w w w . r b h h . o r g
Hematology,
Transfusion
and
Cell
Therapy
Original
article
ZAP-70
expression
is
associated
with
increased
CD4
central
memory
T
cells
in
chronic
lymphocytic
leukemia:
cross-sectional
study
Rodolfo
Patussi
Correia
a,b,∗,
Flávia
Amoroso
Matos
e
Silva
c,
Nydia
Strachman
Bacal
b,d,
Paulo
Vidal
Campregher
a,b,
Nelson
Hamerschlak
a,b,
Gustavo
Pessini
Amarante-Mendes
c,faInstitutoIsraelitadeEnsinoePesquisa,SãoPaulo,SP,Brazil bHospitalIsraelitaAlbertEinstein,SãoPaulo,SP,Brazil
cInstitutodeCiênciasBiomédicas,UniversidadedeSãoPaulo(ICBUSP),SãoPaulo,SP,Brazil dCentrodeHematologiadeSãoPaulo(CHSP),SãoPaulo,SP,Brazil
fInstitutoNacionaldeCiênciaeTecnologia(INCT),Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8March2017 Accepted28March2018 Availableonline11June2018
Keywords:
Chroniclymphocyticleukemia ZAP-70protein-tyrosinekinase MemoryTcells
a
b
s
t
r
a
c
t
Background:AlthoughchroniclymphocyticleukemiaisbasicallyaBcelldisease,its patho-physiologyandevolutionarethoughttobesignificantlyinfluencedbyTcells,astheseare probablythemostimportantinteractionpartnerofneoplasticBcells,participatingintheir expansion,differentiationandsurvival.ChroniclymphocyticleukemiaBcellsmayalsodrive functionalandphenotypicchangesofnon-malignantTcells.Therearefewdataaboutthe associationbetweenmemoryTcellsandprognosis,especiallyrelatedtoZAP-70,acommon reliablesurrogateofthegoldstandardchroniclymphocyticleukemiaprognosticmarkers.
Objective:Theaimofthisstudy wastoinvestigatewhethertheexpressionofZAP-70in chroniclymphocyticleukemiapatientsisassociatedwithabnormalpatternsofthe distri-butionofnaïveandmemoryTcellsrelatedtocrosstalkbetweenthesecells.
Methods:In this cross-sectional, controlled study, patients with chronic lymphocytic leukemiawerecomparedwithhealthyblooddonorsregardingtheexpressionofZAP-70 andthedistributionofnaïveandmemoryTcellsubsetsinperipheralbloodasmeasuredby flowcytometry.
Results:ZAP-70positivepatientspresentedanincreasedfrequencyandabsolutenumberof centralmemoryCD4+Tcells,butnotCD8+Tcells,comparedtoZAP-70negativepatients
andage-matchedapparentlyhealthydonors.
∗ Correspondingauthorat:LaboratoryofClinicalPathology,FlowCytometryDivision,HospitalIsraelitaAlbertEinstein,Av.AlbertEinstein,
627,Morumbi,SãoPaulo,SP,CEP:05652-900,Brazil. E-mailaddress:rodolfoptc@gmail.com(R.P.Correia).
https://doi.org/10.1016/j.htct.2018.03.008
Conclusions: BecausecentralmemoryCD4+Tcellsarelocatedinlymphnodesandexpress
CD40L,weconsiderthatmalignantZAP-70-positiveBcellsmayreceivebeneficialsignals fromcentralmemoryCD4+Tcellsastheyaccumulate,whichcouldcontributetomore
aggressivedisease.
©2018Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Chronic lymphocytic leukemia (CLL) is the most common lymphoproliferativeneoplasminWesterncountries;itis char-acterized by an accumulation of mature CD5+ B cells in
hematopoietic tissuedue to the imbalance in the rates of proliferationandapoptosis.Ithasbeenreportedthatgenetic predisposition, mutations, familial history, environmental factorsandclonalevolutioninducedbyantigensand/or auto-antigensareelementsrelatedtotheetiologyofthedisease.1–3
The clinical course of CLL is heterogeneous and varies betweenindividuals.1,4 Several clinical and laboratory data
areconsidered prognosticmarkers,includingRaiand Binet staging,lymphocytecountdoublingtime,cytogenetics, differ-entgeneticmutations,zeta-chain-associatedproteinkinase 70(ZAP-70)expressionandtheIgheavychainV-IIIregionVH26
(IgVH)genemutationalstatus.3,5–8 Ofthese,ZAP-70
expres-sion, detected by flow cytometry, is commonly used as a reliablesurrogateforgoldstandardprognosticmarkers.8
AlthoughCLLisbasicallyaBcelldisease,ithasbeen pro-posedthatitspathophysiologyandevolutionaresignificantly influencedbyTcellsastheyparticipateintheirexpansion, differentiationandsurvival,whichmayalsoinfluenceT lym-phocytefunctionandphenotype,9,10 withtheaccumulation
ofmemoryTcellsinCLLpatients.11,12Howevertherearefew
dataabouttheassociationbetweenmemoryTcellsandthe prognosisofCLL,especiallyrelatedtoZAP-70.
Thus,thisstudy analyzedtheperipheralTcell compart-mentofCLLpatients,toevaluatewhetherZAP-70expression isassociatedwithanabnormaldistributionofnaïveand mem-oryTcellsrelatedtothecrosstalkbetweenthesecells,and consequentlytotheprognosisofthedisease.
Methods
Studydesign,ethicsandsetting
Thiscontrolledcross-sectionalstudycomparedCLLpatients withhealthyblooddonorsregardingthedistributionofnaïve andmemoryT-cellsubsetsinperipheralblood.Thestudywas conductedintworeferralcentersforhematologyand oncol-ogyin Brazil, one private and the other a public teaching institution.
Allhealthyblooddonorsandpatientssignedinformed con-sentformstherebyagreeingtoparticipateinthisstudy.The reviewboardsofbothinstitutionsreviewedandapprovedthe studyprotocol.
Healthydonorsandpatients
Thecasegroupofthisstudywerealladultpatientsadmitted forthediagnosisofCLL fromSeptember2011toSeptember 2012. The control groupcomprised blood donorsfrom the sameinstitutionsduringthesameperiod.
Avolumeofapproximately10mLofperipheralblood(PB) was collected from all participantsintubes with anticoag-ulantheparinorethylenediaminetetraaceticacid(EDTA)for flowcytometry.Inthebloodbanks,thesamplewascollected aftervoluntarydonations.
Flowcytometryassay
Theflowcytometryassay usedinthis studyforthe evalu-ation ofthe ZAP-70expressionwasperformed intheFlow CytometryLaboratoryoftheClinicalPathologyLaboratoryof Hospital Israelita Albert Einstein.Thetechnical procedures andflowcytometrysettingsfollowedstandardoperating pro-cedures(SOP)previouslyvalidatedinthelaboratoryandthe qualitywasmonitoredbyinternalandexternalquality con-trolsaspublishedbytheAmericanCollegeofPathology(CAP) and UnitedKingdomNational ExternalQualityAssessment Service(UKNEQAS).
TheexpressionofZAP-70inB-cellCLLpatientswasalways evaluatedinparalleltoasampleofperipheralbloodfroma healthydonorinordertovalidatetheintracytoplasmic reac-tionandtheZAP-70antigenicexpressioninT-cells(positive) andBcells(negative).
All fluorochrome-conjugated antibodies were validated beforeuseaccordingtothereactionspecificityandthebest volumecapableofprovidingtheantigen–antibodybinding sat-uration(titration).
CharacterizationofnaïveandmemoryTcells
Peripheral blood mononuclear cells (PBMC) from healthy donors and CLL patients were obtainedand purified using Ficoll-Paque PLUS(GEHealthcareBio-SciencesAB, Sweden) anddensitygradientcentrifugation.13
Thecellconcentrationandviabilitywereassessedusingan opticalmicroscope,Neubauerchamberandtrypanblue.An aliquotof1×106cellswerestainedwithanti-CD3,anti-CD4,
105 105
CD3+CD4+ 32,07TCM
8,47 TCM
TEMRA 10,24
TEMRA 43,28 TEM
22,97
TEM 14,75
34,72
Naïve
33,50
Naïve
CD3+CD4+
CD3+CD8+
CD3+CD8+
105
105
105
105
101 101
101
101
102 102
150K
150K
SSC
100K
100K 50K
FSC
CD4
CD45RA CD8
50K
CD3 CD62L
200K
200K 250K
250K
102
102
102
103 103
103
103
103
104
105
101
102
103
104
104
104
105
101 102 103 104 101 102 103 104 105
104
104
Figure1–CharacterizationofnaïveandmemoryTcellsinperipheralbloodofhealthydonorsandchroniclymphocyte leukemia(CLL)patientsbyflowcytometry.Thelymphocyteregionwasevaluatedconsideringtheforwardandsidescatter ofcells.CD4+andCD8+TcellswererespectivelydeterminedbydoublepositivityofCD3+CD4+andCD3+CD8+,andthe
CD45RAandCD62LexpressionpatternswereusedtoidentitynaïveTcells(CD45RA+CD62L+),centralmemoryTcells
(CD45RA−CD62L+),effectormemoryTcells(CD45RA−CD62L−)andterminaleffectormemoryTcells(CD45RA+CD62L−).For
standardization,50,000eventswereacquiredintheCD3+T-cellpopulation.Thisstrategywasrepresentativeforhealthy
donorsandCLLpatients.
Afterincubationandwashing,sampleswereanalyzedbyflow cytometryinaBDFACSCantoIIapparatus(Becton,Dickinson, SanJose,CA,USA)usingtheFlowJosoftware–(TreeStar,Inc.). CD4+ and CD8+ T cells were respectively determined
by double positivity of CD3+CD4+ and CD3+CD8+.
More-over,CD45RAandCD62L expressionswere usedtoidentify naïve T cells (CD45RA+CD62L+), central memory T cells
(TCM; CD45RA−CD62L+), effector memory T cells (TEM;
CD45RA−CD62L−) and terminal effector memory T cells
(TEMRA;CD45RA+CD62L−)(Figure1).Thisstrategywasusedfor
bothhealthydonorsandCLLpatients.
ZAP-70expressioninCLLpatients
For the ZAP-70flow cytometry assay, 5×106 PB cells from
healthy donors and CLL patients were stained with anti-CD3andanti-CD19mAbsandconjugatedwithphycoerythrin cyanine (PE-Cy5) and PE, respectively (Beckman Coulter). For cytoplasmic staining, cells were fixed, permeabilized
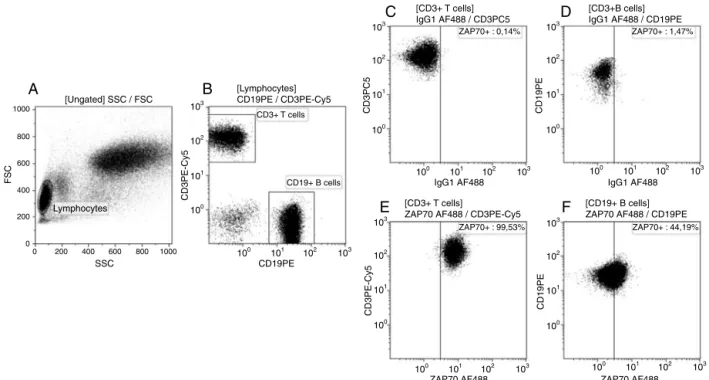
and stained withanti-ZAP-70 mAbconjugated with Alexa-Fluor488(clone1E7.2,Invitrogen).Sampleswereanalyzedina CytomicsFC500apparatus(BeckmanCoulter–Figure2).
Thecut-offforZAP-70positivitywasbasedonpublished data,whichrecommendspositivitywhenmorethan20%of neoplasticBlymphocytesexpressZAP-70.8
Tostandardizethetechnique,theZAP-70expressionwas alwaysanalyzedinthesamplesofhealthydonorsinparallel toCLLpatients.Furthermore,theisotypecontrolfor Alexa-Fluor488wasusedasanegativecontrolofthereaction,while theexpressionofZAP-70onnormalTlymphocyteswas con-sideredthepositivecontrol.14
Statisticalanalysis
A
[Ungated] SSC / FSC
[Lymphocytes] CD19PE / CD3PE-Cy5
[CD3+ T cells] IgG1 AF488 / CD3PC5
[CD3+ T cells]
ZAP70 AF488 / CD3PE-Cy5
ZAP70+ : 1,47% ZAP70+ : 0,14%
ZAP70+ : 44,19% ZAP70+ : 99,53%
ZAP70 AF488 ZAP70 AF488 [CD3+B cells] IgG1 AF488 / CD19PE
[CD19+ B cells] ZAP70 AF488 / CD19PE
1000
1000
SSC Lymphocytes
CD3+ T cells
CD19+ B cells
CD19PE
IgG1 AF488 IgG1 AF488
CD3PE-Cy5
CD3PE-Cy5
CD3PC5 CD19PE
CD19PE
FSC
800
800 600
600 400
400 200
200 0
0
103
103
103 102
102
102 101
101
101 100
100
103
102
101
100
103
102
101
100 103
102
101
100
100
103
102
101
100 100 101 102 103
103
102
101
100
103
102
101
100
B
C
E
F
D
Figure2–DetectionofZAP-70expressioninhealthydonorsandchroniclymphocyteleukemia(CLL)patients.Analiquotof 5×105peripheralbloodcellswerestainedwithmonoclonalantibodies(CD19PE,CD3PE-Cy5,intracellularmouseIgG1
AF488andintracellularZAP-70AF488)andanalyzedbyflowcytometry.(A)Firstagateinthelymphocyteregionwas createdaccordingtoforwardandsidescattertobetteridentifythespecificpopulations.(B)Followingthegatestrategy, CD19+BcellsandCD3+Tcellswerecharacterizedinthelymphocytegate.Thedefinitionofnegativefluorescencebymouse
IgG1AF488(C)inCD3+Tcellsand(D)inCD19+Bcells.(E)ZAP-70positiveexpressioninnormalCD3+Tcellsand(F)in
neoplasticCD19+Bcells.TheZAP-70expressioninnormalTcellsofCLLpatientswasusedastheinternalcontrolof
stainingreaction.Atotalof50,000eventswasacquiredforallcounts.
usingthepairedStudentt-testandthecomparisonbetween thecellsubpopulationswasperformedusingrepeated mea-sures analysis ofvariance (ANOVA) followed byBonferroni multiplecomparison.Comparisonbetweengroups(healthy and case) and cell types or subtypes were achieved using two-wayANOVAfollowedbyBonferronimultiplecomparison whennecessary.Statisticaldifferenceswereconsidered sig-nificantwhenp-valueswere<0.05.
Results
In the study period, 21 CLL patients and 43 controls were enrolled.Inthecontrolgroup,17patientswereover40years old. Table 1 summarizes the characteristics ofthe control groupandtheclinicalandlaboratorydataoftheCLLpatients aresummarizedinTable2.
DistributionprofileofnaïveandmemoryTcells
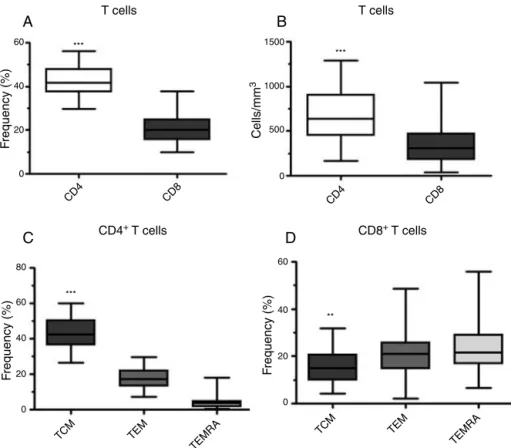
Asexpected,thefrequencyandtheabsolutenumberofCD4+
TcellswerehigherthanthoseofCD8+TcellsintheControl
Group(Figure3).TheTcell compartmentwasalso charac-terizedandthe CD4and CD8subpopulationshaddifferent distributions according to memory T cell subsets. Central memoryTcells(TCM)werepredominantintheCD4+ Tcell
Table1–Characteristicsofhealthydonors.
Characteristic Total Over40-yearolds
Patients–n(%) 43(100) 17(100)
Sex–n(%)
Male 26(60.5) 9(52.9)
Female 17(39.5) 8(47.1)
Age(years)
Median 36 55
Range 19–72 41–72
compartment,whereas TCM werefewerofthe CD8+ T
lym-phocyteswithTEMandTEMRAbeingdistributedequallyamong
CD8+Tlymphocytes(Figure3CandD).
CharacterizationofTcellcompartmentinchronic lymphocyticleukemiapatients
TheanalysisoftheTcellcompartmentinCLLpatientsshowed that,duetotheincreasednumbersofneoplasticB lympho-cytesinPB,thefrequenciesofCD4+andCD8+Tlymphocytes
C
D
B
A
CD4+ T cells CD8+ T cells
T cells T cells
CD4
TCM TEM TCM TEM
TEMRA TEMRA
CD4
CD8 CD8
0 20 40
60 ***
***
** ***
0 20 40 60
0 20 40 60 80
1500
1000
0 500
F
requency (%) Cells/mm
3
F
requency (%) Frequency (%)
Figure3–DistributionprofileofTcellsinhealthydonors.(A)Frequencyand(B)absolutenumberofperipheralbloodCD4+
andCD8+Tcellsfromhealthyindividualsasassessedbyflowcytometry.Frequencyofmemory(C)CD4+and(D)CD8+Tcell
subpopulationsaccordingtotheCD45andCD62Lexpression[centralmemoryTcells(TCM–CD45RA−CD62L+),effector
memoryTcells(TEM–CD45RA−CD62L−)andterminaleffectormemoryTcells(TEMRA–CD45RA+CD62L−)].Thevalueswere
statisticallysignificantwithp-value<0.001(***)andp-value=0.003(**).
B
A
T cells T cellsCLL patients Healthy donors
Cells/mm
3
F
requency (%)
CD4 CD8 CD4 CD8
0 20 40
60 ***
***
1500
1000
0 500
Figure4–AbsolutenumberofCD4andCD8Tcellsisnotchangedinchroniclymphocyteleukemia(CLL)patients.(A) FrequencyofperipheralbloodCD4+andCD8+TcellsinCLLpatientscomparedtohealthydonors.Thevalueswere
statisticallysignificantwithp-value<0.001(***).(B)AbsolutenumberofperipheralbloodCD4+andCD8+TcellsfromCLL
patientscomparedtothehealthydonors.
DistributionpatternofnaïveandmemoryTcellsin chroniclymphocyticleukemiapatients
Nodifferencesinthefrequencyandtheabsolutenumberof naïve,TCM, TEM and TEMRA were observedinthe CD4+ and
CD8+Tcellpopulations(Figure5).However,ZAP-70positive
patients had significant differences in the frequencies of naïveandabsolutenumber ofTCM intheCD4+ Tcell
com-partment.Therewasno differenceinthe frequencyorthe
absolutenumberofCD8+TcellsinCLLpatientscomparedto
age-matchedapparentlyhealthyindividuals(Figure6).
Age-dependentdifferencesinthedistributionofnaïveand memoryTcells
Cells/mm
3
CD4+T cells
CD8+T cells CD8+T cells
CD4+T cells
B
A
D
C
CLL patients Healthy donors (group 2)
CLL patients Healthy donors (group 2)
Cells/mm
3
F
requency (%)
F
requency (%)
0
50
50 100 150 200
10
0 0
20 30 40
0
20 200
40 400
60 600
80 800
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
Figure5–NaïveandmemoryTcelldistributionsinchroniclymphocyteleukemia(CLL)patients.PeripheralbloodTcell subsetsinCLLpatientsandage-matchedhealthydonors(Group2)weredeterminedbyflowcytometry.(A)Frequencyand (B)absolutenumberofCD4+naïveandmemoryTcellssubsets.(C)Frequencyand(D)absolutenumberofCD8+naïveand
memoryTcellssubsets.TCM:centralmemoryTcells;TEM:effectormemoryTcells;TEMRA:terminaleffectormemoryTcells.
CD4+T cells CD4+T cells
A
D
C
B
CD8+T cells CD8+T cells
Cells/mm
3
Cells/mm
3
F
requency (%)
F
requency (%)
CLL ZAP-70 positive CLL ZAP-70 negative Healthy donors (group 2)
CLL ZAP-70 positive CLL ZAP-70 negative Healthy donors (group 2) 0
20 40 60 80
0 500 1000 1500
***
***
50 100 150 200
0 50
10
0 20 30 40
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
TEMRA TEM TCM Naiv
e
Figure6–NaïveandmemoryCD4+TcelldistributionsarerelatedtotheZAP-70expressioninchroniclymphocyteleukemia
(CLL)patients.(A–C)Frequencyand(B–D)absolutenumberofCD4+andCD8+naïveandmemoryTcellssubsetsinCLL
patientswithandwithoutZAP-70expressionincomparisontoage-matchedhealthydonors.Differencesinthefrequencies ofnaïveandabsolutenumberofcentralmemoryTcellsintheCD4+Tcellcompartmentwerestatisticallysignificantwith
p-value<0.001andp-value=0.008,respectively.TCM:centralmemoryTcells;TEM:effectormemoryTcells;TEMRA:terminal
p=0,769 r=0,002
p=0,963 r=0,000
p=0,0004 r=0,263
p=0,2776 r=0,028 p=0,0337 r=0,105
p=0,0465 r=0,093 p=0,473 r=0,126 p=0,948 r=0,000
Age (years) Age (years)
Age (years)
Age (years)
Age (years) Age (years)
Age (years) Age (years)
B
C
F
E
H
G
D
A
0 20
30 50
20
0 15
5
0
10
10 30 40
20
0 10 30 40
20 20 40
40 60
60 70 20 30 40 50 60 70
30 50
20 40 60 70 20 30 40 50 60 70
30 50
20 40 60 70 20 30 40 50 60 70
30 50
20 40 60 70 20 30 40 50 60 70
80
0 20 40 60 80
0 20 40 60 80
0 20 40 60
0 20 40 60
CD4 Tcm
CD4 TEM CD4 TEMRA
CD8 TCM
CD8 TEM CD8 TEMRA
CD4 naïve
CD8 naïve
F
requency (%)
F
requency (%)
F
requency (%)
F
requency (%)
F
requency (%)
F
requency (%)
F
requency (%)
F
requency (%)
Figure7–DistributionofnaïveandmemorysubtypesofCD8+Tcells,butnotCD4+Tcells,isagedependent.Analysisof
thefrequencydistributionof(A–D)CD4+and(E–F)CD8+naïve,centralmemoryTcells(T
CM),effectormemoryTcells(TEM)
andterminaleffectormemoryTcells(TEMRA)inhealthydonors(19–72yearsold).Linearregressionwasusedtoevaluatethe
Table2–Clinicalandlaboratorydataofchronic lymphocyteleukemiapatients.
Characteristic Value
n 21
Sex–n(%)
Male 16(76.2)
Female 5(23.8)
Age(years)
Median 64
Range 46–88
Leucocytes(×103/L)
Median 24.46
Range 2.60–61.00
Lymphocytes(×103/L)–n(%)
Median 20,465.0(78.3)
Range 1.609(41.7)-53,741.0(92.0)
Hemoglobin(g/dL)
Median 13.6
Range 8.5–16.7
Hematocrit(fL)
Median 39.8
Range 25.6–54.2
Platelets(×109/L)
Median 205
Range 32,000–265,000
ZAP-70expression–n(%)
Negative 16(76.2)
Positive 5(23.8)
distributions.6 Therefore, thenext stepwastoruleout the
possibilitythattheincreaseinTCMCD4+cellswasdueto
dif-ferencesintheagesofthepatients.Onanalyzingthehealthy group(19–72yearsold),nodifferenceswereobservedinthe naïveandmemoryCD4+Tcellswithage(Figure7),butinthe
CD8+Tcellcompartment,thenaïveTcellsdiminishedand
TCMandTEMRAcellsincreasedwithage(Figures7E–H).
Discussion
The involvement of T cells in the pathophysiology of hematologicmalignancies,especially inCLL, iswidely dis-cussed. The interaction of neoplastic B cells with their microenvironment, particularly CD4+ T lymphocytes and
withextracellularcomponents,couldregulatetheexpansion, differentiation, and survivalofneoplastic Bcells,and pos-siblyinfluencetheTlymphocytegene expression,function andphenotype.9,10 Thisstudy showedthat ZAP-70positive
patientspresentedincreasedTCM CD4+ Tcellscomparedto
ZAP-70negativepatientsandtoage-matchedhealthydonors (Figure5).
MemoryTcellsrepresentaheterogeneousgroupofcells characterizedbytheirphenotypicdiversity,proliferativeindex versuseffectorfunction,and migratorycapabilitytolymph nodesand/or toperipheraltissues.They areclassified into three major groups: TCM, TEM, and TEMRA. Briefly, TCM are
locatedinlymphnodesandhaveloweffectorfunctionwith
ahighproliferativeindex.Ontheotherhand,TEMandTEMRA
arecharacterizedbyhigheffectorfunctionintheliver,lung, and gutand withalowproliferativeindex. Together,these cellsprovideimmediateprotectioninperipheraltissuesand efficientsecondaryresponseinlymphnodes.15–18
Itiswellestablishedthatthesignalingandantigenic stim-ulationbyBcellreceptors(BCRs)iscrucialforsurvivaland growthofCLLcells,eventhoughitisadiseasewithalow pro-liferativeindex.19Inthiscontext,T
CMCD4+cellscanmigrate
tothelymphnodesduetotheexpressionofCD62LandCCR7, and interactwithBcells byCD40L andCXCR5,that conse-quentlycouldcontributetostrongerBCRsignaling,expansion andevolutionofthedisease.18Thesedatacouldsupportthe
findingthatZAP-70positiveCLLpatientshaveincreasedTCM
CD4+Tcells.
In addition to the modulation of BCR signaling, the cytokinesproducedbymemoryTcells,especiallyinterleukin (IL)-4andinterferon-gamma(IFN-␥),couldpromoteincreased
survivaloftheCLLclone.20,21 Recently,astudyshowedthat
ZAP-70expressioninCLL isassociatedwithareductionof naïveCD4+Tcellsandincreasesinactivation/differentiation
ofCD4+TcellstoamemoryprofilethatexpressesIL-4or
IL-10.Inaddition,thesecytokinesmightfavorthegrowthand survivalofCLLcells.22
Furthermore,as neoplastic CLLBcells are inefficientas antigenpresentingcells,theevidencethatincreasedTCMCD4+
TcellscouldbeaCLLmechanismissupportedbythetheoryof thegenerationofmemoryTcells;aweakstimulationofnaïve Tcellsduringantigenpresentationdrivesthedifferentiation ofthesecellsintoTCMcells.23,24
ToratifythatthechangesdetectedintheCD4+memoryT
cellcompartmentareaCLLintrinsicmechanismandnotan age-dependentevent,thefrequencyofthesecellswas evalu-atedinhealthydonorsstratifiedbyage.Indeed,eventsrelated toage,suchashomeostaticregulation,thymusinvolutionand antigenic stimulation were observed,6 but this mechanism
onlyaffectedtheCD8+Tcellcompartment(Figure7).
There-fore, ourfinding ofincreasedTCM CD4 cellsisaconsistent
resultanddirectlyassociatedwithCLL,inparticular, associ-atedtoZAP-70expressioninthisdisease.
This study has limitations in evaluating the real asso-ciation between the results with CLL prognosis, especially due to absent ofexperimental verification and correlation with other clinical and laboratory data. Still, we believe the increasein TCM CD4+ T cells might be a resultof the
crosstalk mechanism between CLL ZAP-70 positive B cells with normal CD4+ T cells, in which the neoplastic clone
could obtain greater survivalandexpansion signalsdueto cytokinesandcostimulatorysignalsasdescribedabove. Inter-estingly, it is reported that murine models using animals deficientinBcellshaveimpairedgenerationofCD4+memory
Tcells.15,25
These findings could be important to understand the pathophysiology,andconsequently,theycouldhelpdrive fur-therinvestigationsintheprognosisandtreatmentofCLL.
Conflicts
of
interest
r
e
f
e
r
e
n
c
e
s
1. SwerdlowSH,CampoE,HarrisNL,JaffeES,PileriAS,SteinH, etal.WHOclassificationoftumoursofhaematopoieticand lymphoidtissues.4thed.Lyon:InternationalAgencyfor ResearchonCancer;2008.
2. InamdarKV,Bueso-RamosCE.Pathologyofchronic lymphocyticleukemia:anupdate.AnnDiagnPathol. 2007;11(5):363–89.
3. PuenteXS,PinyolM,QuesadaV,CondeL,Ordó ˜nezGR, VillamorN,etal.Whole-genomesequencingidentifies recurrentmutationsinchroniclymphocyticleukaemia. Nature.2011;475(7354):101–5.
4. RichesJC,RamsayAG,GribbenJG.Chroniclymphocytic leukemia:anupdateonbiologyandtreatment.CurrOncol Rep.2011;13(5):379–85.
5. RaiKR,SawitskyA,CronkiteEP,ChananaAD,LevyRN, PasternackBS.Clinicalstagingofchroniclymphocytic leukemia.Blood.1975;46(2):219–34.
6. BinetJL,AuquierA,DighieroG,ChastangC,PiguetH, GoasguenJ,etal.Anewprognosticclassificationofchronic lymphocyticleukemiaderivedfromamultivariatesurvival analysis.Cancer.1981;48(1):198–206.
7. DamleRN,WasilT,FaisF,GhiottoF,ValettoA,AllenSL,etal. IgVgenemutationstatusandCD38expressionasnovel prognosticindicatorsinchroniclymphocyticleukemia. Blood.1999;94(6):1840–7.
8. CrespoM,BoschF,VillamorN,BellosilloB,ColomerD, RozmanM,etal.ZAP-70expressionasasurrogatefor immunoglobulin-variable-regionmutationsinchronic lymphocyticleukemia.NEnglJMed.2003;348(18):1764–75.
9. CorreiaRP,MatosE,SilvaFA,BacalNS,CampregherPV, HamerschlakN,etal.InvolvementofmemoryT-cellsinthe pathophysiologyofchroniclymphocyticleukemia.RevBras HematolHemoter.2014;36(1):60–4.
10.ScrivenerS,GoddardRV,KaminskiER,PrenticeAG.Abnormal T-cellfunctioninB-cellchroniclymphocyticleukaemia.Leuk Lymphoma.2003;44(3):383–9.
11.TinhoferI,WeissL,GassnerF,RubenzerG,HollerC,GreilR. DifferenceintherelativedistributionofCD4+T-cellsubsets inB-CLLwithmutatedandunmutatedimmunoglobulin(Ig) VHgenes:implicationforthecourseofdisease.J
Immunother.2009;32(3):302–9.
12.HofbauerJP,HeyderC,DenkU,KocherT,HollerC,TrapinD, etal.DevelopmentofCLLintheTCL1transgenicmouse
modelisassociatedwithsevereskewingoftheT-cell compartmenthomologoustohumanCLL.Leukemia. 2011;25(9):1452–8.
13.BöyumA.Isolationofmononuclearcellsandgranulocytes fromhumanblood.Isolationofmonuclearcellsbyone centrifugation,andofgranulocytesbycombining centrifugationandsedimentationat1g.ScandJClinLab InvestSuppl.1968;97:77–89.
14.VillamorN.ZAP-70staininginchroniclymphocyticleukemia. CurrProtocCytom.2005;6:19.Chapter6:Unit619.
15.JamesonSC,MasopustD.DiversityinTcellmemory:an embarrassmentofriches.Immunity.2009;31(6):859–71.
16.PepperM,JenkinsMK.OriginsofCD4(+)effectorandcentral memoryTcells.NatImmunol.2011;12(6):467–71.
17.SallustoF,GeginatJ,LanzavecchiaA.Centralmemoryand effectormemoryTcellsubsets:function,generation,and maintenance.AnnuRevImmunol.2004;22:745–63.
18.SallustoF,LenigD,FörsterR,LippM,LanzavecchiaA.Two subsetsofmemoryTlymphocyteswithdistincthoming potentialsandeffectorfunctions.Nature.
1999;401(6754):708–12.
19.StevensonFK,Caligaris-CappioF.Chroniclymphocytic leukemia:revelationsfromtheB-cellreceptor.Blood. 2004;103(12):4389–95.
20.BuschleM,CampanaD,CardingSR,RichardC,HoffbrandAV, BrennerMK.Interferongammainhibitsapoptoticcelldeath inBcellchroniclymphocyticleukemia.JExpMed.
1993;177(1):213–8.
21.PanayiotidisP,GaneshaguruK,JabbarSA,HoffbrandAV. Interleukin-4inhibitsapoptoticcelldeathandlossofthe bcl-2proteininB-chroniclymphocyticleukaemiacellsin vitro.BrJHaematol.1993;85(3):439–45.
22.MonserratJ,SánchezMÁ,dePazR,DíazD,MurS,ReyesE, etal.Distinctivepatternsofnaive/memorysubset
distributionandcytokineexpressioninCD4Tlymphocytesin ZAP-70B-chroniclymphocyticpatients.CytomBClinCytom. 2014;86(1):32–43.
23.LanzavecchiaA,SallustoF.Understandingthegenerationand functionofmemoryTcellsubsets.CurrOpinImmunol. 2005;17(3):326–32.
24.DazziF,D’AndreaE,BiasiG,DeSilvestroG,GaidanoG,Schena M,etal.FailureofBcellsofchroniclymphocyticleukemiain presentingsolubleandalloantigens.ClinImmunol
Immunopathol.1995;75(1):26–32.