w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
l-Amino
acid
oxidase
isolated
from
Calloselasma
rhodostoma
snake
venom
induces
cytotoxicity
and
apoptosis
in
JAK2V617F-positive
cell
lines
Cristiane
Tavares
a,
Thaís
Maciel
a,
Sandra
Burin
a,
Luciana
Ambrósio
a,
Sandro
Ghisla
b,
Suely
Sampaio
a,
Fabíola
Castro
a,∗aFaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSãoPaulo(USP),RibeirãoPreto,SP,Brazil
bUniversitätKonstanz,Konstanz,Germany
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14January2016 Accepted30March2016 Availableonline14April2016
Keywords:
Myeloproliferativeneoplasms Apoptosis
l-Aminoacidoxidase
Calloselasmarhodostoma
Januskinase2mutation
a
b
s
t
r
a
c
t
Background:MyeloproliferativeneoplasmsarePhiladelphiachromosome-negativediseases characterizedbyhyperproliferationofmaturemyeloidcells,associatedornotwiththeJanus kinase2tyrosinekinasemutation,JAK2V617F.Asthereisnocurativetherapy,researchers havebeeninvestigatingnewdrugstotreatmyeloproliferativeneoplasms,includingl-amino acidoxidasefromCalloselasmarhodostomasnakevenom(CR-LAAO),whichisatoxincapable ofelicitingapoptosisinseveraltumorcelllines.
Objective:Toevaluatetheeffectsofl-aminoacidoxidasefromC.rhodostomasnakevenom intheapoptoticmachineryofJAK2-mutatedcelllines.
Methods:TheHEL92.1.7andSET-2celllineswereculturedwithl-aminoacidoxidaseand catalasefor12hat37◦Cin5%carbondioxide.Thecellviabilitywasassessedbythe
multi-tabletournamentmethod,thelevelofapoptosiswasmeasuredbyflowcytometry,andthe expressionofcysteine-dependentaspartate-specificproteasesandcleavedPoly(ADP-ribose) polymerasewereanalyzedbyWesternblotting.
Results:l-AminoacidoxidasefromC.rhodostomasnakevenomwascytotoxictoHEL92.1.7 andSET-2cells(50%inhibitoryconcentration=0.15g/mLand1.5g/mL,respectively)and
induced apoptosisin a concentration-dependent manner.Cell treatment withcatalase mitigatedthel-aminoacidoxidasetoxicity,indicatingthathydrogenperoxideisakey com-ponentofitscytotoxiceffect.Theactivatedcaspases3and8expressionandcleavedPARP inHEL92.1.7andSET-2cellsconfirmedtheapoptosisactivationbyCR-LAAO.
Conclusions:l-Aminoacid oxidasefromC. rhodostoma snake venomis a potential anti-neoplasticagentagainstHEL92.1.7andSET-2JAK2V617F-positivecellsasitactivatesthe extrinsicapoptosispathway.
©2016Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:LaboratóriodeHematologia,FaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSãoPaulo
(USP),AvenidadoCafé,s/n,14040-903RibeirãoPreto,SP,Brazil. E-mailaddress:castrofa@fcfrp.usp.br(F.Castro).
http://dx.doi.org/10.1016/j.bjhh.2016.03.004
Introduction
Myeloproliferativeneoplasms(MPN)arehematological neo-plasms with similar phenotypic features, preserved cell maturation,andhyperproliferationofoneormorebloodcell types.MPNcomprisechronicmyeloidleukemia(CML), poly-cythemiavera(PV),essentialthrombocythemia(ET),primary myelofibrosis(PMF), chronic neutrophilicleukemia,chronic eosinophilic leukemia, mastocytosis, and non-classifiable MPN.1 Patients with PV, ET, and PMF are negative for the
BreakpointClusterRegion-AbelsonLeukemia(Bcr-Abl)oncogene, and their hematopoietic progenitors are independent and hypersensitive to numerous cytokines.2,3 Mutation in the Janus kinase 2 (JAK2) tyrosine kinase is the predominant molecularalterationinMPN;itischaracterizedbya guanine-to-thyminetransversionatnucleotide1849ofexon14ofthe gene(chromosome9),resultinginthesubstitutionofvaline forphenylalanineatposition617(JAK2V617F).4
TheaminoacidexchangeoccursintheJH2pseudokinase domainandleadstothelossoftheautoinhibitorycontrolof theJH2domainovertheJH1domain,andtheconsequent con-stitutiveactivationoftheprotein.5Jekarletal.6reportedthat theJAK2V617Fmutationisfoundin95%ofPVpatientsandin 50%ofpatientswithETandMF.InadditiontotheJAK2V617F mutation,other genemutationssuchasJAK2exon12,MPL andcalreticulinmayhelpinthedifferentialdiagnosis, patho-genesis,andprognosisofPhiladelphiachromosome-negative (Ph−)MPN.7ItiswellknownthatthepathogenesisofMPNis
alsolinkedtogeneticandepigeneticalterationsandmyeloid cellresistancetoapoptosis.5,8,9
Apoptosisisthephysiologicalprocessofprogrammedcell deaththatplaysa role inthemaintenanceofcell number andintegrity,andtissuedevelopmentinavarietyofbody sys-tems.Activationofapoptosisoccursmainlyviatheintrinsic and extrinsic pathways,10 both of which require the par-ticipationofcysteine-dependentaspartate-specificproteases (caspases). Caspases are proteases bearing a catalytic cys-teine residue that cleaves other proteins at their aspartic acidresidue.Caspasesaresynthesizedasinactiveprecursors (zymogens)thatrequirecleavagebyproteasestoformactive enzymes,whichinturntriggerareactioncascadethat culmi-natesincellapoptosis.10
Many types of stimuli can elicit the intrinsic or mito-chondrialapoptosispathway,includinghypoxia,intracellular stress,lackofgrowthfactors,irradiation,chemotherapeutic agents,bacteria,andviruses.Thesestimuliinducetherelease of cytochrome c, apoptosis inducing factor (AIF), second mitochondriaderivedactivatorofcaspases/directinhibitorof apoptosis-bindingproteinwithlowpI(SMAC/DIABLO),which culminatesintheactivationofapoptoticproteaseactivating factor-1(APAF-1).ThebindingofAPAF-1todeoxyadenosine triphosphate(ATP)/2 deoxy-ATP(dATP) induces the forma-tionoftheapoptosome,amultimericcomplexthatactivates theinitiatorcaspase-9andsubsequentlyactivatesthe execu-tionercaspases-3,-6,and-7.11
Theextrinsicapoptosispathwayistriggeredbythebinding ofligandstodeathreceptors.TheseincludeFAS(FAS/CD95), tumornecrosisfactor(TNF)deathreceptors1and2(TNF-R1 andR2,respectively),TNF-relatedapoptosis-inducingligand
receptors(TRAIL) R1 and R2(DR4 and DR5,respectively); a family of transmembrane proteins bearing a cysteine-rich extracellular domain and an intracellular domain named “deathdomain”(DD),whichisresponsiblefortransducingthe apoptotic signal.Thereceptor-ligand bindingrecruits adap-tor moleculessuchas FADD(FAS associated withDD) and TRADD(TNFR1associatedwithDD);italsoelicitsintracellular signalingpathwaysthatactivatetheinitiatorcaspases-8,-9, and-10andtheexecutionercaspases-3,-6,and-7,and pro-motestheformationofapoptoticbodies(celldeath).Finally, macrophagesphagocytosetheapoptoticbodies.12
DespiteextensiveknowledgeonthepathogenesisofMPN, scientistshaveneitherstratified thediseasenordiscovered effective treatments tocureit yet. Thecurrent treatments for PV, ET, and PMF rely on palliative therapies (bleeding, hydroxycarbamide, interferon-␣, busulfan, corticoids, and
androgens),supportivetherapies(bloodtransfusion,growth factors,erythropoietin,andantibiotics),allogeneicbone mar-rowtransplant,andJAK2inhibitors.13Bonemarrowtransplant istheonlytreatmentthatcanchangethecourseofthe dis-ease; however,thesuccessofthis therapyisrestrictedtoa smallgroupofyoungpatientswhoreceivebonemarrowfrom a human leukocyte antigen (HLA)-compatible donor. JAK2 inhibitorshaveexertedpromisingeffectsinpatientswithPMF, including those who arenegativeforthe JAK2V617F muta-tion.Recentstudieshaveevidencedthebeneficialeffectsof JAK2inhibitorsonthetreatmentofpatientswithPMF,such asdecreasedspleenvolume,bodyweightgain,delayedbone marrowfibrosis,andimprovedoverallsurvival.14
In this sense, researchers have sought for new drugs and therapiesto treat and curepatients with MPN. Snake venoms are one of the antitumor agents under investi-gation; these proteins exert a variety of pharmacological activities that influence the clinical manifestations of the disease,suchasapoptosis induction.15Snakevenomshave complex compositions with different contents of proteins, peptides,carbohydrates,lipids,biogenicamines,nucleotides, andaminoacids.Themajorcomponentofthevenomofthe
Calloselasma rhodostoma, a snake from Vietnam, Cambodia, ThailandandMalaysiaisl-aminoacidoxidase(LAAO),which isaflavoenzymethatcatalyzestheoxidativedeaminationofl -aminoacidsto␣-ketoacidandproducesammoniaandH2O2as
byproducts.16ThesecondaryeffectsofH
2O2seemtoaccount
formostofthebiologicaleffectsofC.rhodostomaLAAO (CR-LAAO),includingapoptosisinduction.17
Toimprovetheunderstandingonthebiologicalactivityand pharmacologicalpotentialofLAAOs,thepresentstudyaimsto examinetheeffectsofCR-LAAOontheapoptosismachinery ofJAK2V617F-positivecelllinesobtainedfromMPNpatients.
Method
Celllinesandculture
withCR-LAAO,incompleteRPMI1640mediumsupplemented with10%fetalbovineserum,2mMglutamine,100U/mL peni-cillin,100mg/mLstreptomycin, and 25mM Hepes, under a humidified5%CO2atmosphereat37◦C.
Determinationoftheenzymaticactivityofthel-amino
acidoxidase
Dr.SandroGhisla(UniversityofKonstanz,Germany) kindly providedtheCR-LAAO,whichwasobtainedaccordingtothe methoddescribedbyMacherouxetal.18Theenzymatic activ-ityofCR-LAAOwasdeterminedin0.1MTris–HClbuffer pH 7.2, at 25◦C.The qualitative test was performed to verify
whethertheproteinwasactive,whichisarequisitefor bio-logicalassays.
l-Aminoacidoxidasecytotoxicityassay
The ability of living cells to reduce 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to insolublevioletformazancrystalswasusedastheparameter todeterminethecytotoxicityofCR-LAAOtoHEL92.1.7and SET-2cells.Bothcelllines(1×105cells/well)wereculturedin
96-wellplates,inthepresenceofCR-LAAO(0.03–3.0g/mL),
25Metoposide(VP-16;positivecontrol),orbuffer(negative
control),for12hunderahumidified5%CO2atmosphereat
37◦C.ToassessthecontributionofH
2O2totheoveralleffect
ofCR-LAAO, bothcell lines were treatedwith CR-LAAO in combinationwith150U/mLcatalase.
Theplateswere centrifugedat240×g for10minat4◦C
and200LofMTT(5mg/mL)wereaddedtoeachwell.After
4hofincubationat37◦Cunder5%CO
2,theformazan
crys-talsweredissolvedbyadding100Lofalysingsolution(10%
sodiumdodecylsulfateplus0.01MHCl)tothewells.Thefinal absorbancewasrecordedat540nm.Controlsintheabsenceof CR-LAAOhad100%ofcellviability.Thepercentageofcell via-bilitywascalculatedandusedtodeterminethe50%inhibitory concentration(IC50)values.
Measurementofapoptosis
HEL92.1.7andSET-2cells(2×105cells/well)wereculturedin
24-wellplates inthepresenceofCR-LAAO(0.05–2.5g/mL),
for12hunderahumidified5%CO2atmosphereat37◦C.The
cellswerewashedwithphosphatebufferedsalineatpH7.4, centrifuged,and suspendedin400Lofhypotonic
fluores-centsolution(50g/mLpropidiumiodide;Sigma–Aldrich,St.
Louis,MO,USA).After45minofincubationat4◦Cinthedark,
thesampleswere analyzedinaFACSCanto flowcytometer (Becton-Dickinson,SanJose,CA,USA).Tenthousandevents were collected and analyzed bythe Diva softwareand the resultsareexpressedaspercentageofcellswithhypodiploid nuclei,whichrepresentapoptoticcells.
Westernblottinganalysisofcaspases-3,-8,and-9and
cleavedPoly(ADP-ribose)polymerase
The HEL 92.1.7 and SET-2 cells (1×106) treated with
CR-LAAO(0.03–0.15g/mLand1.5–3.0g/mL,respectively)were
suspendedin200Lofthe Westernblotting samplebuffer
supplementedwith5%mercaptoethanol,4%sodiumdodecyl sulfate (SDS),20% glycerol,and 100mMTris–HClatpH6.0. Thesampleswereanalyzedbyelectrophoresisandtransferred ontopolyvinylidenefluoride(PVDF)membranes.
The membrane was sequentially labeled overnight with anti-caspase-3 polyclonal rabbit antibody (code 9662, Cell Signaling Technology), anti-tubulin (code T3320, Sigma–Aldrich), anti-caspase-9 polyclonal rabbit antibody (code9502,CellSignalingTechnology),anti-caspase-8mouse monoclonalantibody(code9746,CellSignalingTechnology), and anti-cleaved Poly(ADP-ribose)polymerase (PARP– code 9541, Cell Signaling Technology). The antibodies had been diluted in a blocking solution containing 5% skim milk and 0.01% sodium azide, according to the manufacturers’ instructions.
Afterlabelingwitheachprimaryantibody,themembrane was incubated with the peroxidase-labeled anti-mouse or anti-rabbitsecondaryantibodyfor1hatroomtemperature. The labeled proteinswere detected bychemiluminescence (AmershamECLPlus,GEHealthcareLifeScience,Pittsburgh, BREAK).Then,themembranewaswashedwiththestripping bufferbeforebeingrelabeledwiththenextprimaryantibody.
Statisticalanalysis
Thepercentagesofcellviabilityandapoptosisof CR-LAAO-treatedHEL92.1.7andSET-2cellswerestatisticallycompared withtheirrespectiveuntreatedcontrols,usingone-way anal-ysisofvariance(ANOVA)andDunnett’spost-test.Toanalyze theeffectofH2O2oncellviability,thecellgroupstreatedor
notwithcatalasewerestatisticallycomparedusingStudent’s
t-test.DatawereanalyzedusingtheGraphPadPrismsoftware (v.5.0;GraphPadSoftware,LaJolla,CA,USA).Ap-value<0.05 wasconsideredsignificant.
Results
C.rhodostomal-aminoacidoxidasedecreasesthe
viabilityofHEL92.1.7andSET-2cellsbyreleasingH2O2
Comparedwiththecontrol,CR-LAAOdiminishedthe viabil-ityofHEL92.1.7andSET-2cellsinaconcentration-dependent manner,yieldingtheIC50valuesof0.15and1.5g/mL,
respec-tively(Figure1).ThestandardcompoundVP-16decreasedthe viabilityofbothcelllinesbynearly96.7%.
Toassesswhether H2O2mediatesthecytotoxic effectof
CR-LAAO,thecellsweretreatedwiththetoxinin combina-tionwithcatalase.Catalasemarkedlychangedthecytotoxic profileofCR-LAAObyincreasingtheviabilityofbothcelllines to80–100%(Figure2).Together,theseresultsdemonstratethat (i)CR-LAAOismorecytotoxictoHEL92.1.7cellsthantoSET-2 cells,and(ii)theH2O2releasedduringtheenzymaticreaction
catalyzedbythisoxidasemediatesitscytotoxicaction.
C.rhodostomal-aminoacidoxidaseinducesapoptosisin
HEL92.1.7andSET-2cellsbyactivatingcaspases
120
∗ ∗ ∗
∗ ∗ ∗
∗ ∗ ∗ ∗ ∗
∗ ∗ ∗
∗ ∗
∗ ∗ ∗ ∗
∗
HEL 92.1.7 SET-2
A B
CR-LAAO (µg/mL) 100
80
60
% of viability 40 % of viability
20
0
120
100
80
60
40
20
0 C
0.03 0.05 0.15 0.25 0.350.50 1.00 1.50 2.00 2.50 3.00VP-16 0.35
CR-LAAO (µg/mL) C
0.03 0.05 0.15 0.25 0.50 1.00 1.50 2.00 2.50 3.00VP-16
Figure1–Cytotoxicityofl-aminoacidoxidasefromCalloselasmarhodostomasnakevenom(CR-LAAO)to(A)HEL92.1.7and (B)SET-2JAK2V617Fpositivecells.C:negativecontrol(culturemedium).VP-16:25Metoposide(positivecontrol).
Resultsareexpressedasmeans±standarddeviation/standarderrorofthemeanoftriplicateassays.*p-value<0.05vs.C (one-wayANOVA).
120 ∗
∗ ∗ ∗ ∗ ∗ ∗ ∗ ∗ ∗ ∗
∗
∗ ∗ ∗ ∗ ∗ ∗
HEL 92.1.7
A
B
SET-2100
80
60
% of viability 40 % of viability
20
0
120
ns
Catalase (–) Catalase (+) 100
80
60
40
20
0 C
0.03 0.05 0.15 0.25 0.35 0.50 1.00 1.50 2.00 2.50 3.00 C 0.03 0.05 0.15 0.25 0.35 0.50 1.00 1.50 2.00 2.50 3.00 CR-LAAO (µg/mL) CR-LAAO (µg/mL)
Figure2–l-AminoacidoxidasefromCalloselasmarhodostomasnakevenom(CR-LAAO)cytotoxicity,aloneorin combinationwithcatalaseto(A)HEL92.1.7and(B)SET-2JAK2V617F-positivecells.
C:negativecontrol(culturemedium),ns:non-significant.Resultsareexpressedasmeans±standarddeviation/standard errorofthemeanoftriplicateassays.*p-value<0.05vs.catalase+(Student’st-test).
usingflowcytometry.CR-LAAOinducedtheformationofcells withhypodiploidnucleiinHEL92.1.7cellsmorestronglythan intheSET-2cellline,inaconcentration-dependentmanner (Figure3).
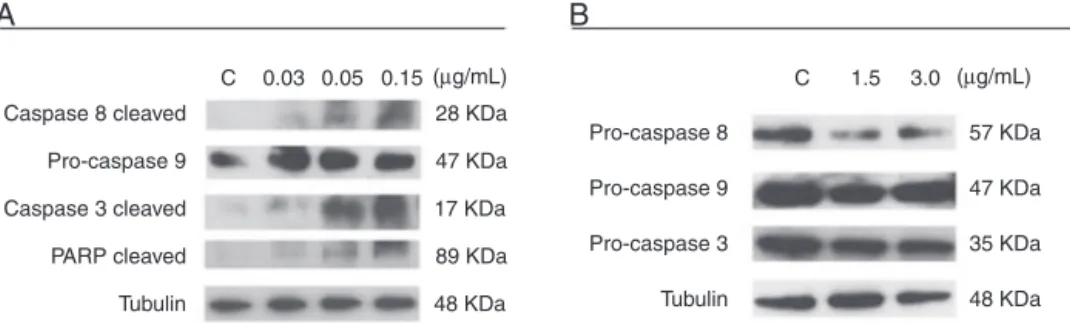
Next, to assess whether CR-LAAO-induced apoptosis occurred via the intrinsic or extrinsic pathway, the activa-tion ofcaspases -3,-8,-9and cleavedPARP were analyzed intheHEL92.1.7andSET-2celllinestreatedwithCR-LAAO
for 12h. The reduced intensity of pro-caspases -3 and -8 bands associatedwiththe increasedintensityofcaspase-3 andcleavedPARPbandsindicatedtheactivationofcaspases (Figure4).
Therefore, the activation of caspases mediates the apoptosis-inducingeffectofCR-LAAObytriggeringthe extrin-sic pathway,which ismore pronouncedin HEL.92.1.7 cells thaninSET-2cells.
HEL 92.1.7
A
B
SET-2120
100
80
60
40
20
0
120
100
80
60
40
20
0
CR-LAAO (µg/mL)
% apoptosis
(Hypodiploid n
uclei)
% apoptosis
(Hypodiploid n
uclei)
C 0.05 0.25 0.50 1.00 2.50 VP-16
CR-LAAO (µg/mL) C 0.05 0.25 0.50 1.00 2.50
VP-16 ∗
∗ ∗
∗
∗ ∗
∗ ∗ ∗
∗ ∗
∗
Figure3–Quantificationofapoptosisbydeterminingthepercentageofcellswithhypodiploidnucleiinducedbyl-amino acidoxidasefromCalloselasmarhodostomasnakevenom(CR-LAAO)of(A)HEL92.1.7and(B)SET-2JAK2V617F-positivecells. C:negativecontrol(culturemedium).VP-16:25Metoposide(positivecontrol).
Caspase 8 cleaved
Pro-caspase 9
Pro-caspase 8
Pro-caspase 9
Pro-caspase 3
Tubulin Caspase 3 cleaved
PARP cleaved
Tubulin C
A
B
0.03 0.05 0.15 C 1.5 3.0
28 KDa
57 KDa
47 KDa
35 KDa
48 KDa 47 KDa
17 KDa
89 KDa
48 KDa
(µg/mL) (µg/mL)
Figure4–CaspaseactivationinHEL92.1.7andSET-2cellstreatedwiththel-aminoacidoxidasefromCalloselasma
rhodostomasnakevenom(CR-LAAO),detectedbywestern-blotting.(A)Activationofcaspases-3,-8andcleavedPARPinHEL 92.1.7cells.(B)Activationofpro-caspases3and8inSET-2cells.C:negativecontrol(culturemedium).
Discussion
PatientswithPh−MPNsuchasPV,ET,andPMFcandisplay
extramedullary hematopoiesis, hyperplasia of megakary-ocytesinthebonemarrow,andvaryingdegreesofdysplasia. ProgressionoftheseMPNtypesismarkedbytheappearanceof medullaryfibrosis(inpatientswithPVandET)or transforma-tionintoeithermyelodysplasticsyndromeoracuteleukemia: 10%ofthecasesprogresstoacutemyeloidleukemia.19
DescriptionoftheJAK2V617Fmutationin2005prompted themolecularstudyofthephysiopathologicalmechanismsof Ph−MPN.4ExpressionofJAK2V617Finducestheproliferation
ofcelllinesindependentlyoftheirstimulationbycytokines andgrowthfactors.Inmurineexperimentalmodels,the retro-viralexpressionofJAK2V617Finhematopoieticcellselicitsthe developmentofahematopoieticdiseasewhoseclinicalsigns andsymptomsresemblesthoseofPV.4 Thepresenceofthe JAK2V617Fmutationcorrelateswiththemostsevereclinical andlaboratoryparametersinpatientswithPV,ET,andPMF, whichischaracterizedbyincreasedhematocrit,hemoglobin levels, leukocyte count, and neutrophil count; diminished erythropoietinlevels;hepatomegaly;splenomegaly;and ele-vatedincidenceofthromboembolicevents,whencompared withJAK2V617F-negativepatients.20Althoughthe pathogen-esisofMPNarewellknown,scientistshavestillnotdiscovered aneffectivedrugtotreatandcuremostofthepatientswith PV,ET,andPMF.
Several researchers have described the resistance of mononuclear cells to apoptosis and deregulation of the expressionofapoptosis-regulatinggenesinpatientswithPV, ET,andPMF.9,21
Ruxolitinib,aJAK2inhibitor,activatescaspases-3,-8,and -9inspecificcelllines,22whilediosgenincleavescaspases-3, -8,and-9inHEL92.1.7cells.23Thesereportsunderscorethe relevanceofapoptosisactivationfortheantitumoreffectof newdrugsagainstJAK2-mutatedcells.
Inthepresentstudy,thepro-apoptoticandcytotoxic poten-tialofCR-LAAOwasexaminedinHEL92.1.7andSET-2cells withtheJAK2V617FmutationfrompatientswithPVandET whoprogressedtoacuteleukemia.
Recent studies have demonstrated that H2O2 accounts
for the biological effects of CR-LAAO, including apoptosis induction, which is an interesting therapeutic strategy to treat neoplasms.24 The increased production of H
2O2 and
other reactiveoxygen specieselicitsapoptosis bydamaging the mitochondrialmembraneand promotingthereleaseof cytochromec intothecytosol.25 Inthepresentstudy,a 12-htreatmentwithCR-LAAOsignificantlyreducedtheviability of HEL92.1.7 and SET-2 cells; it indicates that CR-LAAOis cytotoxictoJAK2V617F-positivecellsfrompatientswithPV, ET,andPMF.CatalasemitigatedthecytotoxicityofCR-LAAO, demonstratingthatH2O2playsacentralroleinthecelldeath
processinJAK2-mutatedcelllines.ThefactthattheHEL.92.1.7 cellsweremoresensitivetothecytotoxicactionofCR-LAAO thantheSET-2cellsisextremelyrelevantbecausetheformer ishomozygousfortheJAK2V617Fmutation,whichmakesit moreleukemogenicthantheSET-2cellline.Thesedataalso stress thatanalysisoftheoverall effectofCR-LAAOin dif-ferentcellularcontextscanhelptopredicttheeffectofthis toxininothermutatedcelllinesandincellsobtainedfrom JAK2V617F-positivepatientswithPV,ET,andPMF.
The mononuclear cells of healthy individuals are more resistant totheactionofCR-LAAOthan theHEL.92.1.7 and SET-2cells,whichsuggeststhatCR-LAAOselectivelyinduces cytotoxicity in tumor cell lines.26 Although the antitumor potentialofsnakevenomLAAO(SV-LAAO)hasalreadybeen reported,15thisisthefirstreportontheactionofCR-LAAOin celllinesobtainedfrompatientswithPh−MPN.
CR-LAAO alsoelicitedapoptosis inHEL.92.1.7 and SET-2 cellsbyactivatingcaspases-3,-8andcleavedPARP.Cleavage ofpro-caspase-8caneitheractivatecaspase-3orelicit apo-ptosisviathemitochondrialpathwayorviaBIDcleavage.The activationofpro-caspases-3and-8suggestsactivationofthe extrinsicpathwayandBIDcleavage.Theextrinsicapoptosis pathwayistriggeredbybindingdeathreceptorstotheir lig-andsFAS(FAS/CD95),TNF-R1and-R2,TRAILR1(DR4)andR2 (DR5),afamilyoftransmembraneproteinsbearinga cysteine-richextracellulardomainandanintracellularDD.Thesignal transductionviaDDactivatestheworkingcaspases-3,-6,and -7andelicitsthesubsequentformationofapoptoticbodies (celldeath).12
Bcr-Abltyrosine-kinase activity usedto treatpatients with CML–inBcr-Abl-positiveCMLcelllines.28Inlinewiththese data,severalstudiesreportedthatSV-LAAOsexerttheir cyto-toxicactionbyreleasingH2O2,whichcausesoxidativestress
andconsequentcelldeathintumorcelllines.26,29 However, catalaseonlypartiallyinhibitedthecytotoxicityofLAAOfrom
Bothropsleucurus.30Therefore,notonlythereleaseofH
2O2but
alsoothermechanismsmaymediatethecytotoxicactionof SV-LAAO.
In agreement with the aforementioned literature, the resultsofthepresentstudyshowthatSV-LAAOsactinH2O2
-dependent and -independent manners, depending on the cellularcontext.Althoughthe mechanism ofactionof CR-LAAOispoorlyunderstood,theresultsofthecurrentstudy indicate that this toxin is a potential antineoplastic agent againstJAK2V617F-positivecelllines,asdemonstratedbyits abilitytoinducecytotoxicityandapoptosisofHEL92.1.7and SET-2cells.Thesefindingsmayhelptodevelopnoveldrugs totreatPh−MPNaswellastoimprovecurrentknowledgeon
thesnakeenvenomationmechanism.Inaddition,LAAOscan beusefultoolstoinvestigatethebiochemicalpathwaysofthe apoptosisprocessintumorcells.
Itisalsoimportanttopointoutthatthepresent investiga-tionshowedresultsofaCR-LAAOcytotoxicitypotentialjust againstJAK2V617F-positivecelllines.Thus,inordertoconfirm theCR-LAAOtherapeuticpotentialagainstPh− MPN,future
studiesusingMPNmicemodelsandinprimarysamplesfrom myeloproliferativepatientsmustbeconductedtoevaluatethe toxicityofthedrugandtheeffectofthecompoundonprimary cells.
Conclusions
TheresultsofthisstudysuggestthatCR-LAAOhasan anti-neoplasticpotentialagainstHEL92.1.7andSET2-JAK2positive celllinesdemonstrated bytheactivation ofextrinsic apop-toticpathway,whichmaycontributetothedescriptionofnew drugsforthetreatmenttheMPN.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsthanktoSãoPauloResearchFoundation(FAPESP, grants #2011/23236-4 and 2014/19127-3)and Research Sup-portCenteronAnimalToxinsofUSP(NAP-TOXAN-USP,grant #12.1.17615.1.5).
r
e
f
e
r
e
n
c
e
s
1. CampoE,SwerdlowSH,HarresNL,PileriSA,SteinH,JaffeES. The2008WHOclassificationoflymphoidneoplasmsand beyond:evolvingconceptsandpracticalapplications.Blood. 2011;117(19):5019–32.
2. SpivakJL.Narrativereview:thrombocytosis,polycythemia vera,andJAK2mutations–thephenotypicmimicryof
chronicmyeloproliferation.AnnIntMed.2010;152(5): 300–6.
3.MilosevicJD,KralovicsR.Geneticandepigeneticalterations ofmyeloproliferativedisorders.IntJHematol.2013;97(2): 183–97.
4.JamesC,UgoV,LeCouédicJP,StaerkJ,DelhommeauF,Lacout C,etal.AuniqueclonalJAK2mutationleadingtoconstitutive signallingcausespolycythaemiavera.Nature.
2005;434(7037):1144–8.
5.VainchenkerW,DelhommeauF,ConstantinescuSN,Bernard OA.Newmutationsandpathogenesisofmyeloproliferative neoplasms.Blood.2011;118(7):1723–35.
6.JekarlDW,HanSB,KimM,LimJ,OhEJ,KimY,etal.JAK2 V617Fmutationinmyelodysplasticsyndrome,
myelodysplasticsyndrome/myeloproliferativeneoplasm, unclassifiable,refractoryanemiawithringsideroblastswith thrombocytosisandacutemyeloidleukemia.KoreanJ Hematol.2010;45(1):46–50.
7.TefferiA,PardananiA.GeneticsCALRmutationsandanew diagnosticalgorithmforMPN.NatRevClinOncol.
2014;11(3):125–6.
8.TognonR,GasparottoEP,LeroyJM,OliveiraGL,NevesRP, CarraraRC,etal.Differentialexpressionofapoptosis-related genesfromdeathreceptorpathwayinchronic
myeloproliferativediseases.JClinPathol.2011;64(1): 75–82.
9.GasparottoEP,TognonR,FerreriraAF,OliveiraGL,PalmaPV, ZanichelliMA,etal.DeregulatedexpressionofA1,BCL-2, BCL-XLandMCL-1antiapoptoticproteinsandBID,BADand
BAXproapoptoticgenesinPolycythemiaverapatients.BrazJ PharmSci.2011;47(4):873–86.
10.DejeanLM,RyuSY,Martinez-CaballeroS,TeijidoO,Peixoto PM,KinnallyKW.MACandBCL-2familyproteinsconspirein adeadlyplot.BiochimBiophysActa.2010;1797(6–7):1231–8.
11.Hail-JuniorN,KimHJ,LotanR.Mechanismsof fenretinide-inducedapoptosis.Apoptosis. 2006;11(10):1677–94.
12.JinZ,El-DeiryWS.Overviewofcelldeathsignalingpathways. CancerBiolTher.2005;4(2):139–63.
13.JabbourE,CortesJ,GilesF,KantarjianH.Currentperspectives onthetreatmentofpatientswithchronicmyeloidleukemia: anindividualizedapproachtotreatment.CancerJ.
2007;13(6):357–65.
14.ChoiCW,BangSM,JangS,JungCW,KimHJ,KimHY,etal. Guidelinesforthemanagementofmyeloproliferative neoplasms.KoreanJInternMed.2015;30(6):771–88.
15.CostaTR,MenaldoDL,SilvaCP,SorrechiaR,AlbuquerqueS, PietroRC,etal.Evaluatingthemicrobicidal,antiparasiticand antitumoreffectsofCR-LAAOfromCalloselasmarhodostoma venom.IntJBiolMacro.2015;80:489–97.
16.GuoC1,LiuS,YaoY,ZhangQ,SunMZ.Pastdecadestudyof snakevenoml-aminoacidoxidase.Toxicon.
2012;60(3):302–11.
17.RodriguesRS,SilvaJF,Franc¸aB,FonsecaFP,OtavianoAR,Silva FH,etal.StructuralandfunctionalpropertiesofBp-LAAO,a newl-aminoacidoxidaseisolatedfromBothropspauloensis
snakevenom.Biochimie.2009;91(4):490–501.
18.MacherouxP,SethO,BollschweilerC,SchwarzM,KurfürstM, AuLC,etal.l-Amino-acidoxidasefromtheMalayanpitviper
Calloselasmarhodostoma–comparativesequenceanalysisand characterizationofactiveandinactiveformsoftheenzyme. EurJBiochem.2001;268(6):1679–86.
19.ThepotS,ItzyksonR,SeegersV,RaffouxE,QuesnelB,ChaitY, etal.TreatmentofprogressionofPhiladelphia-negative myeloproliferativeneoplasmstomyelodysplasticsyndrome oracutemyeloidleukemiabyazacitidine:areporton54cases onthebehalfoftheGroupeFrancophonedes
20.SpeletasM,KatodritouE,DaiouC,MandalaE,PapadakisE, KioumiA,etal.CorrelationsofJAK2-V617Fmutationwith clinicalandlaboratoryfindingsinpatientswith
myeloproliferativedisorders.LeukRes.2007;31(8): 1053–62.
21.TognonR,GasparottoEP,NevesRP,NunesNS,FerreiraAF, PalmaPV,etal.Deregulationofapoptosis-relatedgenesis associatedwithPRV1overexpressionandJAK2V617Fallele burdeninEssentialThrombocythemiaandMyelofibrosis.J HematolOncol.2012;5:2.
22.Szyma ´nskaJ,SmolewskiP,MajchrzakA,Cebula-ObrzutB, ChojnowskiK,Treli ´nskiJ.Pro-apoptoticactivityofruxolitinib aloneandincombinationwithhydroxyurea,busulphan,and PI3K/mTORinhibitorsinJAK2-positivehumancelllines.Adv ClinExpMed.2015;24(2):195–202.
23.CailleteauC,LiagreB,BeneytoutJL.Aproteomicapproachto theidentificationofmoleculartargetsinsubsequent apoptosisofHELcellsafterdiosgenin-induced megakaryocyticdifferentiation.JCellBiochem. 2009;107(4):785–96.
24.CostaTR,BurinSM,MenaldoDL,CastroFA,SampaioSV. Snakevenoml-aminoacidoxidases:anoverviewontheir
antitumoreffects.JVenomAnimToxinsInclTropDis. 2014;20:23.
25.RakshitS,MandalL,PalBC.InvolvementofROSin chlorogenicacid-inducedapoptosisofBcr-Abl+CMLcells. BiochemPharmacol.2010;80(11):1662–70.
26.AndeSR,KommojuPR,DraxlS,MurkovicM,GhislaS, FröhlichKU,etal.Inductionofapoptosisinyeastbyl-amino
acidoxidasefromtheMalayanpitviperCalloselasma rhodostoma.Yeast.2008;25(5):349–57.
27.FungSY,LeeML,TanNH.Molecularmechanismofcelldeath inducedbykingcobra(Ophiophagushannah)venoml-amino
acidoxidase.Toxicon.2015;96:38–45.
28.BurinSM,AyresLR,NevesRP,AmbrosioL,MoraisFR, Dias-BaruffiM,etal.l-Aminoacidoxidaseisolatedfrom
BothropspirajaiinducesapoptosisinBCR-ABL-positivecells andpotentiatesimatinibmesylateeffect.BasicClin PharmacolToxicol.2013;113(2):103–12.
29.Bregge-SilvaC,NonatoMC,deAlbuquerqueS,HoPL,Azevedo ILMJ,DinizMRV,etal.Isolationandbiochemical,functional andstructuralcharacterizationofanovell-aminoacid
oxidasefromLachesismutasnakevenom.Toxicon. 2012;60(7):1263–76.
30.NaumannGB,SilvaLF,SilvaL,FariaG,RichardsonM, EvangelistaK,etal.Cytotoxicityandinhibitionofplatelet aggregationcausedbyanl-aminoacidoxidasefromBothrops