w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Allogeneic
hematopoietic
stem
cell
transplantation
in
patients
with
advanced
indolent
lymphoproliferative
disorders
Ana
Marcela
Rojas
Fonseca-Hial
a,b,∗,
Katya
Parisio
b,
Jose
Salvador
Rodrigues
Oliveira
a,baHospitalSãoPaulo,UniversidadeFederaldeSãoPaulo(UNIFESP),SãoPaulo,SP,Brazil bHospitalSantaMarcelina,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received9May2015 Accepted24February2016 Availableonline19March2016
Keywords:
Hematopoieticstemcelltransplant Indolentlymphoproliferative disorder
Reducedintensityconditioning Graft-versus-lymphoma Allogeneic
a
b
s
t
r
a
c
t
Background:Theroleofallogeneichematopoieticstemcelltransplantationforadvanced indolentlymphoproliferativedisordersremainstobeestablished.
Objective:This paperaimstodescribethe resultsofallogeneichematopoieticstem cell transplantationinpatientswithadvancedindolentlymphoproliferativedisorders. Methods:Thisarticlereportson29adultpatientssubmittedtoallogeneictransplantations from1997to2010.
Results:Most had follicular non-Hodgkin lymphoma (n=14) or chronic lymphocytic leukemia(n=12).Themedianagewas44years(range:24–53years)and65%ofpatients weremale.Only21%hadhadaccesstorituximaband45%tofludarabine.Allhadadvanced disease(stageIV)withpartialresponseorstabledisease.Mostunderwentmyeloablative conditioningn=17–59%).Inthisscenario,refractorydiseasewasobservedinseven(24%) patients,the100-daymortalityratewas17%(n=5)andrelapseoccurredinfourpatients (18%).Themaincauseofdeaththroughoutthefollowupwasrefractorydiseaseinsixof the12patientswhodied.Moderateandseverechronicgraft-versus-hostdiseasewas fre-quent;about41%of24patientsanalyzed.Theoverallsurvivalratesanddiseasefreesurvival at42monthswere56.7%and45.4%,respectively.AccordingtoKaplan–Meyeranalysis,the mediantimefromdiagnosistotransplantpredictedtheoverallsurvival;howeverage, gen-derandconditioningregimendidnotpredicttheprognosis.Itwasimpossibletoreachother conclusionsbecauseofthesmallsamplesizeinthisstudy.
Conclusions: Theroleofallogeneictransplantationsshouldbere-evaluatedintheeraof targetedtherapy.
©2016Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:Hematologia–TransplantedeMedulaÓssea,HospitalSãoPaulo,UniversidadeFederaldeSãoPaulo(UNIFESP), RuaLoefgreen,2018,SãoPaulo,SP,Brazil.
E-mailaddress:amarcela@terra.com.br(A.M.R.Fonseca-Hial).
http://dx.doi.org/10.1016/j.bjhh.2016.02.006
Introduction
HematopoieticStemcelltransplantation(HSCT)isfrequently consideredforeligiblepatientswithnon-Hodgkinlymphoma (NHL).1–6 AutologousHSCT(autoHSCT)isrecommendedfor
patientswitheitherrelapsedNHLorthoseinfirstremission asconsolidativetherapy.NHLpatientsareusuallyconsidered forallogeneic HSCT(alloHSCT) becauseofthe highrateof relapseseenevenafterchemotherapyandautoHSCT,andthe potentialbenefitofagraft-versus-lymphoma(GvL)effectafter alloHSCT.Theoutcomesforthesepatientsinlarge prospec-tivestudiesarelacking,andcurrentdirectionsandtimingof selectionforautooralloHSCTareinfluencedbyavarietyof factors,includingpatient-or disease-relatedfactors, physi-cianpreference,andinstitutionalpractices.7–10
AlloHSCTinindolentlymphoproliferativedisordersallows aGvLeffect,andtheriskofgraftcontaminationbyresidual tumorcellsisavoided.However,thehightransplant-related mortality(TRM)and nonrelatedmortality(NRM)associated withmyeloablative(MA)allo HSCTgreatlylimitstheuseof thisapproach. AlloHSCTisassociatedwithalower riskof relapse, butthis reducedriskfrequently doesnottranslate toasurvivalbenefitbecauseoftheexcessiveTRM.11–14 Allo
HSCThasbeenusedassalvagetherapyinrelapsedlymphoma afterpreviousautoHSCT;limitedsuccesswasconfinedmostly topatientsinremissionwithgoodperformancestatusanda humanleukocyteantigen(HLA)-matchedsiblingdonor.15
Reduced-intensityconditioning (RIC) regimens arebeing increasingly used in patients with NHL.5,6,16 These
lower-intensityconditioningregimensreportedlyhavelowerNRM, and can be used in older patients with comorbidities. Lower-intensityregimens forallo HSCTuse lower doses of conditioning chemotherapy and radiation and rely on an immune-mediatedGvLeffectfordiseasecontrol.17,18 Inthe
eraofemergingnoveltherapies,the actualtiming,optimal conditioningregimens,andlong-termeffectsofthetypeof stemcelltransplantationareunclear.
Inthis scenario, this paperdescribes theresults ofallo HSCTin29patientswithadvancedindolent lymphoprolifera-tivedisordersintwoinstitutionsinBrazilfrom1997to2010.
Materials
and
Methods
Twenty-nine consecutive over 18-year-old patients with advanced indolent lymphoproliferative disorders who received allo HSCT between April 1997 and October 2010 in Hospital São Paulo (Universidade Federal de São Paulo – UNIFESP) and Hospital Santa Marcelina adult transplant program,wereretrospectivelyincludedinthisstudy(Table1) bythe analysisofpatientmedicalrecords.Only21%ofthe patientswithBcellNHL receivedplanned rituximab-based chemotherapy before allo HSCT. Patients were required to have chemotherapy-sensitive disease (or nonbulky stable disease) documentedbefore allo HSCTand after induction or salvage chemotherapy. This study was approved bythe ResearchEthicsCommitteesofbothinstitutions.Allpatients provided informed consentin accordance with the Decla-ration of Helsinki. Clinical information was reviewed, and baseline characteristics were recorded, including data of
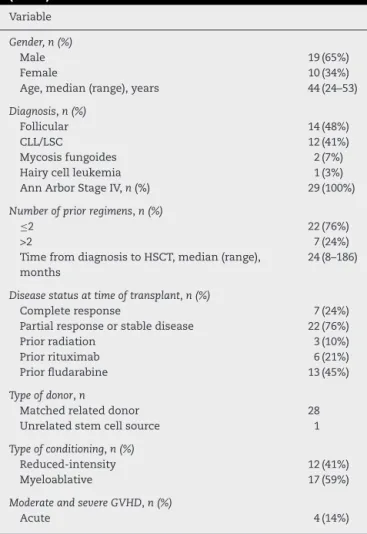
Table1–Patients,diseaseandtransplantcharacteristcs
(n=29).
Variable
Gender,n(%)
Male 19(65%)
Female 10(34%)
Age,median(range),years 44(24–53)
Diagnosis,n(%)
Follicular 14(48%)
CLL/LSC 12(41%)
Mycosisfungoides 2(7%)
Hairycellleukemia 1(3%)
AnnArborStageIV,n(%) 29(100%)
Numberofpriorregimens,n(%)
≤2 22(76%)
>2 7(24%)
TimefromdiagnosistoHSCT,median(range), months
24(8–186)
Diseasestatusattimeoftransplant,n(%)
Completeresponse 7(24%)
Partialresponseorstabledisease 22(76%)
Priorradiation 3(10%)
Priorrituximab 6(21%)
Priorfludarabine 13(45%)
Typeofdonor,n
Matchedrelateddonor 28
Unrelatedstemcellsource 1
Typeofconditioning,n(%)
Reduced-intensity 12(41%)
Myeloablative 17(59%)
ModerateandsevereGVHD,n(%)
Acute 4(14%)
CLL:Chroniclymphocyticleukemia;LSC:lymphomaofthe sper-maticcord;GVHD:Graft-versus-hostdisease.
commonpre-transplantandtransplantvariables.All patho-logicanalyseswerereviewed,anddiagnosis wasconfirmed attheinstitution.
Definitionsandresponsecriteria
Responsecriteriawerebasedontheguidelinesofthe Inter-nationalWorkshoponNon-HodgkinLymphoma.18Complete
remission(CR)wasdefinedascompleteradiologicregression ofallpreviousmeasurablediseaseandbonemarrow involve-ment.Partialresponse(PR)wasdefinedasareductionof50% inthe sumofthe productsofthe longestand perpendicu-lardiametersofmeasurablelesions.Progressionwasdefined asanincreaseof25%ormoreinthesitesoflymphomaor developmentofnewsitesoflymphomaatanytimeafter trans-plantation.Relapsewasdefinedasrecurrenceoflymphoma afteracomplete response.Basedonthesecriteria, alldata wereverifiedindividuallyregardingthebestresponsestatus beforeHSCT.
Otheroutcomesanalyzedincludeacuteandchronicgraft versus-hostdisease(GVHD)andcauseofdeath.AcuteGVHD (aGVHD)wasdefinedand gradedbasedonthepattern and severity of organ involvement using established criteria.19
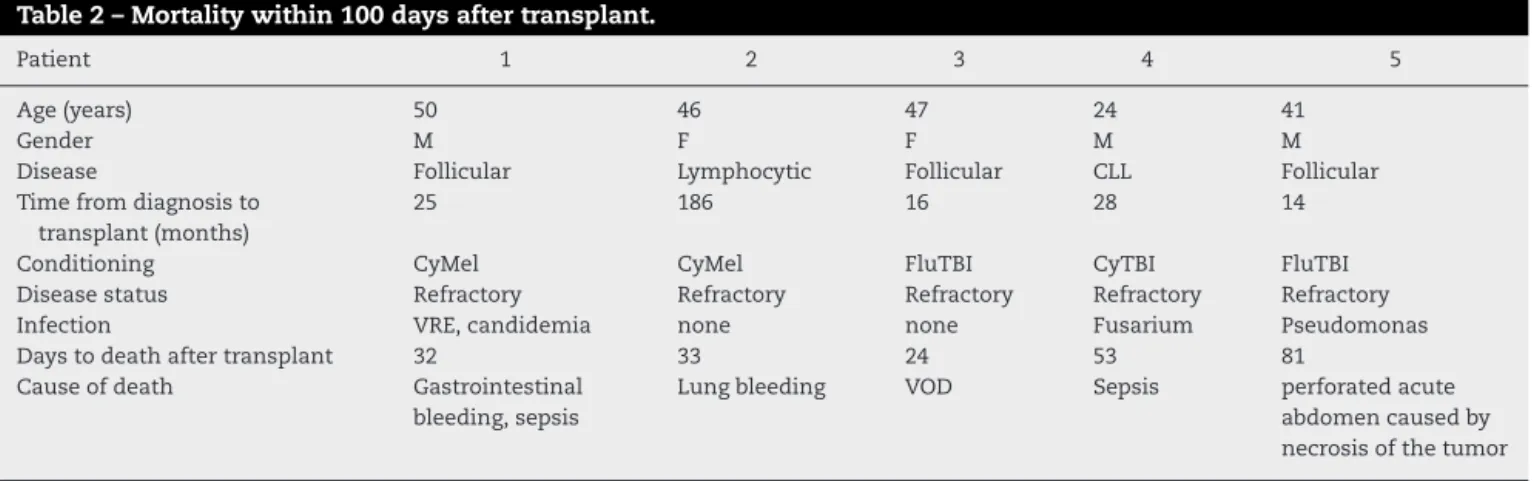
Table2–Mortalitywithin100daysaftertransplant.
Patient 1 2 3 4 5
Age(years) 50 46 47 24 41
Gender M F F M M
Disease Follicular Lymphocytic Follicular CLL Follicular
Timefromdiagnosisto transplant(months)
25 186 16 28 14
Conditioning CyMel CyMel FluTBI CyTBI FluTBI
Diseasestatus Refractory Refractory Refractory Refractory Refractory
Infection VRE,candidemia none none Fusarium Pseudomonas
Daystodeathaftertransplant 32 33 24 53 81
Causeofdeath Gastrointestinal bleeding,sepsis
Lungbleeding VOD Sepsis perforatedacute abdomencausedby necrosisofthetumor
M:male;F:female;CyMel:cytarabineandmelphalan;FluTBI:fludarabineandtotalbodyirradiation;CLL:chroniclymphocyticleukemia;VRE: vancomycin-resistantenterococci;VOD:venousoclusivedisease.
chronicGVHDbasedonclinicalcriteria.20,21Boththeseevents
weresummarizedbythecorrespondingcumulativeincidence estimate,withdeathwithoutthedevelopmentofGVHDasthe competingrisk.TheWorldHealthOrganizationcriteriawere usedtodefinethehistologicclassificationofNHLafter2001.
Transplantprocedures
Thirteen patients received RIC [fludarabine and total body irradiation(n=9),fludarabineandcyclophosphamide(n=2), fludarabine and busulfan (n=1) or carmustine, etoposide, cytarabineandmelphalan–BEAM(n=1)],and16receivedaMA regimen[cyclophosphamideandmelphalan(n=8),busulfan andcyclophosphamide(n=6),orcyclophosphamideandtotal bodyirradiation(n=2)].Therewasonlyoneunrelateddonor stemcelltransplantation.AllpatientsreceivedGVHD prophy-laxiswithacalcineurininhibitorandeithermethotrexate(MA regimen)ormycophenolatemofetil(RICregimen).
Supportivecare
All patients received standard supportive care as per institutionalguidelines.Standardantimicrobialprophylaxis, surveillancecultures,andtreatmentwereadministeredasper protocol.
Statisticalanalysis
Variables are described as medians and range for con-tinuous variables, and percentage of total for categorical variables (Table 1). Occurrenceofacuteand chronicGVHD, relapse and mortality were calculated using cumulative incidenceestimates,adjustedforthecompeting risk. Prob-abilitiesofprogressionfreesurvival(PFS)andoverallsurvival (OS)wereestimatedfromthetimeoftransplantationusing Kaplan–Meier analysis. Univariate analysis was performed withdiseaseandtransplant-relatedvariablestoseetheeffect onlong-termoutcomeofpatients.Thechi-squaretestwas usedto determinethe relationship between all categorical variables.TheMann–WhitneyUranksumtestwasusedto identifyassociationsbetweencontinuousvariablesand cat-egories.All reportedp-valuesare two-sidedwithstatistical
significancedeclaredatap-value<0.05.Statisticalanalyses were performedusingthe StatisticalPackagefortheSocial Sciencessoftware(version20;IBM-SPSS,Chicago,IL,USA).
Results
Variablesrelatedtothepatient,disease,andtransplant out-comesarepresentedinTable1.Twenty-nineeligiblepatients who underwent allo HSCT foradvanced indolent lympho-proliferativedisordersfrom January1997toDecember2010 wereincludedinthisanalysis.Themedianagewas44years (range:24–53years).HalfofthepatientshadfollicularNHL.All patientswerestagedasAnnArborIVAorIVB.Mostpatients received less than three therapeutic regimens before allo HSCT,andonly21%hadreceivedrituximab.Themediantime fromdiagnosisuntiltransplantwas24months.Seventy-six percentofpatientshadpartialresponseorstablediseaseat thetimeofthetransplant.MAconditioningwasusedin59%, especiallyinthefirstyearsofthisstudy.
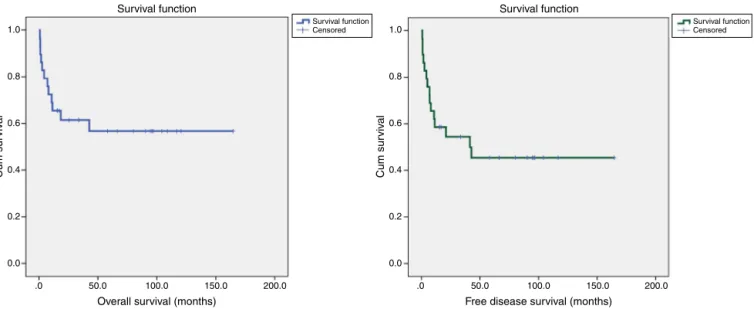
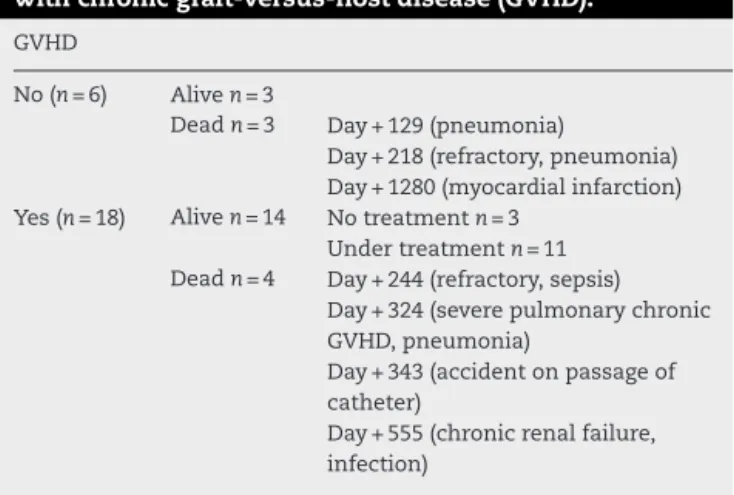
TRM was associatedwith refractory disease in the five patientsthatdiedbefore100days(Table2).Sixteenpatients had somegradeofcutaneous, hepatic and gastrointestinal aGVHD, but only four had grade III or IV (Table 3). Of 24 patients, 18 had cGVHD, although just 12 with moderate or severeone. Fourdied ofinfection or other immunosup-pression complications (Table 4). TheOS and disease free survival(DFS) at42monthswere 56.7% and45.4%, respec-tively(Figure1).Age,gender,andconditioningregimenhad no impact on the OS (p-value=0.0091, p-value=0.123 and p-value=0.290,respectively).Onlylesstimebetweenthe diag-nosisandtransplantationimprovedtheOS(p-value=0.031–
Figure2).Themediantimefromdiagnosistotransplantwas twoyears(range:8–186months).Youngerpatientshavebetter
Table3–Acutegraft-versus-hostdiseasein29patients.
Site GradeI–IV GradeIIIorIV
Cutaneous 8 2
Hepatic 8 3
Gastrointestinaltract 7 1
1.0
0.8
0.6
0.4
0.2
0.0
.0 50.0 100.0
Overall survival (months)
Cum sur
v
iv
al
1.0
0.8
0.6
0.4
0.2
0.0
Cum sur
v
iv
al
150.0 200.0 .0 50.0 100.0
Free disease survival (months) Survival function Survival function
Survival function Censored
Survival function Censored
150.0 200.0
Figure1–Overallanddiseasefreesurvivalinadvancedlymphoproliferativedisordersandnon-Hodgkinlymphomaafter
allogeneichematopoieticstemcelltransplantation.
1.0
0.8
0.6
0.4
0.2
0.0
.0 50.0 100.0
Overall survival (months) Survival functions
Cum sur
viv
al
150.0 200.0
1.0
0.8
0.6
0.4
0.2
0.0
.0 50.0 100.0
Overall survival (months) Survival functions
Cum sur
viv
al
150.0 200.0
Age (years)
≤44
≤44-censored >44
>44-censored
Time of disease (months)
≤24
≤24-censored >24
>24-censored
k_condicionam MA NMA
NMA-censored MA-censored Gender
Male Female
Female-censored Male-censored
1.0
0.8
0.6
0.4
0.2
0.0
.0 50.0 100.0
Overall survival (months) Survival functions
Cum sur
viv
a
l
150.0 200.0
1.0
0.8
0.6
0.4
0.2
0.0
.0 50.0 100.0
Overall survival (months) Survival functions
Cum sur
viv
a
l
150.0 200.0
Table4–Outcomesatfiveyearsof24patientsinrelation
withchronicgraft-versus-hostdisease(GVHD).
GVHD
No(n=6) Aliven=3
Deadn=3 Day+129(pneumonia)
Day+218(refractory,pneumonia) Day+1280(myocardialinfarction) Yes(n=18) Aliven=14 Notreatmentn=3
Undertreatmentn=11 Deadn=4 Day+244(refractory,sepsis)
Day+324(severepulmonarychronic GVHD,pneumonia)
Day+343(accidentonpassageof catheter)
Day+555(chronicrenalfailure, infection)
OSandmenhadbetterresponsesthanwomen(p-value>0.05 forboth).
Discussion
Inthepresentstudy,29patientswithadvancedindolent lym-phoproliferativedisorderswere analyzedafterallo HSCTin twotransplantcentersinBrazilbetweenApril1997and Octo-ber2010.About42monthsafterthetransplant,56.7%were aliveandtheDFSwas45.4%.
Indolentlymphoproliferativedisorders,mainlyNHL, com-priseavarietyofdiseaseswithdifferentincidencepatterns inpopulations.Approximately390,000newcasesand199,000 NHLdeathswereestimatedintheworldin2012.Therewere 9800newcasesjustinBrazilin2014,thatis,aboutfivenew casesforevery100,000inhabitants.22Likemostcancers,the
riskofNHLincreaseswithage,oftenwhenpatientsareunable togetHSCT.
Generally,treatmentofindolentNHLinBrazilisthrough theNationalHealthSystem.Accordingtothecensus depart-ment ofthe BrazilianGovernment, the Instituto Nacionalde GeografiaeEstatística(IBGE),thecoverageofhealthinsurance inBrazilis24.7%.Thiscoverisconcentratedregionally,with 64%ofinsurancein2012beinginthesoutheast.23 Thereis
adelayintheconsolidationoftheuseofnewmedicinesin the BrazilianNHS. Rituximab, forexample,widely used in patientswithindolentCD20+NHL,wasapprovedbytheU.S. FederalandDrugsAdministration(FDA)in1998.InBrazil,the authorizationtoroutinelyusethisdrugwasonlypublishedin 2009.24–26
Registry data from the European Society for Blood and MarrowTransplantation(EBMT)andtheCenterfor Interna-tionalBloodandMarrowTransplantResearch(CIBMTR)show noplateauinrelapseratesafterautografting.11,27,28
Further-more, the risk of second malignancies after auto HSCT is notinsignificant, ranging from 5% to15% inseveral stud-ies. Clinical evidence of a GvL effect after allo HSCT is suggestedby a plateau inrelapse risk that is reached2–5 yearsfollowingalloHSCT,indicatingthatasubstantial pro-portionoflymphomapatientsgetlong-termdiseasecontrol (10–12years),andrelapsesareuniformlylow.Evenpatients withrefractoryorrelapsedlymphoproliferativedisordersare
benefitedwithalloHSCT.EarlyalloHSCTproducesbetterOS andPFSrates.However,alloHSCTincreasespost-transplant complications, suchas cGVHD.Thetiming toperformallo HSCTinthesepatientsisnotwelldefined.29–31TheCIBMTR
observedgoodresultsinpatientswithNHLsubmittedtheallo HSCT.Onanalyzing1248over40-year-oldpatientsafterRICor non-myeloablativeregimens(NMA)foraggressive(n=668)or indolentNHL(n=580),itwasnotedthatalloHSCTisagood therapeuticoptionevenforover55-year-oldpatients,giving anOSof40%inthreeyears.Thereisanincreaseinprolonged remission;thereportconcludedthatalloHSCTisunderused inthisgroupofpatients.32
Inthecohortofadvancedindolentlymphoproliferative dis-orderpatientsofthisstudy,onlythetimefromdiagnosisto transplantwas significantlyimportant(p-value=0.031).The mediantimefromdiagnosistotransplantwastwoyears.The therapeuticoptionofallo HSCTinthesepatientswasonly foradvanceddisease.Afactthatmayhavecontributedtothe delayintimefromdiagnosestotransplantwastheincreased useofrituximabandpossibletransientdiseasecontrol.Atthe beginning ofthis study,whenrituximabwaslessavailable, patientswere treatedwithfirst linetherapy,and according toindication,weredirectlyreferredfortransplant.Inthelater years,thepatientstriedtoreceiverituximabthroughthecourt ofjustice,therebypostponingthetransplant.
Younger patients had better, albeit not statistically sig-nificant, responses than older patients. Womenhad worse resultsthanmen,butagainwithoutstatisticalsignificance. This could indicate that menhave better home carethan women,inthisgroup.
TheconditioningwasMAin59%ofpatients.This repre-sentstheperiodoftransitionfromusingMAtoRICinBrazil. TRMwasassociatedwithrefractorydiseaseinthefivepatients that died before 100 days than the refractoriness was the mostcommon cause oftransplant failureespeciallyin the lastyearsofthisstudywhentheRIChadbecomeroutinein transplant centers.There wasanincrease inalloHSCTfor refractoryandlong-termdisease,particularlywiththe possi-bilityofperformingtransplantsintheelderlyandinpatients withcomorbidities.Thebenefitsthatcouldhavebeenvisible withthedecreaseoftheNRM,endedupbeingmaskedbythe changeintheprofileoftransplantpatients.PossiblyRICwas notenoughtotreatrefractorydiseaseinthesecases.AMD AndersonstudyshowedexcellentDFSandOS(85%and83%, respectively)afteramedianfollow-upoffiveyearsforrelapsed follicularNHLafterRICusingfludarabine,cyclophosphamide andrituximabfollowedbyalloHSCT.Theincidencesofgrade II–IVacuteandchronicGVHDwere11%and0%,respectively. They analyzed 47 indolent NHL patients submitted toallo HSCTfromMarch1999toApril2005.17
autoHSCTiscertainlyveryattractive.Webelievethatfuture clinicaltrialsshouldaimtoconsiderthesenovelagentsinthe transplantandafterthetransplantperiod.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. PasswegJR,BaldomeroH,BregniM,CesaroS,DregerP,Duarte RF,etal.HematopoieticSCTinEurope:dataandtrendsin 2011.EuropeanGroupforBloodandMarrowTransplantation. BoneMarrowTranspl.2013;48(9):1161–7.
2. McCarthyPLJr,HahnT,HassebroekA,BredesonC,GajewskiJ, HaleG,etal.Trendsinutilizationandsurvivalafter
autologoushematopoieticcelltransplantationinNorth Americafrom1995to2005:Significantimprovementin survivalforlymphomaandmyelomaduringaperiodof increasingrecipientage.BiolBloodMarrowTranspl. 2013;19(7):1116–23.
3. ReddyN,GreerJP,GoodmanS,KassimA,MorganDS, ChinratanalabW,etal.Consolidativetherapywithstemcell transplantationimprovessurvivalofpatientswithmantle celllymphomaafteranyinductionregimen.ExpHematol. 2012;40(5):359–66.
4. ReddyN,SavaniBN.Treatmentoptionsfortransformed lymphoma:incorporatingallogeneicstemcell
transplantationinamultimodalityapproach.BiolBlood MarrowTranspl.2011;17(9):1265–72.
5. ChinratanalabW,ReddyN,GreerJP,MorganD,EngelhardtB, KassimA,etal.Immunomodulatorynonablative
conditioningregimenforB-celllymphoidmalignancies.Exp Hematol.2012;40(6):431–5.
6. KhouriIF.Allogeneicstemcelltransplantationinfollicular lymphoma.2011;24(2):271–7.
7. ChaudharyL,Kharfan-DabajaMA,HariP,HamadaniM.Is hematopoieticcelltransplantationstillavalidoptionfor mantlecelllymphomainfirstremissioninthe
chemoimmunotherapy-era?BoneMarrowTranspl. 2013;48(12):1489–96.
8. OlianskyDM,CzuczmanM,FisherRI,IrwinFD,LazarusHM, OmelJ,etal.Theroleofcytotoxictherapywithhematopoietic stemcelltransplantationinthetreatmentofdiffuselargeB celllymphoma:updateofthe2001evidencebasedreview. BiolBloodMarrowTranspl.2011;17(1):20–47.
9. OlianskyDM,GordonLI,KingJ,LaportG,LeonardJP, McLaughlinP,etal.Theroleofcytotoxictherapywith hematopoieticstemcelltransplantationinthetreatmentof follicularlymphoma:anevidence-basedreview.BiolBlood MarrowTranspl.2010;16(4):443–68.
10.PapageorgiouSG,CwynarskiK,KottaridisPD.Theroleof allogeneichematopoieticprogenitorcelltransplantationin patientswithdiffuselargeB-cellnon-Hodgkinlymphomas (DLBCL).BoneMarrowTranspl.2013;48(10):1271–8.
11.JantunenE,SuredaA.Theevolvingroleofstemcell transplantsinlymphomas.BiolBloodMarrowTranspl. 2012;18(5):660–73.
12.AyalaE,TomblynM.Hematopoieticcelltransplantationfor lymphomas.CancerControl.2011;18(4):246–57.
13.HariP,CarrerasJ,ZhangMJ,GaleRP,BolwellBJ,BredesonCN, etal.Allogeneictransplantsinfollicularlymphoma:higher riskofdiseaseprogressionafterreduced-intensitycompared tomyeloablativeconditioning.BiolBloodMarrowTranspl. 2008;14(2):236–45.
14.BacherU,KlyuchnikovE,Le-RademacherJ,CarrerasJ,Armand P,BishopMRJ,etal.Conditioningregimensforallotransplants fordiffuselargeB-celllymphoma:myeloablativeorreduced intensity?Blood.2012;120(20):4256–62.
15.WrenchD,GribbenJG.Stemcelltransplantationfor Non-Hodgkin’slymphoma.HematolOncolClinNAm. 2008;22:1051–79.
16.BlaiseD,FarnaultL,FaucherC,MarchettiN,FürstS,ElCheikh J,etal.Reduced-intensityconditioningwithFludarabin,oral Busulfan,andthymoglobulinallowslong-termdisease controlandlowtransplant-relatedmortalityinpatientswith hematologicalmalignancies.ExpHematol.
2010;38(12):1241–50.
17.KhouriIF,McLaughlinP,SalibaRM,HosingC,KorblingM,Lee MS,etal.Eight-yearexperiencewithallogeneicstemcell transplantationforrelapsedfollicularlymphomaafter nonmyeloablativeconditioningwithfludarabine,
cyclophosphamide,andrituximab.Blood.2008;111(12):5530–6.
18.ChesonBD,PfistnerB,JuweidME,GascoyneRD,SpechtL, HorningSJ,etal.Revisedresponsecriteriaformalignant lymphoma.JClinOncol.2007;25(5):579–86.
19.PrzepiorkaD,WeisdorfD,MartinP,KlingemannHG,BeattyP, HowsJ,etal.1994ConsensusConferenceonacuteGVHD grading.BoneMarrowTranspl.1995;15(6):825–8.
20.ShulmanHM,SullivanKM,WeidenPL,McDonaldGB,Striker GE,SaleGE,etal.Chronicgraft-versushostsyndromeinman. Along-termclinicopathologicstudyof20Seattlepatients. AmJMed.1980;69:204–17.
21.AraiS,JagasiaM,StorerB,ChaiX,PidalaJ,CutlerC,etal. Globalandorgan-specificchronicgraft-versus-hostdisease severityaccordingtothe2005NIHConsensusCriteria.Blood. 2011;118(15):4242–9.
22.InstitutoNacionaldeCâncerJoséAlencarGomesdaSilva: Estimativa2014,incidênciadecâncernoBrasil,Linfomanão Hodgkin[cited2September2014].Availablefrom:
http://www.inca.gov.br/estimativa/2014/sintese-de-resultados-comentarios.asp.
23.InstitutoBrasileirodeGeografiaeEstatística.Pesquisa NacionalporAmostrasdeDomicílios.Availablefrom:[cited 15September2014].http://www.ibge.gov.br/home/estatistica/ populacao/trabalhoerendimento/pnad98/saude/analise.shtm. 24.LegetGA,CzuczmanMS.Useofrituximab,thenew
FDA-approvedantibody.CurrOpinOncol.1998;10(6):548–51.
25.BaerIiWH,MainiA,JacobsI.Barrierstotheaccessanduseof rituximabinpatientswithNon-Hodgkin’slymphomaand chroniclymphocyticleukemia:aphysiciansurvey. Pharmaceuticals(Basel).2014;7(5):530–44.
26.SecretariadeCiência,TecnologiaeInsumosEstratégicos. ParecerTécnico:ousodoRituximabenotratamentodo Linfomanão-Hodgkin,decélulaB,baixograuCD20.Brasília: MinistériodaSaúde;2009.
27.PasquiniMC,WangZ.Currentuseandoutcomeof
hematopoieticstemcelltransplantation:CIBMTRSummary Slides,2012[cited19May2013].Availablefrom:
http://www.cibmtr.org.
28.PeniketAJ,RuizdeElviraMC,TaghipourG,CordonnierC, GluckmanE,deWitteT,etal.AnEBMTregistrymatched studyofallogeneicstemcelltransplantsforlymphoma: allogeneictransplantationisassociatedwithalowerrelapse ratebutahigherprocedure-relatedmortalityratethan autologoustransplantation.BoneMarrowTranspl. 2003;31(8):667–78.
29.RezvaniAR,SandmaierBM.Allogeneichematopoieticcell transplantationforindolentnon-Hodgkinlymphoma: indicationsandoutcomes.CurrOpinHematol. 2013;20(6):509–14.
31.RezvaniAR,StorerB,MarisM,SorrorML,AguraE,MaziarzRT. Nonmyeloablativeallogeneichematopoieticcell
transplantationinrelapsed,refractory,andtransformed indolentNon-Hodgkin’slymphoma.JClinOncol. 2008;26(2):211–7.
32.McCluneBL,AhnKW,WangHL,AntinJH,ArtzAS,CahnJY, etal.Allotransplantationforpatientsage≤40yearswith