-2),ıH(1/2)4.25/4.19(m,H-3)andıH(1/2)2.90/2.86
(m,H-4)suggestingthepresenceofa flavan-3-olmoietyinthe molecule.ThetypicalABXspinsystemduetothearomatic pro-tonsoftheB-ring,werealsopresentaspairsofsignals,atıH(1/2)
6.97/6.84(d,J=1.7/1.9Hz,H-2′),ı
H(1/2)6.75/6.69(d,J=8.2/8.1Hz,
H-5′)andı
H(1/2)6.80(dd,J(1)=1.7,7.9)/6.62(dd,J(2)=2.1,8.1Hz) (H-6′). Two singlet signals at ıH(1/2) 6.20/6.21 suggested a tri-substituted A-ring. The presence of a phenylpropanoid moiety relatedwithcaffeicacidwasindicatedbyanextraaromaticABX systemwithsignalsatıH6.64(d,J=7.6Hz, 1H,H-5′′),6.44(dd, J=8.0,2.2Hz,1H,H-6′′),6.55(d,J=2.2Hz,1H,H-2′′),inaddition toan aliphaticABX systemdue to themethine CH-7′′ and the methyleneCH2-8′′,atıH4.55(dd,J=6.8,1.3Hz,1H,H-7′′),and3.0 (m,2H,H-8′′).
Theabovedataagreewiththosepublishedforthemixtureof cinchonainsIa(1)andIb(2),isolatedfromTrichiliacatigua, Meli-aceae (Pizzolatti et al.,2002). The13C NMR dataand 2D NMR experiments(COSY,HSQC,HMBC,NOESY;300MHz,acetone-D6) confirmedtheaboveassignments.HMBCcorrelationofH-8′′and H-7′′ withthetertiarycarbonC-8(ıC1/2 105.7/105.9),indicating thatthephenylpropanoidunit wasattachedtotheC-8position oftheepicatechinmoiety.TherelativeconfigurationattheC-7′′ stereogeniccenterofcompounds1and2wasestablishedbased onthecorrelationsobservedintheNOESYexperiment.Thus,for compound1acorrelationbetweenH-2andH-2′′/6′′wasobserved;
whilefor compound2NOESY correlationbetweenH-7′′ and H-2′/6′wasobserved.ThesameNOESYinteractionswerepreviously reportedbyResendeetal.(2011)forcinchonainsIaandIb.
Catechin-3-O-rhamnopyranoside(3)
Compound 3 wasobtained as a yellowish amorphous solid, the molecular formula C21H24O10 was deduced from the ESI-MS[M+H]+ion atm/z437.314and 435.73[M−H]−.The1Hand 13CNMRspectraand2DNMRexperiments(COSY,HSQC,HMBC,
NOESY,TOCSY;500MHz,CD3OD)indicatingthepresenceof cate-chinandrhamnosemoietiesinthemolecule.The1HNMRspectrum showedtheABXaromaticsystemwithsignalsatıH/C6.84/115.1
(d,J=2.0Hz,1H,H-2′/C-2′),6.76/116.1(d,J=8.0Hz,1H,H-5′/C-5′), 6.71/119.8(dd,J=8.0,1.8Hz,1H,H-6′/C-6′)duetotheB-ring pro-tons.AnAXsystemassociatedwithtwo-metacoupledaromatic protonswithsignalsatıH/C 5.94/96.4(d,J=2.5Hz,1H,H-6/C-6)
and5.86/95.5 (d,J=2.5Hz,1H, H-8/C-8)andanAMX2 spin sys-temwithresonancesatıH/C4.62/81.1(d,J=8.0Hz,1H,H-2/C-2),
3.93/75.9(m,1H, H-3/C-3),andat2.64/27.9 (dd,J=16.5, 8.5Hz, 1H,H-4␣/C-4),and2.88/27.9(dd,J=16.5,5.5Hz,1H,H-4/C-4), indicatedthepresenceofaflavan3-olskeleton.TheJ2,3coupling of8.0HztogetherwiththechemicalshiftofC-2atıH/C4.62/81.1
indicatea 2,3-transstereochemistry ofthecatechin. The HMBC spectrumshows correlationof H-3(ıH3.93)withtheanomeric carbon signalatıC 102.2(C-1′′)indicating a3-O-linkageof the
rhamnosetotheflavan.Alltheabovedataagreedwiththosefor catechin-3-O-rhamnopyranoside (Ishimaru et al., 1987). ESI-MS (positiveionmode):[M+H]+m/z437.314.
Epicatechin(4)
Compound4wasobtainedasaredamorphouspowder. ESI-MSpositiveionmode:[M+H]+291.152and289.58[M
−H]−.The
1HNMRrevealedanABX-typearomaticspinsystemwithproton
signalsatıH/C 7.01/115.3(d,J=2.0Hz,1H, H-2′/C-2′),6.78/115.9
(d,J=8.0Hz, 1H,H-5′/C-5′),6.83/119.4(dd,J=8.0,1.8Hz,1H, H-6′/C-6′),andanABmetacoupledaromaticsystemwithsignalsat ıH/C5.94/96.4(d,J=2.5Hz,1H,H-6/C-6)and5.91/95.8(d,J=2.5Hz,
1H,H-8/C-8).ThepresenceofanAMX2 systemwithresonances at lower frequencies ıH/C 4.90/79.8 (d, J=3.5Hz, 1H, H-2/C-2),
4.18/67.5(ddd,J=1.54,3.10,4.64,1H,H-3/C-3),and2.74/29.2(dd,
J=16.8,2.8Hz,1H, H-4␣)and 2.88/29.2(dd, J=16.6, 4.3Hz, 1H, H-4),indicatethepresenceofaflavan3-olskeleton.TheJ2,3 cou-plingof3.5HztogetherwiththechemicalshiftofthemethineC-2 atıH/C4.90/79.8,indicatinga2,3-cisstereochemistry.Thus,
Lyonisideandnudiposide(5and6)
Compounds5and6wereisolatedasabrownamorphoussolid. TheUVspectrumshowedabsorptionsat236,and276nm(MeOH). Themolecularformulafor5and6C27H36O12wasdeducedfromthe
pseudo-molecularionpeaksatm/z575.446[M+Na]+,and551.65 [M−H]−, obtained by ESI-MS in positive and negative modes, respectively.Theidentificationof5and6waspossiblebycareful analysisof1Hand13CNMRspectra,included1Dand2D exper-iments(COSY, HSQC,HMBC,TOCSY);at 500MHz,using D2Oas solvent.The13CNMRspectrumofthemixtureshowedsomedual signals,whilethe1HNMRspectrumshoweddifferencesonlyinthe chemicalshiftsoftheanomericprotonH-1′′,andsomealicylic pro-tonsofthexylosemoiety.The1Hand13CNMRspectradisplayed thetypicalsignalsofalignanskeleton,includinganaromatic pro-tonsingletatıH/C6.81/108.8(s,1H,H-2′/C-2′),andtwoequivalent
aromaticprotonssingletatıH/C6.53/106.9(s,2H,H-2,H-6/C-2,C-6)
for5;andatıH/C6.81/108.7(s,1H,H-2′/C-2′),and6.55/106.9(s,2H,
H-2,H-6/C-2,C-6)for6.The1Hand13CNMRspectraalsoshowed foursingletsignalsduetofivemethoxylgroups,twoofthem sym-metricallyequivalents[ıH/C3.91/57.1(s,3H,OMe-3′),3.81/57.3(s,
6H,OMe-3,OMe-5),3.43/60.7(s,3H,OMe-5′)for5;3.91/57.1(s, 3H,OMe-3′), 3.82/57.3(s,6H, OMe-3, OMe-5),3.42/60.7(s, 3H, OMe-5′)for6].TheNMRspectraof5and6revealedothersignals, consistentwiththepresenceofaxylosemoiety[ıH/C4.37/104.7(d, J=7.8Hz,H-1′′,anomericproton),3.34/73.9(dd,J=8.5Hz,16.5Hz, 1H,H-2′′/C-2′′),3.47/76.5(t,J=9.2Hz,1H,H-3′′/C-3′′),3.63/70.2(m, 1H,H-4′′/C-4′′),3.59/66.0(dd,J=11.3Hz,4.3Hz,1H,H-5␣′′/C-5′′), 3.95/66.0(m,1H,H-5′′/C-5′′)for5;and4.07/103.4(d,J=7.5Hz, H-1′′,anomericproton),3.28/73.7(m,1H,H-2′′/C-2′′),3.63/70.1(m, 1H,H-3′′/C-3′′),3.09/76.6(t,J=11.1Hz,1H,H-4′′/C-4′′),3.59/66.1 (dd,J=11.3Hz,4.3Hz,1H,H-5␣′′/C-5′′),3.95/66.1(m,1H,H-5′′ /C-5′′)for6].Thevalueofthecouplingconstant(J=7.5Hz)between theanomericprotonandC-2′′position,suggestedthe-orientation oftheglycosidiclinkagein5and6.TheHMBCcorrelationfrom H-1′′(ıH4.37for5;4.07for6)toC-9(ıC71.6for5and6)indicated thatthexyloseunitwaslinkedtotheoxygenatC-9.Theabovedata
wereinagreementwiththoseforlyonisideandnudiposide(5and
6)(Sadhuetal.,2007).
Themajor metabolitesisolated(3–6)of ME,wereidentified throughtheelaborationofanHPLC–ESI-MSchromatographic pro-fileofWaterextractofR.mangle.
Efficacyindiabeticrats
WeconfirmedthattheStz-Namodelissuitableforachronic experimentinwhichglucosevaluesareevaluated.Theglucose val-uesforthegroup1(normal)remainstablearound125mg/dlover the42daysofexperimentation,whereasthevaluesforthegroup 2(hyperglycemic)werearound170mg/dlinthesameperiod;the group2 presentedstatisticallysignificanthighervalues as com-pared to the group 1 (Table 1). The glycated hemoglobin and triacylglycerides(Table2)arealsohigherinthegroup2in con-trasttothegroup1andtheincreaseinHb1Acandtriacylglycerides levelsissignificantafter14daysoftheinjection.
Thestandardhypoglycemicagentglibenclamidecouldcontrol theglucoselevelsfromday7andtheHb1Acafter28days.The Rm-EWextractcontrolstheglucosevalues fromday7,andthe Hb1Acafter28days,theHb1Acresultsarestatisticallydifferent fromtheirowntime0butnotfromgroup2,theRm-EWextract alsocontrolsthetriacylglyceridesandtheVLDLlevelsafter28days
(Tables1and2).
R.mangletraditionalusagetotreattype2diabetesisthrough drinkingthedecoctionthroughouttheday,thiswayof administra-tionisnoticeableanditisnotassociatedwithmeals,thismeans theplantisnotusedinthepostabsorptivestate.Thisfactcouldbe relatedtotheactionmechanism.
Someof themaincomponentsofR. manglecortexwere iso-latedfromthemethanolsolublefraction(MSF)byrepeatedcolumn chromatographyinsilicagelandpurified byHPLC.Thisprocess allowed the isolation and identification of two flavalignans: 1 and2(cinchonainsIaandIb),twoflavanols:3(catechin-3-O-␣ -rhamnopyranoside)and4(epicatechin),andtwolignanglycosides: 5and6(lyonisideandnudiposide).Thesecompoundshadnotbeen previouslyreportedforR.mangle.Thechromatographicprofilefor
Table1
ChronichypoglycemiceffectofRhizophoramanglecortexonSTZ-NAinduceddiabeticrats.Thevaluesrepresentthemean±SEM.Superscriptedlettersinthesamerow indicatestatisticallysignificantdifferencescomparedwithtime0.Superscriptednumbersinthesamecolumnindicatestatisticallysignificantdifferencescomparedwith therespectivecontrolgroup(a,1)(p<0.05).
Groups Glucose
T0(mg/dl) T7(mg/dl) T14(mg/dl) T21(mg/dl) T28(mg/dl) T35(mg/dl) T42(mg/dl)
1Norm. 123±3 129±1 124±2 127±1 125±4 118±6 131±3
2Hyperg. 175±01 171
±21 171
±101 154
±41 168
±71 162
±41 168
±91 3Glib.5mg/kg 179±2 129±5a1 148
±9a 132
±6a1 150
±9a 134
±11a1 153
Table2
ChronichypoglycemiceffectsoftheRhizophoramanglecortexonSTZ-NAinduceddiabeticrats.Thevaluesrepresentthemean±SEM.Superscriptedlettersinthesamerow indicatestatisticallysignificantdifferencescomparedwithtime0.Superscriptednumbersinthesamecolumnindicatestatisticallysignificantdifferencescomparedwith therespectivecontrolgroup(a,1)(p<0.0).
Groups T0 T14 T28 T42
HbA1c(%) Tg (mg/dl)
vLDL (mg/dl)
HbA1c (%)
Tg (mg/dl)
vLDL (mg/dl)
HbA1c(%) Tg(mg/dl) vLDL (mg/dl)
HbA1c(%) Tg(mg/dl) vLDL (mg/dl)
1Norm. 3.6±0.1 71±4.0 14±1 3.6±0.1 79±16 16±3 3.54±0.1 63±4 12±1 3.62±0.1 61±10 12±2 2Hyperg. 3.7±0.1 52±11 10±11 4.1±0.21 77.6±14 15±3 4.2±0.11a 119±151a 24±31a 4.3±0.11a 113±26a 23±5a 3Glib.5mg/kg 3.8±0.2 69±11 14±2 4.2±0.2 89±13. 18±3 3.9±0.2 100±14 20±3 3.9±0.21 115
±19 23±4a 4Rm-EW90mg/kg 3.4±0.2 78±10 15±21 4.1
±0.2a 76
±13 15±3 3.9±0.1 65±41 13
±11 4.0
±0.2 72±10 14±2
allextracts(Fig.1)indicatesthat3–6arepartofthemain com-poundsfoundinR.mangle;however,itisstillnecessarytocontinue thechemicalanalysisoftheothersubfractionsforabetter under-standingofthechemicalprofileofthisplant.
Amongplantmetabolites,phenolsarefoundtopossessawide range of biological effects. In recent years, plant polyphenols includingphenolicacids,flavonoids,stilbenesandlignans,based oninvitrostudies,animal modelsand someclinicaltrials,have beenproposedaseffectivesupplementsfordiabetesmanagement andpreventionofitslong-termcomplications(Bahadoranetal.,
2013).
Based on several in vitro, animal models and some human studies,dietaryplantpolyphenolsandpolyphenol-richproducts, modulatecarbohydrateandlipidmetabolismaswellas attenu-atehyperglycemia,dyslipidemiaandinsulinresistance(Bahadoran
etal.,2013).
Sietal.(2011)showsthatepicatechintreatmentcausedchanges
indiabeticmice.Thesechangesareassociatedwithahealthierand longerlifespan,includingimprovedskeletalmusclestressoutput, reducedsystematicinflammationmarkersandserumLDL choles-terol,increasedhepaticantioxidantglutathioneconcentrationand totalsuperoxidedismutaseactivity,decreasedcirculating insulin-likegrowthfactor-1,andimprovedAMP-activatedproteinkinase activityin theliverandskeletalmuscle.Recentstudies(Litterio etal.,2015)showedthatepicatechinpreventedhypertensionina
invivomodeldietwith10%(w/v)fructoseinthedrinkingwater (high fructose, HF) for eight weeks on rats, decreasing super-oxideanion production and elevatingNOSactivity,favoring an increase in NO bioavailability. Epicatechin and epicatechin-rich foodsimprovesinsulinsensitivityinhighfatdiet-fedinamouse modelofobesityandT2Dtriggeredbyhighfatconsumptioninmice
(Cremoninietal.,2016).
Cinchonain Ib, from Eriobotrya japonica (Thunb.) Lindl., Rosaceae,leavesenhancedinsulinsecretionfromINS-1cells(rat insulinomacell), aswellasreduced plasmainsulinlevelinrats after108mg/kgoraladministration,however,itdidnotinduceany changesinbloodlevels(Qa’danetal.,2009).
Indiabeticratstheethanol–waterextractexertsahypoglycemic effectaftersevendaysofadministration,theeffectwassustained untilday42,thedecreaseinglucoselevelswerereflectedinthe Hb1Aclevelsafter28days,despitebeingsignificantnotbefore42 daysafterthebeginningoftheexperiment.Thereasonforthisis; Hb1Acisformedinanon-enzymaticglycationwhenhemoglobin isexposedtoplasmaglucose,whentheaverageamountofplasma glucoseincreases,thefractionofglycatedhemoglobinincreases. Thisservesasamarkerforaveragebloodglucoselevelsoverthe previousthreemonths.
Insulindeficiencyandinsulinresistancecauseinconsequence anincreasein lipolysisconductedby adipocytes.Theactivation ofthis pathwaycontributes todyslipidaemia, conditionpresent in type 2 diabetics (De Fronzo et al., 2015). In the present study,decreaseoftriacylglyceridesandplasmaglucoselevelswas observed,suggestingthecontrolofinsulinresistanceasapossible mechanismofaction.Suchdecreasemaybeduetotheactivation
oftheAKTkinasepathwaywhichisresponsibleforinhibitingthe enzymesinvolvedin lipolysiswherebytheconcentrationof tri-glyceridesintheblooddecreases.Anotherimportantfactoristhat AKTalsoisresponsibleforinhibitingtheactivityofGSK3 increas-ingglycogensynthesiswhichdecreasesthereleaseofglucoseby theliver(Suniletal.,2012),forthis reasonwesuggestthatthe hypoglycemiceffectoftheplantmaybelinkedtotheliverglucose output.
Insummary, thedecoctionofR.mangle exertsachronic(42 daysof treatment)hypoglycemicandhypolipidemiceffect.This effects couldbeassociated withthe compoundspresent in the waterextract:catechin-3-O-rhamnopyranoside,lyoniside, nudipo-sideandespecially byepicatechin.However,furtherstudiesare neededtotacklethemechanismofactionresponsibleofitseffects.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
AA-Cidealizedthestudy,writethemanuscriptandgetthe finan-cialsupport;GMT-Vperformthepharmacologicalexperiments; SME-Rperformsthephytochemicalexperiments;LQreviewedthe phytochemicalexperimentaldata.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This project was partially sponsored by the DGAPA, PAPIIT IN28216andCONACyTCB-0151264.
References
ADA,2015.Classificationanddiagnosisofdiabetes.DiabetesCare38,S8–S16.
Alarcon-Aguilara,F.J.,Roman-Ramos,R.,Perez-Gutierrez,S.,Aguilar-Contreras,A., Contreras-Weber,C.C.,Flores-Saenz,J.L.,1998.Studyoftheanti-hyperglycemic effectofplantsusedasantidiabetics.J.Ethnopharmacol.61,101–110.
Andrade-Cetto,A.,Heinrich,M.,2005.Mexicanplantswithhypoglycaemiceffect usedinthetreatmentofdiabetes.J.Ethnopharmacol.99,325–348.
Andrade-Cetto,A.,Mares,M.,2012.HypoglycemiceffectoftheRhizophoramangle
Bahadoran, Z., Mirmiran, P., Azizi, F.,2013. Dietary polyphenols as potential nutraceuticalsinmanagementofdiabetes:areview.J.DiabetesMetab.Disord. 12,43.
CommitteefortheUpdateoftheGuidefortheCare,UseofLaboratoryAnimals, Insti-tuteforLaboratoryAnimalResearch,CommitteefortheUpdateoftheGuide fortheCareandUseofLaboratoryAnimals,InstituteforLaboratoryAnimal Research,2011.GuidefortheCareandUseofLaboratoryAnimals,Divisionon EarthandLifeStudies,NationalResearchCouncil,8thed, doi:10.1163/1573-3912islamDUM3825.
Cremonini,E.,Bettaieb,A.,Haj,F.G.,Fraga,C.G.,Oteiza,P.I.,2016.(−)-Epicatechin improvesinsulinsensitivityinhighfatdiet-fedmice.Arch.Biochem.Biophys. 599,13–21.
De Fronzo, R.A., Ferrannini, E., Groop, L., Henry, R.R., Herman, W.H., Holst, J.J., Weiss, R., 2015. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers.,
http://dx.doi.org/10.1038/nrdp.2015.19.
IDF,2015.DiabetesAtlas,7thed.InternationalDiabetesFederation,Brussels.
Ishimaru,K.,Nonaka,G.I.,Nishioka,I.,1987.Flavan-3-olandprocyanidineglycosides fromQuercusmiyagii.Phytochemistry26,1167–1170.
Litterio,M.C.,VazquezPrieto,M.A.,Adamo,A.M.,Elesgaray,R.,Oteiza,P.I.,Galleano, M.,Fraga,C.G.,2015.(−)-Epicatechinreducesbloodpressureincreasein high-fructose-fedrats:Effectsonthedeterminantsofnitricoxidebioavailability.J. Nutr.Biochem.26,745–751.
Masiello,P.,Broca,C.,Gross,R.,Roye,M.,Manteghetti,M.,Hillarire-Buys,D.,Novelli, M.,Ribes,G.,1998.Developmentofanewmodelinadultratsadministered streptozotocinandnicotinamide.Diabetes47,224–229.
Pennington,T.D.,Sarukhán,J.,1998.TropicalTreesofMexico,1sted.UNAM-FCE, México.
Pizzolatti,M.G.,Venson,A.F.,SmâniaJúnior,A.,Smânia,E.D.F.A.,Braz-Filho,R.,2002.
TwoepimericflavalignansfromTrichiliacatigua(Meliaceae)withantimicrobial activity.ZeitschriftfurNaturforsch.-Sect.CJ.Biosci.57,483–488.
Qa’dan,F.,Verspohl,E.J.,Nahrstedt,A.,Petereit,F.,Matalka,K.Z.,2009.Cinchonain IbisolatedfromEriobotryajaponicainducesinsulinsecretioninvitroandinvivo. J.Ethnopharmacol.124,224–227.
Resende,F.O.,Rodrigues-Filho,E.,Luftmann,H.,Petereit,F.,PalazzodeMello,J.C., 2011.Phenylpropanoidsubstitutedflavan-3-olsfromTrichiliacatiguaandtheir
invitroantioxidativeactivity.J.Braz.Chem.Soc.22,2087–2093.
Sadhu,S.K.,Khatun,A.,Panadda,P.,Ohtsuki,T.,Ishibashi,M.,2007.Lignan glyco-sidesandflavonoidsfromSaracaasocawithantioxidantactivity.J.Nat.Med.61, 480–482.
Schroeter,H.,Heiss,C.,Balzer,J.,Kleinbongard,P.,Keen,C.L.,Hollenberg,N.K.,Sies, H.,Kwik-Uribe,C.,Schmitz,H.H.,Kelm,M.,2006.(−)-Epicatechinmediates ben-eficialeffectsofflavanol-richcocoaonvascularfunctioninhumans.Proc.Natl. Acad.Sci.U.S.A.103,1024–1029.
Si, H., Fu, Z., Velayutham, P., Babu, A., Zhen, W., Leroith, T., Meaney, M.P., Voelker,K.a.,Jia,Z., Grange, R.W.,Liu, D.,2011. DietaryEpicatechin Pro-motes Survival ofObese DiabeticMice and Drosophilamelanogaster 1–3.,
http://dx.doi.org/10.3945/jn.110.134270.health-promoting(online0–5). Sunil,C.,Agastian,P.,Kumarappan, C.,Ignacimuthu,S.,2012. Invitro
antiox-idant,antidiabetic andantilipidemicactivitiesofSymplocos cochinchinensis
(Lour.) S. Moore bark. Food Chem. Toxicol. 50, 1547–1553, http://dx. doi.org/10.1016/j.fct.2012.01.029.