w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Special
article
Guidelines
on
neonatal
screening
and
painful
vaso-occlusive
crisis
in
sickle
cell
disease:
Associac¸ão
Brasileira
de
Hematologia,
Hemoterapia
e
Terapia
Celular
Project
guidelines:
Associac¸ão
Médica
Brasileira
–
2016
Josefina
Aparecida
Pellegrini
Braga
a,
Mônica
Pinheiro
de
Almeida
Veríssimo
b,
Sara
Teresinha
Olalla
Saad
c,
Rodolfo
Delfini
Canc¸ado
d,e,
Sandra
Regina
Loggetto
f,∗aEscolaPaulistadeMedicina,UniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil bCentroInfantilBoldrini,Campinas,SP,Brazil
cFaculdadedeCiênciasMédicas,UniversidadeEstadualdeCampinas(Unicamp),Campinas,SP,Brazil dFaculdadedeCiênciasMédicasdaSantaCasadeSãoPaulo(FCMSCSP),SãoPaulo,SP,Brazil eHospitalSamaritano,SãoPaulo,SP,Brazil
fCentrodeHematologiadeSãoPaulo(CHSP),SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12February2016
Accepted4April2016
Availableonline8April2016
Introduction
Theguidelines projectisajoint initiative ofthe Associac¸ão
MédicaBrasileiraandtheConselhoFederaldeMedicina.Itaims
to bring together information in medicine to standardize
conductinordertohelpdecision-makingduringtreatment.
The data contained in this article were prepared by and
∗ Correspondingauthorat:AvenidaBrigadeiroLuísAntônio,2533,JardimAmérica,01401-000SãoPaulo,SP,Brazil.
E-mailaddress:loggetto.sr@hotmail.com(S.R.Loggetto).
are recommended by the Associac¸ão Brasileira de
Hematolo-gia,HemoterapiaeTerapiaCelular(ABHH).Evenso,allpossible
medical approaches should be evaluated by the physician
responsiblefortreatmentdependingonthepatient’s
charac-teristicsandclinicalstatus.
Thisarticlepresentstheguidelinesonneonatalscreening
andpainfulvaso-occlusivecrisisinsicklecelldisease(SCD).
http://dx.doi.org/10.1016/j.bjhh.2016.04.001
1516-8484/©2016Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.PublishedbyElsevierEditoraLtda.Thisisan
Description
of
the
method
used
to
gather
evidence
TheseGuidelineswerepreparedbyelaboratingeightclinically
relevantquestions,tworelatedtoneonatalscreeningforSCD
andsixrelated topainfulvaso-occlusive crisisinSCD. The
questionswere structuredusingthe Patient/Problem,
Inter-vention,ComparisonandOutcome(PICO)system(Appendix
A),allowingthegenerationofevidencesearchstrategiesin
the key scientific databases (MEDLINE PubMed, Lilacs,
Sci-ELO, Embase, CochraneLibrary, Premedline via OVID). The
datarecoveredwerecriticallyanalyzedusingdiscriminatory
instruments(scores)accordingtothetypeofevidence–JADAD
forrandomizedclinicaltrialsandtheNewcastleOttawascale
for non-randomized studies.After identifying studies that
potentially substantiaterecommendations,thelevel of
evi-denceanddegreeofrecommendationwerecalculatedusing
theOxfordClassification.1
Degree
of
recommendation
and
level
of
evidence
A:Majorexperimentalandobservationalstudies
B:Minorexperimentalandobservationalstudies
C:Casereports(non-controlledstudies)
D:Opinionwithoutcriticalevaluationbasedonconsensus,
physiologicalstudiesoranimalmodels
Background
SCDisagroupofinheriteddiseasesinwhichthesynthesisof
hemoglobin(Hb)isimpairedbecauseofamutationinthebeta
globinchainoftheHbgeneonchromosome16.This
muta-tionleadstothesubstitutionofaglutamicacidforvalineat
position6ofthebetachain,resultingintheproductionofHb
Swhoseexpressioncausessicklingofredbloodcells,
poly-merizingHbwithresultingvaso-occlusion,painandchronic
organdamage.1–3(D)HbSisthemostcommonabnormalHb
inBrazil.2(D)
Sicklecellanemiaoccurswhenthepatientishomozygous
for the Hb S gene (Hb SS). Moreover, Hb S can be
associ-atedwithotherabnormalHb,suchasHbS/beta-thalassemia,
Hb SC, Hb SD, persistence of Hb fetal (Hb F) with Hb S,
amongothers. Theterm SCD defines both sickle cell
ane-miaand these associations. Thecombination ofthe Hb S
genewithnormalHb(HbA)characterizesthesicklecelltrait
(HbAS).1–3(D)
ThediagnosisofSCD,aconditionwithhighmorbidityand
mortality rates, is made atbirth with neonatal screening.
Thepathophysiologyiscomplexandevolveswithacuteand
chroniccomplicationsthat affect differentorgans and
sys-tems.
BecauseofthecomplexityofSCD,manyquestionswere
askedduringthedevelopmentoftheseguidelinesandsoit
was decidedtopresent them in threeparts. Thefirst part
discusses diagnosis by neonatal screening and aspects of
vaso-occlusivecrisis,thesecondpartanswersquestionsabout
splenicsequestrationand thecentralnervous systemfrom
diagnosis to treatment and the third part deals with the
preventionofinfections,diagnosisandtreatmentoffever,
pri-apismandbonemarrowtransplantation.
Objective
Theaimofthefirstpartoftheseguidelinesistheapproachto
diagnosisbyneonatalscreeningandsubsequentconfirmation
ofSCDandquestionsrelatedtothediagnosisandtreatment
ofthevaso-occlusivecrisis.
What
is
the
prevalence
of
sickle
cell
disease
and
how
are
the
results
of
neonatal
screening
for
hemoglobinopathies
interpreted?
P:Allnewbornbabies
I:Resultsofneonatalscreening
C:
O:Interpretationoftheresults
The laboratory techniques used to identify hemoglobin
in the Newborn Screening Program are high-performance
liquid chromatography(HPLC)and isoelectricfocusing(IEF)
because thesetestscanquantifysmallamountsofHb;the
main Hb in the newbornis Hb F.4–16 (A),17 (B) Several
dis-eases areinvestigatedduringneonatalscreening, including
SCD,phenylketonuriaandcongenitalhypothyroidism.4–8,12–16
(A) The Hb with the highest concentration is shown
first in the neonatal screening results and so Hb F will
alwaysbefollowedbytheotherhemoglobins.4–6,14(A)1–3(D)
Table 1showshow tointerpret neonatalscreeningresults.
The most common hemoglobinopathies in Brazil are Hb
FAS, Hb FS, Hb FSA, Hb FSC, Hb FSD and Hb FSA+Hb
Bart’s.
TheprevalenceofHbSinBrazilisfrom1.2to10.9%
depend-ingontheregionofthecountry,5,6,13–16(A),17–19(B)whilethe
prevalence of Hb FS (sickle cell anemia) ranges from <0.1
to 0.2%6,7,13–16 (A)17,19 (B) and Hb FSC ranges from <0.1 to
0.9%.5–7,14,15(A)17,18(B)Itisnoteworthythatthestateswiththe
highestnumberofcasesofSCDareBahiaandRiodeJaneiro,
whileParanáand RioGrandedoSulhavethelowest rates.
TheprevalenceofHbCisfrom 0.15to7.4%.5–7,14,15 (A)17–19
(B). HbS/beta-thalassemiahasbeenidentifiedinthestates
ofSãoPaulo,MinasGerais,ParanáandRioGrandedoSul,all
withprevalences<0.1%.5,6,14,16(A)InthecityofRibeirãoPreto,
SãoPaulotheincidenceforsicklecellanemiawasshownto
be1:7,358andforHbSCdisease1:9,365.7(A)InRioGrande
doSulthe incidenceofSCD (HbSS, Hb SC,HbSD and Hb
S/beta-thalassemia)was1:9,1206(A)andinMinasGeraisthe
incidences of Hb FS and Hb FSC were 1:2,800and 1:3,450,
respectively.14(A)
InFrance,SCDprevalencetendstobehigherinthe
pop-ulationofinfantsborntoparentsfromSub-SaharanAfrica,
the Mediterranean, the Arabian Peninsula, French islands
and Indiacompared tothegeneralpopulation(3:10,000 vs.
1:10,000).20 (B) InBrazil,theUSA and theUnitedKingdom,
Table1–Interpretationofneonatalscreeningtestforhemoglobinopathies3(D).
Result Interpretation Clinicalcondition
FAa Normal Asymptomatic
FAS Sicklecelltrait Asymptomatic
FS Sicklecellanemia(HbSS)or
HbS/Beta0-thalassemiaor HbS/HPFH
Hemolyticanemia
FSAorFSb HbS/Beta+-thalassemia Hemolyticanemia
FSC HbSC Hemolyticanemia
FSD HbSD Hemolyticanemia
FSA+HbBart’s HbS/alpha-thalassemia Hemolyticanemia
FSE HbSE Hemolyticanemia
FSVc HbSV Hemolyticanemia
FAC HbCtrait Asymptomatic
FC HbCor
HbC/beta0-thalassemia
Hemolyticanemia
FCA HbC/beta+-thalassemia Hemolyticanemia
FAD HbDtrait Asymptomatic
FD HbD Hemolyticanemia
FDA HbD/beta+-thalassemia Hemolyticanemia
FA+HbBart’s(1–5%) Silentcarrierof
alpha-thalassemia
Asymptomatic
FA+HbBart’s(5–10%) Alpha-thalassemiatrait Mildanemia
FA+HbBart’s(25–50%) HbHdisease Hemolyticanemia
F 0-thalassemia
(thalassemiamajor)–by high-performanceliquid chromatography
Hemolyticanemia
HPFH:hereditarypersistenceoffetalhemoglobin.
a FAbecausefetalHbispredominantatbirth;theresultofthalassemiaminorisalsoHbFA.
b HbFSAisHbSassociatedwithbeta-thalassemia.However,ifthepercentageofHbAisverylow,thephenotypeinneonatalscreeningmay
beHbFS.
c FSVindicatesHbvariantsdifferentfromHbA,HbS,HbC,HbE,HbDandHbBart’s.ThefollowingHbvariantshavebeenidentifiedinBrazil:
HbWoodville,HbChad,HbG-Phil,HbE-Saskatoon,HbRichmond,HbO-Arab,HbBeckman,HbHope.
characteristics.4,10 (A),2 (D) Screening is also universal in
Belgium.11(A)
After the diagnosis of SCD, children and their families
shouldbeprovidedspecialcare,asthereisapossibility of
othercasesofSCDinthefamily.4,8,11,13,15(A),21,22(C)
The interpretation of newborn screening for sickle cell
anemia depends on the initial tests and the confirmation
method used. The proper interpretation and use of these
resultsdependsontheimplementationexperienceof
neona-talscreeningprograms.8(A)
TheNeonatalScreeningProgramforhemoglobinopathies
inBrazilhashadagreatimpactonSCDmortalityand
mor-bidityrates,asearlydiagnosispermitstheuseofprophylactic
antibiotictherapy,specialvaccinationsandthetrainingof
par-entsandcaregiversabouttheclinicalcharacteristicsofSCD,
suchasspleenpalpationforsplenicenlargement.23(D)
Recommendation: The results of neonatal screening
forhemoglobinopathiesshouldbeinterpretedbasedon tests made using HPLC or IEF. As Hb is expressed in decreasingorderofconcentration,HbFwillalwaysbethe firsttobereportedintheresults,followedbyatleastone otherHb.
Is
there
evidence
for
the
need
to
perform
a
confirmatory
electrophoresis
exam
after
the
sixth
month
of
life?
P: Over 6-month-old patients and abnormal hemoglobin resultsinneonatalscreening
I:Bloodcollectionforhemoglobinelectrophoresis C:Resultsofneonatalscreening
O:Interpretationoftheresults
The diagnosis of hemoglobinopathies needs to be con-firmedwhenthechildisaboutsixmonthsoldand,insome situations,astudyoftheparentsormolecularanalysis(DNA) ofthechildshouldbemade.1,2(D),4,6–8,10,11,14–16(A)
Positiveresultsshouldalwaysbeconfirmed.4,5,10–12,24 (A)
Thecombinationofmethods(IEFandHPLC)reducesthe
pos-sibilityoffalsenegativeresultsforSCD,whichcanoccurin
casesofred blood celltransfusions priortosample
collec-tionorprematurity.4,9(A)Falsepositiveresultsforsicklecell
anemiacanbefoundintherarecombinationofHbSandHb
HopedetectedbyIEF.InFrance,twocasesin42infantswith
suspectedHbSS,actuallyhadHbS/HbHope.25(B)
A Brazilian study of 4,635 children from Minas Gerais
screening showed 0.6% of discordant results between the
initialscreeningand anIEFexam after sixmonths oflife.
Sevencaseshadhadbloodtransfusionsbeforeblood
collec-tion,sevencaseshadproblemsinbloodcollectionorinthe
transcriptionoftheexamresults,therewasdifficultyto
dif-ferentiatebetweenHbSandHbDineightcasesandthereason
wasnotidentifiedinfivecases.14(A)
Recommendations: All children identified as having
hemoglobinopathiesduringneonatalscreeningshould be retested by hemoglobin electrophoresis after six monthsoflife.
Is
there
evidence
on
the
factors
that
cause
a
vaso-occlusive
crisis?
P:Patientsbetween0and18yearsoldwithsicklecellanemia andpainfulcrisis
I:Fever,dehydration,infection,metabolicdisorder,exposure toextremecoldandheat,alcoholism,osteomyelitis,illegal drugs(marijuana,cocaine)
C:Withoutsymptoms
O:Triggersofvaso-occlusivecrisis
Several factors have been associated with the vaso-occlusive crisis in patients with SCD, such as infections, climatechange,psychologicalfactors,altitude,acidosis,sleep apnea,stress,dehydration,hypoxiaandphysicalexhaustion. However,inmostcasesthetriggeringfactorisnotidentified.26 (D)
Pain crises in these patients have variable intensities
and frequencies, being higher in winter. Higher
tempera-turesduringwinterwereassociatedwithlesspainintensity
andfrequency.27(B)However,anotherstudydidnotconfirm
the association between changes in temperature and pain
frequency.28(B)Moreover,thehospitalizationrateforpain
cri-sisincreaseswiththeintensityofwindandairhumidity.29,30
(B)
Somefactorsincreasethelikelihoodofhospitalizationfor
painfulcrisisinpatientswithSCD.Inamultivariateanalysis
offactorsassociatedwithcrisis,increasedriskof
hospitaliza-tionwasobservedinsubjectswithHbSS(hazardratio:3.1),in
thoseexposedtosmoking(hazardratio:1.9)andthosewith
ahistory ofasthma (hazard ratio:1.3).31 (B)Another study
demonstratedtheimportanceofrespiratorysymptomsand
asthmainpain crisis.32 (B)Anassessmentofnocturnal O
2
saturationandpainfulcrisisin90patientswithsicklecell
ane-miaconcludedthatanocturnalO2saturation<90%(p-value
<0.0001),Hbbelow8.8g/dL(p-value<0.01)andahematocrit
below28%(p-value<0.0012)areassociatedwiththeonsetof
symptoms.33(B)
Otherclinicalsituationsareassociatedwithpaincrisisin
patientswithSCD, including high bloodviscosity34 (B)and
menstruation(61.5%ofcasesofcrisisinwomenoccurduring
menstruation).35(C)
Recommendation: Factors associated with pain
cri-sis can be environmental, suchas temperature, wind and humidity or clinical such asrespiratory diseases, increased blood viscosity, anemia and menstruation. Smokingincreasestheriskofhospitalizationandsome infections increase the risk of having apainful vaso-occlusivecrisis.
Is
there
evidence
that
the
use
of
pain
assessment
scales
is
a
good
method
to
monitor
pain
related
to
vaso-occlusive
crisis?
P:Patientsbetween0and18yearsoldwithsicklecellanemia andpainfulcrisis
I:Followinguptreatmentusingpre-establishedpainscales C: Following up treatment without using pre-established painscales
O:Adequatepaincontrol
Thereareseveralchallengestopainmanagementinsickle cell anemia, such as disregarding the level of pain felt by patients,thedifficultyto‘quantify’thispain,thebest instru-menttoassesspain,thediscrepancybetweenpainandpatient behavior,theinadequateprescriptionofanalgesiaandthefear thatthepatientbecomesdependentonopioids.Onlyby eval-uatingthetrueintensityofthepain,isitpossibletoofferthe besttreatment.36(B)
Three methodsare used toevaluatepainintensity. The
African-American Oucher scale is designed for children
between 3 and 12 years and gives scores of0–100 for the
intensityofpain;itconsistsofaseriesofpicturesofchildren
expressing different levels ofpain. TheWong-Baker FACES
scaleusespainscoresbetween0and5,andcanbeusedin
over3-year-oldchildren;itiscomprisedofaseriesofdrawings
offacesexpressingdifferentlevelsofpain.Thevisualanalog
scale(VAS)usesahorizontal10-cmlineonpaper,whereone
endisdesignatedasnopainandtheotherisdesignatedas
extremepain;thepatientindicatesatwhatlevelhis/herpain
isonascaleof0–10.37(B)
Acriticalissueinthemanagementofpaininsicklecell
anemia is precisely which scale is most appropriate. An
assessmentofthepainof100childrenwithsicklecellanemia
and vaso-occlusivecrisisusingthethreemethods
(African-AmericanOucherscale,VASandtheWong-BakerFACESscale)
showedthattheFACESandOucherscaleswereequallyvalid
andreliableasinstrumentstoevaluatepain,but56%of
chil-drenand adolescentspreferredthe FACESscale.Thevisual
analogscalehadthelowestdegreeofreliability.37(B)
Aretrospectivestudyof3-to21-year-oldpatientsusedthe
VAS tocompare152episodesofpainduetovaso-occlusion
in77 patientswithSCDversus221episodesofpainin219
patientswithlong bonefracture. Thepain scoreswere
sig-nificantlyhigherinthechildrenwithpainfulvaso-occlusive
crisis(7.7±2.5vs.6.7±3.0;p-value=0.005).InSCDpatients,
therewasnorelationshipbetweenanypainassessmentscale
A retrospective study of 279 episodes of painful
vaso-occlusivecrisis(initiallytreatedwithonedoseofmorphine)
in105over 8-year-old childrenwithSCD foundthat
appli-cationofthe Wong-BakerFACES painscale (0–5)canguide
analgesiamanagement.Theinitialscorewashigherin
hos-pitalizedcomparedtonon-hospitalizedchildren(4.4vs.3.9;
p-value=0.002). The FACES scale allowed a more accurate
assessmentofthenecessityofhospitalizationinover
8-year-oldSCDchildrenwithpainfulcrisiswhowerebeingtreated
withmorphine.39(B)
Aprospectivestudyof232over16-year-oldSCDpatients
whoself-reportedpaineverydayduringsixmonthsusinga
painscalebetween0and9,reportedthatthemeanpain
inten-sityincreasedasthepercentageofdayswithpainincreased
(p-value<0.001).Painwasreportedon56%ofthetotal
patient-days; pain crises without attending amedical service was
reportedon13%ofthedaysandmedicalcarewasusedonly
in3.5%ofthedays.About30%ofpatientsreportedpainon
morethan95%ofthedays.Basedinthescale,theuseof
opi-oidswashigherondayswithmorepain(p-value<0.001).Thus,
thepainscalealsoallowscontrolofpainoutofthehospital
withouttheindiscriminateuseofopioids.40(B)
Themedicationquantificationscale(MQS)associatedwith
apainscaleappliedin27SCDchildrenhospitalizedforpainful
crisis,allowsmonitoringregardingtheuseofanalgesicsand
painintensity.Inthisprospectivestudy,59.3%describedthe
onsetofpainassuddenandthatpaincontinuedtobeconstant
for70.4%ofpatientsfromthetimeofonsetuntiladmission
tothehospital.UsingtheAfrican-AmericanOucherscale,the
meanscoreofpainintensityonthedayofhospitalizationwas
84±9.9(range:63.8–100),andtheinitialmeanscoreoftheMQS
was15.7±4.9(range:6–24).Afterdrugtherapy(morphinewas
themostfrequent),thisscoredropped1.2±0.5pointsforeach
dayofhospitalization(range:0.9–2.5;p-value<0.0001).There
wasacorrelationbetweenthepainlevelanddrugdose;the
MQSprovedtobeasensitiveandusefultooltoquantifythe
utilizationofanalgesicsinSCD.41(B)
ThepainlevelmeasuredusingtheVAS(scoresof0–100)
in74SCDadultswithvaso-occlusivecrisisidentifiedamean
scoreof80[95%confidenceinterval(CI):75.99–82.95]onarrival
athospital.Thereductioninpainfollowingtheuseof
anal-gesics was monitoredusing the VAS. Inpatients reporting
a significant improvementin pain, the change in the VAS
was23.4(95%CI:15.4–31.4),whileitwasonly13.5(95%CI:
11.25–15.74)inthoseinwhomtheimprovementwasminimal.
Thestudyconcludedthattheminimumclinicallysignificant
scoretoassessimprovementinpainduringtreatmentwith
analgesiawas13.5;thisalsoallowsanevaluationofpainrelief
inadultswithSCD.42(B)
Apainintensityscale(numericratingscale)wasusedto
predicthospitalizationof65patientsagedbetween13and53
yearsold(mean:23years)withSCDandvaso-occlusivecrisis
and80acuteeventsthatresultedin49hospitalizations.The
meaninitialscoreforpain was8.5inhospitalizedpatients
and5.1forthosewhoweredischarged(p-value<0.001).When
consideringascoreof6.5asthecutoffpoint,thesensitivity
was0.886andspecificitywas0.762,withpositiveandnegative
predictivevaluesof0.886and0.760,respectively.Therefore,
the pain score allows an indication for hospitalization.43
(B)
In theassessmentofpain in17SCD childrenusing the
FACESscale,thelevelofpainpredictedthelengthof
hospi-talization.Childrenwithahighscore(>2)24hafterreceiving
medicationsspentmoretimeinhospital.44(B)
Recommendation:TheuseofpainscalesinSCDpatients withvaso-occlusivecrisiscanguidetreatment,monitor responseandpredicthospitalization.
What
is
the
best
sequence
of
medications
to
control
painful
vaso-occlusive
crisis?
P:Patientsbetween0and18yearsoldwithsicklecellanemia andpainfulcrisis
I:Treatmentwithmorphineversusmeperidine C:Treatmentwithparacetamolordipyrone O:Adequatepaincontrol
The treatment of painful vaso-occlusive crisis in SCD patients involves correcting triggering factors, such as hypoxia,infection,acidosis,dehydration,physicalexhaustion andexposuretoextremecold.Themanagementofpainful cri-sisisbasedonnon-randomizedclinicalstudies45,46(A),47(B),
andmainlyconsistsofhydrationandanalgesia.48 (A)Thus,
manyguidelineshavebeenwrittentoprovidesupportinthe
treatmentofpaininsicklecellanemia.49,50(D)
Theproperuseofanalgesicsisimportantandthe
prescrip-tion should suitthe intensityofpain, witha fixeddoseat
specificintervals,andnotusingmedications‘asnecessary.’
Theuseofmedicationsforpainmanagementinover
12-year-old childrenand adults should follow the threesteps
oftheanalgesiascale (Table2)recommendedbytheWorld
HealthOrganization(WHO)51,52(D).
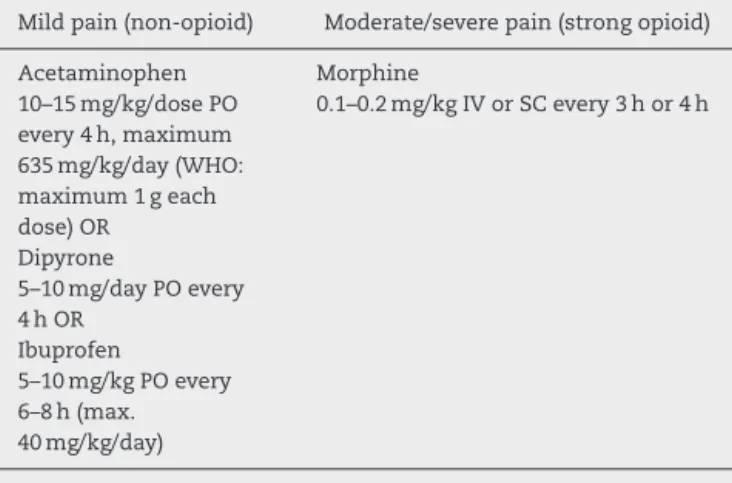
For under 12-year-oldchildren,the use ofdrugs forthe
managementofpainisalittledifferentbecausetheanalgesia
scale,asrecommendedbyWHOguidelinespublishedin2012,
hasonlytwosteps(Table3)53(D).
Thetwo-stepapproachconsiderstheuseoflowdosesof
strongopioidanalgesicsforthetreatmentofmoderatepain
becauseitisnotsafetousecodeineinchildrendueto
prob-lems relatedtogeneticvariabilityinbiotransformation and
insufficientdatafromclinicalstudiesinchildrenusingother
intermediateopioidssuchastramadol.53(D)
Treatment ofmildto moderatepain inover 12-year-old
childrenand adultsshould startwithanon-opioiddrugat
therecommendeddoseandfrequencytogetherwithadjuvant
medicationsasnecessary.Ifthepaindoesnotstop,addaweak
opioid.Iftheweakopioidcombinedwithanon-opioiddrug
doesnotcontrolthepain,theweakopioidshouldbereplaced
byastrongopioid.48(A)49,52(D)
Inunder 12-year-oldchildrenwithmildpain, treatment
shouldstartwithanon-opioiddrugattherecommendeddose
andfrequency;ifpaindoesnotimprove,alowdoseofastrong
opioidshouldbeaddedwiththe dosebeingincreasedonly
ifthepaindoesnotimprove.Afterastartingdoseaccording
Table2–StepsofpainmanagementlistedintheanalgesiascaleasrecommendedbytheWorldHealthOrganization– over12-year-oldchildrenandadults51,52(D).
Mildpain Visualpainscale(1–4)
Moderatepain Visualpainscale(5–7)
Severepain Visualpainscale(8–10)
Non-opioid Weakopioid Strongopioid
Aspirin
30–60mg/kg/dayPOevery4h maximum3.6g/24h OR
Acetaminophen
10–15mg/kg/dosePOevery4h maximum635mg/kg/day OR
Dipyrone
5–10mg/dayPOevery4h
Codeine
0.5–0.75mg/kg/dosePOevery4h
Codeine
0.75–1mg/kg/doseIVorPOevery 4h
OR Morphine
0.1–0.2mg/kgIVorSCevery3hor 4h
OR Tramadol 0.1–0.25mg/kg/hIV
Ibuprofen
5–10mg/kgPOevery6hto8h
Tramadol 0.5mg/kgIV
maximum5mg/kg/dayevery6h
PO:peros;IV:intravenous;SC:subcutaneous.
Table3–Stepsofpainmanagementlistedinthe analgesiascalerecommendedbytheWorldHealth Organization–under12-year-oldchildren53(D).
Mildpain(non-opioid) Moderate/severepain(strongopioid)
Acetaminophen 10–15mg/kg/dosePO every4h,maximum 635mg/kg/day(WHO: maximum1geach dose)OR
Dipyrone
5–10mg/dayPOevery 4hOR
Ibuprofen
5–10mg/kgPOevery 6–8h(max. 40mg/kg/day)
Morphine
0.1–0.2mg/kgIVorSCevery3hor4h
PO:peros;IV:intravenous;SC:subcutaneous.
beadjustedtothelevelthatiseffective(withnomaximum dose).Themaximumincreaseindosageis50%every24hin outpatientsettings.53(D)
Do not use meperidine because, besides having one
tenth ofthe analgesic power ofmorphine, it is associated
withserious adverseevents,suchas seizuresand physical
dependence.49,50,54,55(D)
Intravenous morphine is considered the treatment of
choicefor the severe pain crisis insickle cell anemia, but
itisassociatedwithseveraladverse eventsincludingacute
chest syndrome. Patient-controlled administration of
mor-phinecomparedtocontinuoususewasnomoreeffectivein
respecttopainmanagementandlengthofhospitalstay,butit
significantlyreducedopioidconsumptionandproducedfewer
adverseevents.56(B)
A randomized, double blind, parallel-group study
com-pared the use oforal versus continuous intravenous
mor-phine.Initialpainmanagementusedintravenousmorphine
(upto0.15mg/kg).Subsequently,acomparisonbetweenoral
morphine(1.9mg/kgevery12h)andcontinuousintravenous
morphine (0.04mg/kg/h)wasmade forthe managementof
painepisodesinsicklecellanemiapatients;therewasno
dif-ference inthe resolutionofpain northe timeofanalgesic
administration.Theneedforrescueanalgesiawassimilar,as
weretheadverseeffects.Oralmorphinemaybeanalternative
totheuseofcontinuousintravenousmorphine.57(B)
Theuseoforalorintravenousmethylprednisoneatadose
of15mg/kgintwodosesinthefirst24hcombinedwith
mor-phine can produce benefits to reduce the time needed to
resolvepaininepisodesofseverepain.However,theuseof
corticosteroidsisassociatedwithareboundeffectwiththe
worsening ofpainwhenits administrationisstopped, and
itsindicationmustbecarefullyconsidered.58 (B)Theuseof
corticosteroidsfavorspainfulcrisiswhenusedinacutechest
syndrome.59(D)
Theuseofnitricoxideinthetreatmentofpainful
vaso-occlusivecrisisofhospitalizedsicklecellanemiapatientsdoes
notshortenthetimerequiredforcrisisresolution,doesnot
reducethelengthofhospitalization,doesnotaffectthe
inten-sity ofpain or chestpain, and does notreduce the useof
opioids.60(A)
Recommendation: Painmanagement ofvaso-occlusive
Is
there
evidence
for
the
use
of
adjuvant
medications
such
as
non-steroidal
anti-inflammatory
drugs,
antihistamines,
antidepressants,
benzodiazepines,
anticonvulsants
or
corticosteroids
in
the
management
of
painful
vaso-occlusive
crisis?
P:Patientsbetween0and18yearsoldwithsicklecellanemia
andpainfulcrisis
I: Treatment with non-steroidal anti-inflammatory drugs
(diclofenac, nimesulide, aspirin, COX 1 inhibitors and
COX2inhibitors),antihistamines,antidepressants,
benzo-diazepinesandanticonvulsants
C:Treatmentwithparacetamolordipyrone
O:Adequatepaincontrol
Therearefewrandomizedclinicaltrialsonthetreatment
ofthepainfulcrisisinsicklecellanemia45(A),46(B),sothat
mostofthetreatmentisbasedondatafromnon-randomized
studies.49,55,61,62(D)
Five studieswith non-steroidalanti-inflammatorydrugs
(NSAIDs)asadjuvantpainmedicationsforchildrenandadults
withSCDwerereviewed.Oraldiflunisalwassimilartoplacebo.
Twostudieswithintravenousketorolacinhospitalizedadults
werefavorabletoketorolacforpaincontrol,whiletwo
oth-erswerenot.Thesmallsamplesizesandtheheterogeneityof
themethodsprecludeanyconclusionintheuseofNSAIDto
treatpaininSCDandmakeanymeta-analysisonthissubject
unrealistic.45 (A)AphaseIIItrial,doubleblind,randomized,
placebo-controlledof66episodesofpainfulvaso-occlusive
cri-sisconcludedthatketorolacwasnoteffectivetocontrolthe
painandtheuseofmorphinewhencomparedtoaplacebo.
Theauthorsdonotrecommendthesystematicuseof
ketoro-lac in these patients.63 (A) No studies were found in the
literatureonotherNSAIDs,suchasacetaminophen.45(A)
A double-blind randomized placebo-controlled study in
Brazilshowednobenefitwiththeuse ofpiracetamin
chil-drenand youngadultswithsickle cellanemia andpainful
vaso-occlusive crisisinrelation tothe use ofopioids,pain
intensityandhospitalization.64(A)Piracetamisineffectivein
thepreventionofvaso-occlusivepaincrisis.64(A)Thesedata
wereconfirmedinameta-analysiswiththreestudies,
includ-ingtheBrazilianstudy,whereitwasconcludedthatthereare
insufficientdatatoindicatepiracetamforpainfulcrisisinSCD
patients.65(A)
Methylprednisoloneorintravenous dexamethasone
sug-gestbenefitsinrelationtopainmanagement,butpatientswho
receivedasingledoseofcorticosteroidshadahigherriskof
painreactivation.45(A)
Magnesiumisavasodilatorthatenhancesthehydrationof
redbloodcells.Forthisreasonitwasinvestigatedina
random-ized,double-blind,placebo-controlledstudy of104children
withSCD hospitalized to receive intravenous analgesia for
painfulcrisis.Intravenousmagnesiumsulfatehadnoeffect
onreducingthelengthofhospitalstay,improvementsinpain
orreductionsinthecumulativedoseofanalgesics.66(A)
Adjuvantmedicationshavebeenusedtoenhancethe
anal-gesiceffectofopioidsinpatientswithcancerandtodecrease
their adverse events. Antihistamines (chlorpheniramine
0.15mg/kg/day orally every 6h with a maximum dose of
12mg/day),antidepressants(amitriptyline10–20mgorallyat
night), benzodiazepines(thedose dependsonthe
benzodi-azepine)andanticonvulsants(carbamazepine5–10mg/kg/day
onceortwiceaday)havemildanalgesiceffects.When
indi-cated,thesemedicationsshouldbeusedcarefully.49,51,52(D)
Recommendations:Thereisnoconsistentevidence
sup-portingtheuseofantidepressants,benzodiazepinesand anticonvulsants in the management of painful vaso-occlusive crisis in sickle cell anemia. There are no benefits with piracetam or magnesium sulfate use in painfulvaso-occlusivecrisis.
Is
there
evidence
for
the
use
of
oxygen
therapy
or
hyperhydration
in
the
management
of
painful
vaso-occlusive
crisis?
P:Patientsbetween0and18yearsoldwithsicklecellanemia andpainfulcrisis
I:Treatmentwithoxygentherapyorhyperhydration C:Treatmentwithoutoxygentherapyorhyperhydration O:Adequatepaincontrol
Althoughinhaledoxygentherapy(50%oxygen)inpatients withSCDreversesthesicklingofredbloodcells,thereisno evidenceintheliteraturethatitsuseinvaso-occlusivepain crisisprovidesbenefitsbyreducingthepain,theuseofopioids orhospitalization.67,68(B)
Dehydrationisanimportantfactorinthesicklingprocess.
Thedecreased abilitytoconcentrateurine, acharacteristic
ofSCDpatients, resultsininadequatecontrolofhydration.
Thus, duringpainful crisis, patientsreceive intravenous or
oralhydration,regardlessoftheirstateofhydration.However,
careisnecessarynottocausecardiacorpulmonaryvolume
overload.Norandomizedcontrolledtrialsreportbenefitsin
respecttoimprovingorresolvingpainwiththe infusionof
intravenousfluidsasadjuvanttreatmentinsicklecellanemia
patientswithpainfulvaso-occlusivecrisis,regardlessofthe
stateofhydration.47(A)
Hydrationinpatientswithsicklecellanemiashouldbe
car-ried out withcaution.Hypotonic solutionsmay beusedto
establishbaselinehydration,maximum1–1.5timesthe
main-tenancevolumeincludingthemedicationsvolume.49,55,61,69,70
(D)Careshouldbetakentoavoidexcessivehydrationbecause
it decreasesthe plasmaoncoticpressureand increasesthe
hydrostatic pressure,withrisk ofpulmonaryedema
(espe-cially in patients with kidney disease, heart disease or
previouslungdisease).70(D)
Recommendations: Thereisno publishedevidenceon
Is
there
evidence
that
bone
scintigraphy
is
a
good
test
to
differentiate
the
painful
vaso-occlusive
crisis
from
osteomyelitis?
Is
there
any
test
that
allows
this
differentiation?
P:Patientsbetween0and18yearsoldwithsicklecellanemia
anddoubtsbetweenpainfulcrisisandosteomyelitis
I:Bonescintigraphy
C:Clinicaldiagnosis
O:Differentialdiagnosisbetweenpainfulvaso-occlusive
cri-sisandosteomyelitis
Insicklecellanemia,boneinfarctionisabout50timesmore
commonthan osteomyelitis.71 (B)However,the
differentia-tionbetweenosteomyelitisandboneinfarctionisdifficult.In
bothsituationsthepatientcanhavepain,fever,edema,
ery-thema,andleukocytosisandimagingtests(X-ray,computed
tomography)arenonspecific.72,73(B)
Aprospectivestudyof42episodesofbonepaininchildren
withSCDshowedthatinmostcasesthe diagnosisofbone
infarctionisclinical.However,scintigraphywithtechnetium
canhelpincaseswherethereisdoubtaboutosteomyelitis.
When bonemarrowscintigraphy withtechnetiumismade
withintendaysofonset,theuptakeisnormalattheinjury
site in the case of osteomyelitis (100% of cases) while in
boneinfarctiontheuptakeisdiminished(93%ofcases).Bone
scintigraphywithtechnetiumhasproveneffectivetoidentify
osteomyelitisin100%ofcasesduetothehigheruptake,butit
isnotspecificforboneinfarction.74(B)
Combinationofsequentialboneandbonemarrow
scintig-raphy withtechnetium showed benefitsin the differential
diagnosisofboneinfarctionandosteomyelitisin39children
withSCDandbonepain.Theseexamsshouldbeperformed
within 72h of the onset ofpain. Decreased bone marrow
uptakeassociated withnormal or reduced boneuptake in
scintigraphysuggestsadiagnosisofboneinfarction,whereas
increasedboneuptakesuggestsinfection.75(B)
Scintigraphyimagesof79 childrenwith SCDperformed
within24hoftheonsetofpainwereretrospectivelyanalysed.
Bonemarrowscintigraphywithtechnetiumwasperformed
followedbybonescintigraphy.Reduceduptakebythebone
marrowand abnormalboneuptakesuggestedbone
infarc-tionandnormaluptakebythebonemarrowwithincreased
boneuptakesuggestedosteomyelitis.Seventycasesofbone
infarctionwerediagnosedwith66beingsuccessfullytreated
withoutantibiotics.Fourcaseswerediagnosedas
osteomyeli-tis,inwhichthreecultureswerepositive;nofalsenegative
resultswereobserved.73(B)
A retrospective study of 22 episodes of suspected
osteomyelitisinSCDchildrenshowedthatthecombination
ofbonescintigraphywithgalliumandtechnetiumcanhelpin
thedifferentialdiagnosisofboneinfarctionand
osteomyeli-tis.Thebestdatafordiagnosisareobtainedwithin24hafter
theonsetofsymptoms.72(B)Theuseofbonemarrow
scintig-raphywithgalliumandtechnetiumwasalsoeffectiveforthis
differentialdiagnosisinastudyof18episodesofpain.76(C)
Aprospectivestudyof53SCDchildrenwiththeneedto
differentiatebetween vaso-occlusive crisis and
osteomyeli-tisfoundultrasoundchangesofthesofttissuesuggestiveof
osteomyelitisin17patients;puswasdrainedfromallpatients.
Theauthorssuggestthatultrasoundshouldbeconsideredas
the first optiontoinvestigateosteomyelitis.77 (B) The
over-all sensitivityof soft tissueultrasound in the diagnosis of
osteomyelitiswas74%withaspecificityof63%.78(C)The
ultra-soundwasnormalincasesofboneinfarction,andperiosteal
elevation,subperiostealorintramedullaryabscessesand
cor-ticalboneerosioncanbeobservedinosteomyelitis.77(B)78(C)
Thediagnosisofosteomyelitisbyultrasoundcanbeenhanced
byinvestigatingreactive proteinCserum levelsand
leuko-cyte counts, as these values are significantly increased in
osteomyelitis.79(B)
Magneticresonanceimaging(MRI)isagoodimagingexam
toevaluatethebonemarrowandtodetect boneinfarction.
However,inSCD,itisdifficulttodifferentiatebetween
infarc-tionandosteomyelitiswithMRI.80(D)
Althoughimagingtestscanhelptodifferentiatebetween
bone infarction and osteomyelitis, a clinical diagnosis is
alwaysbetter.73,74,81(B)
Recommendation: Differentiating bone infarction and
osteomyelitis remainsachallengebecausethe clinical presentationissimilarinbothcasesandlaboratorytests are still of limited use. Bone scintigraphy with tech-netiumdoesnothelptodifferentiateboneinfarctionin sickle cell anemia patients withbone pain. Thus, the combinationofboneandbonemarrowscintigraphywith technetium can assist in the differentiation between osteomyelitis and boneinfarction,ascanscintigraphy usinggalliumandultrasoundofthesofttissues.MRImay playaroleandbesoughtwhereverpossibleincasesof feverandprolongedpain.
Conflicts
if
interest
Theauthorsdeclarenoconflictsofinterest.
Appendix
A.
PICO1
What is the prevalence of sickle cell disease and how
aretheresultsofneonatalscreeningforhemoglobinopathies interpreted?
((Anemia, Hemolytic, Congenital AND screening AND neonatal)) OR (((screening AND neonatal)) AND (Hemoglobinopathies))
PICO2
Isthereevidencefortheneedtoperformaconfirmatory electrophoresisexamafterthesixthmonthoflife?
(Anemia, Sickle Cell OR Hemoglobinopathies OR Hemoglobins) AND (Electrophoresis) AND (Neonatal OR Newborn)
PICO3
SicklecellanemiaAND(veno-occlusivediseaseOR
veno-occlusivesyndromeORpainORvaso-occlusiveORvascular
disease ORcrisis) AND (fever ORdehydration OR infection
OR metabolic syndrome OR cold OR heat OR alcohol OR
osteomyelitisORcocaineORmarihuanaORetiologyORrisk)
PICO4
Isthereevidencethattheuseofpainassessmentscales isagoodmethodtomonitorpain relatedtovaso-occlusive crisis?
SicklecellanemiaAND(veno-occlusivediseaseOR
veno-occlusivesyndromeORpainORvaso-occlusiveORvascular
diseaseORcrisis)AND(SeverityofIllnessIndexORscaleOR
scoreORVASORmeasurement)
PICO5
Whatisthebestsequenceofmedicationstocontrolpainful vaso-occlusivecrisis?
(Therapy/broad[filter]ANDSicklecellanemiaAND
(veno-occlusivedisease ORveno-occlusive syndrome ORpain OR
vaso-occlusiveORvasculardiseaseORcrisis))
PICO6
Isthereevidencefortheuseofadjuvantmedicationssuch as non-steroidal anti-inflammatory drugs, antihistamines, antidepressants, benzodiazepines, anticonvulsants or cor-ticosteroids in the management of painful vaso-occlusive crisis?
(Therapy/broad[filter]ORanti-inflammatoryORHistamine
Antagonists OR anticonvulsant OR Benzodiazepines) AND
Sickle cell anemia AND (veno-occlusive disease OR
veno-occlusivesyndromeORpainORvaso-occlusiveORvascular
diseaseORcrisis)
PICO7
Isthereevidencefortheuseofoxygentherapyor hyperhy-drationinthemanagementofpainfulvaso-occlusivecrisis?
SicklecellanemiaAND(veno-occlusivediseaseOR
veno-occlusivesyndromeORpainORvaso-occlusiveORvascular
diseaseORcrisis)AND(oxygentherapyOROXYGENORFluid
TherapyORInfusions,ParenteralORHYDRATIONOR
DEHY-DRATION)
PICO8
Isthereevidencethatbonescintigraphyisagoodtestto differentiatepainfulvaso-occlusivecrisisfromosteomyelitis? Isthereanytestthatallowsthisdifferentiation?
((RadionuclideImaging))AND(sicklecellanemia)
r
e
f
e
r
e
n
c
e
s
1. NationalInstituteofHealth,NationalHeart,Lung,andBlood Institute,DivisionofBloodDiseasesandResources.The managementofsicklecelldisease;2002.
2. AgênciaNacionaldeVigilânciaSanitária(Anvisa).Manualde
DiagnósticoeTratamentodeDoenc¸asFalciformes;2002.p.
142.Availablefrom:http://bvsms.saude.gov.br/bvs/
publicacoes/anvisa/diagnostico.pdf[cited10.01.15][Internet]. 3. BragaJA,LoggettoSR,CampanaroCM,LyraIM,VianaMB,
AnjosAC,etal.Doenc¸afalciforme.In:LoggettoSR,BragaJA, ToneLG,editors.HematologiaeHemoterapiaPediátrica.São Paulo:Atheneu;2014.p.139–62.
4. ShaferFE,LoreyF,CunninghamGC,KlumppC,VichinskyE, LubinB.Newbornscreeningforsicklecelldisease:4yearsof
experiencefromCalifornia’snewbornscreeningprogram.J PediatrHematolOncol.1996;18(1):36–41.
5.BrandeliseS,PinheiroV,GabettaCS,HambletonI,SerjeantB, SerjeantG.NewbornscreeningforsicklecelldiseaseinBrazil: theCampinasexperience.ClinLabHaematol.2004;26(1):15–9.
6.WagnerSC,deCastroSM,GonzalezTP,SantinAP,ZaleskiCF, AzevedoLA,etal.Neonatalscreeningfor
hemoglobinopathies:resultsofapublichealthsystemin SouthBrazil.GenetTestMolBiomarkers.2010;14(4):565–9.
7.MagalhãesPK,TurcatoMdeF,AnguloIdeL,MacielLM. Neonatalscreeningprogramattheuniversityhospitalofthe RibeirãoPretoSchoolofMedicine,SãoPauloUniversity, Brazil.CadSaudePublica.2009;25(2):445–54.
8.StreetlyA,LatinovicR,HallK,HenthornJ.Implementationof universalnewbornbloodspotscreeningforsicklecelldisease andotherclinicallysignificanthaemoglobinopathiesin England:screeningresultsfor2005-7.JClinPathol. 2009;62(1):26–30.
9.DucrocqR,PascaudO,BévierA,FinetC,BenkerrouM,ElionJ. Strategylinkingseveralanalyticalmethodsofneonatal screeningforsicklecelldisease.JMedScreen.2001;8(1):8–14.
10.AlmeidaAM,HenthornJS,DaviesSC.Neonatalscreeningfor haemoglobinopathies:theresultsofa10-yearprogrammein anEnglishHealthRegion.BrJHaematol.2001;112(1):32–5.
11.LêPQ,FersterA,CottonF,VertongenF,VermylenC, VanderfaeillieA,etal.SicklecelldiseasefromAfricato Belgium,fromneonatalscreeningtoclinicalmanagement. MedTrop(Mars).2010;70(5–6):467–70.
12.Bardakdjian-MichauJ,BahuauM,HurtrelD,GodartC,RiouJ, MathisM,etal.Neonatalscreeningforsicklecelldiseasein France.JClinPathol.2009;62(1):31–3.
13.DinizD,GuedesC,BarbosaL,TauilPL,MagalhãesI. Prevalenceofsicklecelltraitandsicklecellanemiaamong newbornsintheFederalDistrict,Brazil,2004to2006.Cad SaudePublica.2009;25(1):188–94.
14.PaixãoMC,CunhaFerrazMH,JanuárioJN,VianaMB,LimaJM. ReliabilityofisoelectrofocusingforthedetectionofHbSHb C,andHBDinapioneeringpopulation-basedprogramof newbornscreeninginBrazil.Hemoglobin.2001;25(3):297–303.
15.LoboCL,BuenoLM,MouraP,OgedaLL,CastilhoS,deCarvalho SM.NeonatalscreeningforhemoglobinopathiesinRiode Janeiro,Brazil.RevPanamSaludPublica.2003;13(2–3):154–9.
16.WatanabeAM,PianovskiMA,ZanisNetoJ,LichtvanLC, Chautard-Freire-MaiaEA,DomingosMT,etal.Prevalenceof hemoglobinSintheStateofParana,Brazil,basedonneonatal screening.CadSaudePublica.2008;24(5):993–1000.
17.AdornoEV,CoutoFD,MouraNetoJP,MenezesJF,RêgoM,Reis MG,etal.HemoglobinopathiesinnewbornsfromSalvador, Bahia,NortheastBrazil.CadSaudePublica.2005;21(1):292–8.
18.BandeiraFM,LealMC,SouzaRR,FurtadoVC,GomesYM, MarquesNM.Hemoglobin“S”positivenewborndetectedby cordbloodanditscharacteristics.JPediatr(RioJ).
1999;75(3):167–71.
19.deAraújoMC,SerafimES,deCastroWAJr,deMedeirosTM. PrevalenceofabnormalhemoglobinsinnewbornsinNatal, RioGrandedoNorte,Brazil.CadSaudePublica.
2004;20(1):123–8.
20.ThuretI,SarlesJ,MeronoF,SuzineauE,CollombJ,Lena-Russo D,etal.NeonatalscreeningforsicklecelldiseaseinFrance: evaluationoftheselectiveprocess.JClinPathol.
2010;63(6):548–51.
21.MillerFA,HayeemsRZ,BombardY,LittleJ,CarrollJC,Wilson B,etal.Clinicalobligationsandpublichealthprogrammes: healthcareproviderreasoningaboutmanagingtheincidental resultsofnewbornscreening.JMedEthics.2009;35(10):626–34.
23.BainBJ.Haemoglobinopathydiagnosis:algorithms,lessons andpitfalls.BloodRev.2011;25(5):205–13.
24.BoemerF,VanbellinghenJF,BoursV,SchoosR.Screeningfor sicklecelldiseaseondriedblood:anewapproachevaluated on27,000Belgiannewborns.JMedScreen.2006;13(3):132–6.
25.DucrocqR,BévierA,LeneveuA,Maier-RedelspergerM, Bardakdjian-MichauJ,BadensC,etal.Compound
heterozygosityHbS/HbHope(beta136Gly→Asp):apitfallin thenewbornscreeningforsicklecelldisease.JMedScreen. 1998;5(1):27–30.
26.ShapiroBS.Themanagementofpaininsicklecelldisease. PediatrClinNorthAm.1989;36(4):1029–45.
27.SmithWR,BausermanRL,BallasSK,McCarthyWF,Steinberg MH,SwerdlowPS,etal.Climaticandgeographictemporal patternsofpainintheMulticenterStudyofHydroxyurea. Pain.2009;146(1–2):91–8.
28.SlovisCM,TalleyJD,PittsRB.Nonrelationshipofclimatologic factorsandpainfulsicklecellanemiacrisis.JChronicDis. 1986;39(2):121–6.
29.JonesS,DuncanER,ThomasN,WaltersJ,DickMC,HeightSE, etal.Windyweatherandlowhumidityareassociatedwith anincreasednumberofhospitaladmissionsforacutepain andsicklecelldiseaseinanurbanenvironmentwitha maritimetemperateclimate.BrJHaematol.2005;131(4): 530–3.
30.NolanVG,ZhangY,LashT,SebastianiP,SteinbergMH. Associationbetweenwindspeedandtheoccurrenceofsickle cellacutepainfulepisodes:resultsofacase-crossoverstudy. BrJHaematol.2008;143(3):433–8.
31.WestDC,RomanoPS,AzariR,RudominerA,HolmanM, SandhuS.Impactofenvironmentaltobaccosmokeon childrenwithsicklecelldisease.ArchPediatrAdolescMed. 2003;157(12):1197–201.
32.GlassbergJ,SpiveyJF,StrunkR,BoslaughS,DeBaunMR. Painfulepisodesinchildrenwithsicklecelldiseaseand asthmaaretemporallyassociatedwithrespiratory symptoms.JPediatrHematolOncol.2006;28(8):481–5.
33.HargraveDR,WadeA,EvansJP,HewesDK,KirkhamFJ. Nocturnaloxygensaturationandpainfulsicklecellcrisesin children.Blood.2003;101(3):846–8.
34.NeborD,BowersA,Hardy-DessourcesMD,Knight-Madden JM,RomanaM,ReidH,etal.Frequencyofpainfulcrisesin sicklecellanemiaanditsrelationshipswiththe
sympatho-vagalbalance,bloodviscosityandinflammation. Haematologica.2011;96(11):1589–94.
35.YoongWC,TuckSM.Menstrualpatterninwomenwithsickle cellanaemiaanditsassociationwithsicklingcrises.JObstet Gynaecol.2002;22(4):399–401.
36.ZempskyWT.Evaluationandtreatmentofsicklecellpainin theemergencydepartment:pathstoabetterfuture.Clin PediatrEmergMed.2010;11(4):265–73.
37.LuffyR,GroveSK.Examiningthevalidity,reliability,and preferenceofthreepediatricpainmeasurementtoolsin African-Americanchildren.PediatrNurs.2003;29(1):54–9.
38.ZempskyWT,CorsiJM,McKayK.Painscores:aretheyusedin sicklecellpain?PediatrEmergCare.2011;27(1):27–8.
39.Frei-JonesMJ,BaxterAL,RogersZR,BuchananGR. Vaso-occlusiveepisodesinolderchildrenwithsicklecell disease:emergencydepartmentmanagementandpain assessment.JPediatr.2008;152(2):281–5.
40.SmithWR,PenberthyLT,BovbjergVE,McClishDK,RobertsJD, DahmanB,etal.Dailyassessmentofpaininadultswith sicklecelldisease.AnnInternMed.2008;148:94–101.
41.JacobE,MiaskowskiC,SavedraM,BeyerJE,TreadwellM, StylesL.Quantificationofanalgesicuseinchildrenwith sicklecelldisease.ClinJPain.2007;23(1):8–14.
42.LopezBL,FlendersP,Davis-MoonL,CorbinT,BallasSK. Clinicallysignificantdifferencesinthevisualanalogpain
scaleinacutevasoocclusivesicklecellcrisis.Hemoglobin. 2007;31(4):427–32.
43.DoylePortugalR,MorgadoLoureiroM.Evaluationofpain scaletopredicthospitaladmissioninpatientswithsicklecell disease.Haematologica.2003;88(4):ELT11.
44.SporrerKA,JacksonSM,AgnerS,LaverJ,AbboudMR.Painin childrenandadolescentswithsicklecellanemia:a
prospectivestudyutilizingself-reporting.AmJPediatr HematolOncol.1994;16(3):219–24.
45.DunlopRJ,BennettKC.Painmanagementforsicklecell disease.CochraneDatabaseSystRev.2006;(2):CD003350.
46.KavanaghPL,SprinzPG,VinciSR,BauchnrH,WangCJ. Managementofchildrenwithsicklecelldisease:a comprehensivereviewoftheliterature.Pediatrics. 2011;128(6):e1552–74.
47.OkomoU,MeremikwuMM.Fluidreplacementtherapyfor acuteepisodesofpaininpeoplewithsicklecelldisease. CochraneDatabaseSystRev.2012;6:CD005406.
48.LottenbergR,HassellKL.Anevidence-basedapproachtothe treatmentofadultswithsicklecelldisease.HematolAmSoc HematolEducProgram.2005:58–65.
49.U.S.DepartmentofHealthandHumanServices,PublicHealth
Service,NationalInstitutesofHealth,NationalHeart,Lung,
andBloodInstitute.Themanagementofsicklecelldisease.
NIHPublicationNo.02-2117;2002.Availablefrom:
https://www.nhlbi.nih.gov/files/docs/guidelines/scmngt.pdf
[cited10.01.15][Internet].
50.BrunettaDM,CléDV,HaesTM,Roriz-FilhoJS,MorigutiJC. Manejodascomplicac¸õesagudasdadoenc¸afalciforme. Medicina(RibeirãoPreto).2010;43(3):231–7.
51.MinistériodaSaúde,InstitutoNacionaldeCâncer.Cuidados paliativosoncológicos:controledador.INCA:RiodeJaneiro; 2001.p.124.
52.WorldHealthOrganization.Cancerpainreliefandpalliative care.WorldHealthOrganizationTechinicalReportSeries804. Geneva,Switzerland:WorldHealthOrganization;1990.p. 1–75.
53.WHOguidelinesonthepharmacologicaltreatmentof persistingpaininchildrenwithmedicalillnesses.World HealthOrganization;2012.p.36–53.ISBN9789241548120.
54.SCAC(theSickleCellAdvisoryCommittee)ofGENES(The GeneticNetworkofNewYork,PuertoRicoandtheVirgin Islands).Guidelinesforthetreatmentofpeoplewithsickle celldisease;March2002.
55.MinistériodaSaúde,SecretariadeAtenc¸ãoàSaúde, DepartamentodeAtenc¸ãoEspecializada.Manualdeeventos agudosemdoenc¸a.Brasília:EditoradoMinistériodaSaúde; 2009,50p.:il.[SérieA.NormaseManuaisTécnicos].
56.vanBeersEJ,vanTuijnCF,NieuwkerkPT,FriederichPW, VrankenJH,BiemondBJ.Patientcontrolledanalgesiaversus continuousinfusionofmorphineduringvaso-occlusivecrisis insicklecelldisease,arandomizedcontrolledtrial.AmJ Hematol.2007;82(11):955–60.
57.JacobsonSJ,KopeckyEA,JoshiP,BabulN.Randomisedtrialof oralmorphineforpainfulepisodesofsickle-celldiseasein children.Lancet.1997;350(9088):1358–61.
58.GriffinTC,McIntireD,BuchananGR.High-doseintravenous methylprednisolonetherapyforpaininchildrenand adolescentswithsicklecelldisease.NEnglJMed. 1994;330(11):733–7.
59.CabootJB,AllenJL.Pulmonarycomplicationsofsicklecell diseaseinchildren.CurrOpinPediatr.2008;20(3):279–87.
60.GladwinMT,KatoGJ,WeinerD,OnyekwereOC,DampierC, HsuL,etal.Nitricoxideforinhalationintheacutetreatment ofsicklecellpaincrisis:arandomizedcontrolledtrial.JAMA. 2011;305(9):893–902.
61.MinistériodaSaúde,SecretariadeAtenc¸ãoàSaúde,
básicasnadoenc¸afalciforme.Brasília:EditoradoMinistério daSaúde;2006.p.56[SérieA.NormaseManuaisTécnicos].
62.NationalInstituteforHealthandClinicalExcellence(NICE).
ClinicalGuideline143.Sicklecellacutepainfulepisode:
managementofanacutepainfulsicklecellepisodein
hospital;2012.Availablefrom:https://www.nice.org.
uk/guidance/cg143/resources/sickle-cell-disease-managing-acute-painful-episodes-in-hospital-35109569155525[cited
10.01.15][Internet].
63.BartolucciP,ElMurrT,Roudot-ThoravalF,HabibiA,SantinA, RenaudB,etal.Arandomized,controlledclinicaltrialof ketoprofenforsickle-celldiseasevaso-occlusivecrisesin adults.Blood.2009;114(18):3742–7.
64.AlvimRC,VianaMB,PiresMA,FranklinHM,PaulaMJ,Brito AC,etal.Inefficacyofpiracetaminthepreventionofpainful crisesinchildrenandadolescentswithsicklecelldisease. ActaHaematol.2005;113(4):228–33.
65.AlHajeriAA,FedorowiczZ,OmranA,TadmouriGO.Piracetam forreducingtheincidenceofpainfulsicklecelldiseasecrises. CochraneDatabaseSystRev.2007;(2):CD006111.
66.GoldmanRD,MounstephenW,Kirby-AllenM,FriedmanJN. Intravenousmagnesiumsulfateforvaso-occlusive episodesinsicklecelldisease.Pediatrics.2013;132(6):e 1634–1641.
67.ZipurskyA,RobieuxIC,BrownEJ,ShawD,O’BrodovichH, KellnerJD,etal.Oxygentherapyinsicklecelldisease.AmJ PediatrHematolOncol.1992;14(3):222–8.
68.RobieuxIC,KellnerJD,CoppesMJ,ShawD,BrownE,GoodC, etal.Analgesiainchildrenwithsicklecellcrisis:comparison ofintermittentopioidsvs.continuousintravenousinfusionof morphineandplacebo-controlledstudyofoxygeninhalation. PediatrHematolOncol.1992;9(4):317–26.
69.SickleCellSociety,DepartmentofHealth,UKForumon HaemoglobinDisorders.Standardsfortheclinicalcareof adultswithsicklecelldiseaseintheUK;2008.
70.TostesMA,BragaJA,LenCA.Abordagemdacrisedolorosaem crianc¸asportadorasdedoenc¸afalciforme.RevCiêncMéd Campinas.2009;18(1):47–55.
71.KeeleyK,BuchananGR.Acuteinfarctionoflongbonesin childrenwithsicklecellanemia.JPediatr.1982;101(2):170–5.
72.AmundsenTR,SiegelMJ,SiegelBA.Osteomyelitisand infarctioninsicklecellhemoglobinopathies:differentiation bycombinedtechnetiumandgalliumscintigraphy.Radiology. 1984;153(3):807–12.
73.SkaggsDL,KimSK,GreeneNW,HarrisD,MillerJH. Differentiationbetweenboneinfarctionandacute
osteomyelitisinchildrenwithsickle-celldiseasewithuseof sequentialradionuclidebone-marrowandbonescans.JBone JointSurgAm.2001;83A(12):1810–3.
74.RaoS,SolomonN,MillerS,DunnE.Scintigraphic differentiationofboneinfarctionfromosteomyelitisin childrenwithsicklecelldisease.JPediatr.1985;107(5):685–8.
75.KimHC,AlaviA,RussellMO,SchwartzE.Differentiationof boneandbonemarrowinfarctsfromosteomyelitisinsickle celldisorders.ClinNuclMed.1989;14(4):249–54.
76.KahnCEJr,RyanJW,HatfieldMK,MartinWB.Combinedbone marrowandgalliumimaging.Differentiationofosteomyelitis andinfarctioninsicklehemoglobinopathy.ClinNuclMed. 1988;13(6):443–9.
77.Sadat-AliM,al-UmranK,al-HabdanI,al-MulhimF. Ultrasonography:canitdifferentiatebetweenvasoocclusive crisisandacuteosteomyelitisinsicklecelldisease?JPediatr Orthop.1998;18(4):552–4.
78.WilliamRR,HusseinSS,JeansWD,WaliYA,LamkiZA.A prospectivestudyofsoft-tissueultrasonographyinsicklecell diseasepatientswithsuspectedosteomyelitis.ClinRadiol. 2000;55(4):307–10.
79.InusaBP,OyewoA,BrokkeF,SanthikumaranG,Jogeesvaran KH.Dilemmaindifferentiatingbetweenacuteosteomyelitis andboneinfarctioninchildrenwithsicklecelldisease:the roleofultrasound.PLOSONE.2013;8(6):e65001.
80.LonerganGJ,ClineDB,AbbondanzoSL.Sicklecellanemia. Radiographics.2001;21(4):971–94.