Systemic administration of curcumin nanoparticles protects
ischemia-reperfusion injury in ovaries: An animal model study
TAHEREH BEHROOZI-LAK1, MALAHAT EBRAHIMPOUR1, LEILA ZAREI2, MASOUMEH POURJABALI3, NEGIN FARHAD4, HAMIDEH MOHADDESI1*
1Maternal and Childhood Obesity Research Center, Department of Infertility, Urmia University of Medical Sciences, Urmia, Iran
2Department of Anatomical Sciences, Faculty of Medicine and Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran 3Department of Pathology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
4Student Research Committee, Urmia University of Medical Sciences, Urmia, Iran
S
UMMARYStudy conducted at Urmia University of
Medical Sciences, Urmia, Iran
Article received: 4/25/2017
Accepted for publication: 5/20/2017
*Correspondence:
Maternal and Childhood Obesity Research Center, Department of Infertility,
Urmia University of Medical Sciences Address: Nazloo Road
Urmia – Iran Postal code: 5714783734
mohaddesi.h@umsu.ac.ir
http://dx.doi.org/10.1590/1806-9282.64.01.22
Objective: Ovarian torsion must be diagnosed and treated as early as possible. The aim of the present study was to investigate the effects of intraperitoneal
administration of nanocurcumin on ischemia-reperfusion injury in ovaries.
Method: Thirty-five (35) healthy female Wistar rats weighing approximately 250 g were randomized into seven experimental groups (n=5): Group SSG – The rats underwent only laparotomy. Group I: A 3-hour ischemia only. Group I/R: A 3-hour ischemia and 3-hour reperfusion. Group I/C: A 3-hour ischemia only, and 1 mg/kg intraperitoneal administration of curcumin 2.5 hours after induction of ischemia. Group I/R/C: A 3-hour ischemia, 3-hour reperfusion, and 1 mg/kg intraperitoneal administration of curcumin 2.5 hours after induction of ischemia. Group I/NC: A 3-hour ischemia only and 1 mg/kg intraperitoneal administration of nanocurcumin 2.5 hours after induction of ischemia. Group I/R/C: A 3-hour ischemia, 3-hour reperfusion and 1 mg/kg intraperitoneal administration of nanocurcumin 2.5 hours after induction of ischemia.
Results: Nanocurcumin-treated animals showed significantly improved development of ischemia and reperfusion tissue injury compared to those in the other groups (p<0.05). Significant higher values of SOD, tGSH, GPO, GSHRd and GST were observed in I/R/NC animals compared to those in the other groups (p<0.05). The damage indicators (NOS, MDA, MPO and DNA damage level) were significantly lower in I/R/NC animal compared to those of other groups (p<0.05).
Conclusion: Intraperitoneal administration of nanocurcumin can be helpful in minimizing ischemia-reperfusion injury in ovarian tissue exposed to ischemia.
Keywords: Curcumin. Nanoparticles. Ovary.
I
NTRODUCTIONThere are various conditions, such as long mesovarium and adnexal venous congestion, that could result in tor-sion of ovary and subsequently obstruction of the ovar-ian vessels. This causes a life-threatening reduction in tissue blood flow and permanent tissue damage.1
There-fore, ovarian torsion must be diagnosed and treated as early as possible to preserve ovarian function and prevent future infertility.2 Upon detection of ovarian torsion,
de-torsion of the twisted adnexa and evaluation of tissue reperfusion is proposed to prevent future infertility even
in case of cyanotic tissues.2,3 This ovarian torsion-detorsion
process is named ischemia-reperfusion injury.4
Reperfusion of the ischemic tissue leads to much more serious damage to the tissue than the damage caused by ischemia.5 Reperfusion-related damage in the cell is
cre-ated by many factors, mostly including oxygen-derived free radicals, which are rapidly generated in the tissue as a result of reperfusion.6 Due to physiological or pathological
al-terations, oxidative damage takes place with changes favor-ing the oxidation process.7 Prompt diagnosis to reduce
still unachievable with this approach. Therefore, studies on prevention of reperfusion injury seem very important.8
A proposed pathogenesis for tissue injury during reperfusion is the accumulation of activated neutrophils that release reactive oxygen species.9 Lipid peroxidation
in the cell is the most deleterious effects of free radicals that ultimately reduce the membrane potential and sub-sequently cause cell injury. Malondialdehyde (MDA), one of the end products of lipid peroxidation, also results in severe cell damage by inducing polymerization and cross linking in membrane components.10 Free oxygen radicals
react with DNA and form 8-hydroxyguanine (8-OHGua), which is one of the products of DNA damage.11 In spite
of the fact that generation of free oxygen radicals occurs continuously in cells, the presence of endogenous anti-oxidant defense systems preserves tissues from the harm-ful effects of free oxygen radicals.12 Various agents,
anti-inflammatory and antioxidant free radical scavengers have been reported with promising beneficial effects on prevention of ischemic/reperfusion injuries in tissues.13-16
Curcumin is the main phenolic pigments extracted from turmeric, the powdered rhizome of Curcuma longa, along with demethoxycurcumin and bisdemethoxycur-cumin.17 Extensive research indicates that curcumin
pos-sesses potent antioxidant and anti-inflammatory proper-ties, and inhibits lipid peroxidation and scavenges superoxide anion, singlet oxygen, nitric oxide and hy-droxyl radicals.18-20 Administration of curcumin has been
reported to be effective in reversing tissue damage induced by ischemia reperfusion injury in ovarian torsion.21
Curcumin, a naturally-occurring polyphenolic com-pound, is considerably promising; however, its poor wa-ter solubility and fast degradation profile compromise its bioavailability way below the threshold level on ad-ministration. Over a period of time, strong emphasis has been given to improve the biodistribution of native cur-cumin, but only recently the application of the field of nanotherapeutics has significantly improved its thera-peutic efficacy. This is through the development of nano-range formulations of curcumin, popularly known as the nanocurcumin.22
The physiologic characteristic of the peritoneal cav-ity, which helps remove toxic metabolites from the body, has been successfully exploited to provide peritoneal di-alysis in end stage renal disease patients.23 The same
char-acteristics of the peritoneal membrane also provide a use-ful doorway in the body for several pharmacological agents. One advantage would be that the drug achieves therapeu-tic efficacy in the target site while minimizing the sys-temic toxicities. Intraperitoneal administration seems
more effective and may increase drug availability if oral administration poses any difficulties. It is clear that transperitoneal absorption of the drug is much faster than oral administration.24
The present study was different from other studies in the literature for using nanocurcumin on ischemia/ reperfusion injury. Aimed to study peritoneal effects of nanocurcumin on ischemia/reperfusion injury, our study was designed to determine if nanocurcumin could in fact protect against ischemia/reperfusion-induced ovarian damage. The assessments were based on histopathologi-cal and biochemihistopathologi-cal parameters.
M
ETHODStudy design and animals
Two weeks before and during the experiments, the ani-mals were housed in individual plastic cages at room temperature (23±3°C), stable air humidity and a natural day/night cycle. The rats had free access to standard rodent laboratory food and tap water. All measurements were made by two blinded observers unaware of the analyzed groups. The present study was designed and modified based on a method described by Oral et al., 2010. Thirty-five (35) healthy female Wistar rats weighing approximately 250 g were randomized into seven ex-perimental groups (n=5): SSG (SSG Surgery Group) – The rats underwent only laparotomy; group I – A 3-hour ischemia only; group I/R – A 3-hour ischemia and a 3-hour reperfusion; group I/C – A 3-hour ischemia only and 100 mg/kg intraperitoneal administration of cur-cumin (Sigma-Aldrich Chemie Gmbh, Steinheim, Ger-many) 2.5 hours after induction of ischemia; group I/R/C – A 3-hour ischemia, a 3-hour reperfusion and 100 mg/ kg intraperitoneal administration of curcumin 2.5 hours after induction of ischemia; group I/NC – A 3-hour isch-emia only and 1 mg/kg intraperitoneal administration of nanocurcumin (Sigma-Aldrich Chemie Gmbh, Stein-heim, Germany) 2.5 hours after induction of ischemia; group I/R/NC – A 3-hour ischemia, a 3-hour reperfusion and 1 mg/kg intraperitoneal administration of nanocur-cumin 2.5 hours after induction of ischemia. The right ovaries were transferred to a 10% formaldehyde solution for histopathological assessments and the left ovaries were dissected free of surrounding soft tissues and then stored in a freezer at -80°C for biochemical assessments.
Preparation of nanocurcumin
1 mL of this solution was sprayed into boiling water (50 mL) dropwise with a flow rate of 0.2 mL/min for 5 minutes under ultrasonic conditions, with an ultrasonic power of 100 W and a frequency of 30 kHz. After sonication for 10 minutes, the contents were stirred at 200-800 rpm at room temperature for about 20 minutes when a clear orange-colored solution was obtained. The solution was concentrated under reduced pressure at 50°C and then freeze-dried to obtain an orange powder. A co-TLC of the powdered sample with standard curcumin showed both to have the same Rf values. 1H NMR and ultravio-let (UV) spectra of the lyophilized powder confirmed it to be curcumin. Maintaining the drop flow was signifi-cant for both forming nanoparticles and maintaining size uniformity. The mean particle diameter of cur-cumin nanoparticles was measured by dynamic light scattering (DLS) performed on Malvern Zetasizer S90 series. The sample was prepared by taking 1 mg of the lyophilized nanocurcumin powder in 10 mL of distilled water. Transmission electron micrograph (TEM) analy-sis was performed on a Morgagni 268 D from FEI. The sample was prepared by placing a drop of the aqueous dispersion of curcumin nanoparticles on the copper grid and allowing it to air dry. Scanning electron micrograph (SEM) of the aqueous dispersion was recorded on a Jeol JSM 840 microscope by spreading the nanoparticles dispersion over a carbon tape and drying it under nitro-gen stream. The sample was then coated in a sputter coater (EMITECH K 550 x) with a gold layer under vacuum conditions.25
Surgical procedure
Animals were anesthetized by intraperitoneal administra-tion of ketamine-xylazine (ketamine 5%, 90 mg/kg and xylazine 2%, 5 mg/kg). The procedure was carried out based on the guidelines of the Ethics Committee of the International Association for the Study of Pain.26 The
Ethical Committee of the Urmia University of Medical Sciences approved all the experiments.
A longitudinal midline incision was made in the lower abdomen and the uterine horns and adnexa were exposed. In order to induce ischemia, a vascular clamp was applied on the rats’ ovary vessels. After a 3-hour pe-riod of ischemia, both ovaries were surgically dissected for histopathological and biochemical assessments. To induce ischemia/reperfusion, both ovaries underwent ischemia the same way and, at the end of a 3-hour period, the vascular clamps were chosen, removed and a 3-hour reperfusion was obtained. Then, the ovaries were dis-sected for histopathological and biochemical assessments.
Histopathological assessments
The ovaries were fixed in 10% buffered formalin for 24 hours. The tissue samples were then processed and em-bedded in paraffin (5-µm semithin sections). The samples were then dewaxed, rehydrated and stained routinely with hematoxylin and eosin. The sections were then observed under a light photomicroscope. For semithin sections, ovaries were fixed in 2.5% buffered glutaraldehyde and postfixed in 2% OsO4 for 2 h, dehydrated in a graded
ethanol series and embedded in epon resin. Semithin transverse sections (5 μm) were next stained with toluidine blue and examined under a light microscope.
Biochemical assessments
Tissue processing for biochemical assessments of ovaries
The ovary tissue samples were kept at -80ºC for 3 days, and then enzyme activities were determined in rat ovary tissues. The ovary tissues were ground under liquid ni-trogen using a mortar. One half gram was weighed for each group and then treated with 4.5 mL of an appropri-ate buffer. This mixture was homogenized on ice with use of an ultra-turrax homogenizer (IKA, Werke, Ger-many) for 15 minutes. Homogenates were filtered and centrifuged by using a refrigerator centrifuge at 4ºC. Then the supernatants were used to determine the enzymatic activities. All assays were carried out at room temperature.
Superoxide dismutase (SOD) analysis
Superoxide dismutase estimation was based on the gen-eration of superoxide radicals produced by xanthine and the xanthine oxidase system, which reacts with nitroblue tetrazolium to form formazan dye.27 Superoxide dismutase
activity was then measured at 560 nm by the degree of inhibition of this reaction and is expressed as millimoles per minute per milligram of tissue.
Nitric oxide synthase (tNOS) activity
Nitric oxide synthase activity of rat ovaries was measured spectrophotometrically using the oxidation of oxyhemo-globin to methemooxyhemo-globin by NO as described by other authors.28 The absorption difference between 401 and
421 nm was continuously monitored with a dual wave length recording spectrophotometer at 37ºC. For the total NOS (tNOS) assay, the incubation medium contained 1.6 mmol/L oxyhemoglobin, 200 mmol/L CaCl2, 1 mmol/L
MgCl2, 100 mmol/L L-arginine, 100 mmol/L of the
Malondialdehyde (MDA) analysis
Concentrations of ovarian lipid peroxidation were deter-mined by estimating MDA using the thiobarbituric acid test.30 The rat ovaries were rinsed with cold saline. The
corpus mucosa was scraped, weighed and homogenized in 10 mL of 100 g/L KCl. The homogenate (0.5 mL) was added to a solution containing 2-thiobarbiturate (1.5 mL of 8 g/L), acetic acid (1.5 mL of 200 g/L), sodium lauryl sulfate (0.2 mL of 80 g/L) and distilled water (0.3 mL). The mixture was incubated at 98°C for 1 hour. n-butanol: pyridine 5 mL (ratio:15:l) was then added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4,000 rpm. Absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The standard curve was ob-tained by using 1,1,3,3-tetramethoxypropane.
Myeloperoxidase (MPO) analysis
The activity of MPO in the total homogenate was measured according to previously described methods.31 The sample
was weighed and homogenized in 2 mL of 50 mmol/L phos-phate buffer containing 0.5% hexadecyl trimethyl ammo-nium bromide (HDTMAB) and centrifuged at 3,500 rpm for 60 min at 4°C. The supernatant was used to determine MPO activity using 1.3 mL 4-aminoantipyrine-2% phenol (25 mM) solution. 25 mmol/L 4-aminoantipyrine–2% phe-nol solution and 0.0005% 1.5 mL H2O2 were added and
equilibrated for 3-4 minutes. After establishing the basal rate, a sample suspension (0.2 mL) was added and mixed. Increases in absorbance at 510 nm for 4 min at 0.1-min in-tervals were recorded. Absorbance was measured at 412 nm.
Total glutathione (tGSH) analysis
The amount of GSH in the total homogenate was mea-sured according to the previously described methods, with some modifications.32 The sample was homogenized at
pH 7.5, in Tris-HCl buffer (2 mL of 50 mmol/L). The homogenate was precipitated with trichloroacetic acid (0.1 mL of 25%), and the precipitate was removed after centrifugation at 4,200 rpm at 4°C for 40 minutes; the supernatant was used to measure GSH level. A total of 1,500 μL of measurement buffer (200 mmol/L Tris-HCl buffer containing 0.2 mmol/L EDTA at pH 7.5), 500 μL supernatant, 100 μL DTNB (10 mmol/L) and 7,900 μL methanol were added to a tube and vortexed and incu-bated for 30 minutes at 37°C. 5,5-dithiobis (2- nitroben-zoic acid) (DTNB) was used as a chromogen; it formed a yellow-colored complex with sulfhydryl groups. Absor-bance was measured at 412 nm using a spectrophotom-eter (Beckman DU 500, USA). The standard curve was obtained using reduced glutathione.
Glutathione peroxidase (GPO) analysis
GPO activity was determined according to the method of Lawrence and Burk.33 After tissue homogenization,
su-pernatant was used for GPO measurement. Following the addition of KH2PO4, EDTA, GSH, B-NADPH, NaN3 and
GR, the mixture was incubated. As soon H2O2 was added,
the chronometer was turned on and the absorbance at 340 nm was recorded for 5 minutes every 15 seconds.
Glutathione reductase (GSHRd) analysis
GR activity was determined spectrophotometrically by measuring the rate of NADPH oxidation at 340 nm accord-ing to Carlberg and Mannervik method.34 After tissue
ho-mogenization, supernatant was used for GR measurement. After NADPH and GSSG were added, a chronometer was set on and absorbance was measured for 5 minutes with 30 minutes intervals at 340 nm spectrophotometrically.
Glutathione S-transferase (GST) activity
GST activity was determined by Habig and Jakoby.35
En-zyme activity was determined in a 4-mL cuvette contain-ing 30 mM GSH, 30 mM 1-chloro-2,6-dinitrobenzene, 0.1 M PBS (pH 6.5), and tissue homogenate at 340 nm using a spectrophotometer.
Isolation of DNA from ovarian tissue
DNA isolation was performed using a method previously described by other authors.8 In brief, the tissue samples were
cDNA hydrolysis with formic acid
DNA hydrolysis with formic acid was performed based on a modified method described by other authors.8
Brief-ly, 50 mg of DNA were hydrolyzed with 0.5 mL of formic acid (60%, v/v) for 45 minutes at 150°C. The tubes were allowed to cool. The contents were then transferred to Pierce micro-vials, covered with Kleenex tissues cut to size, secured in place using a rubber band and cooled in liquid nitrogen. Formic acid was removed by freeze-drying and prior to analysis by HPLC they were re-dissolved in the eluent, final volume 200 μL.
Measurement of 8-hydroxy-2 deoxyguanine (8-OH Gua)
Measurement of 8-hydroxy-2 deoxyguanine (8-OH Gua) was performed based on a modified method described by others.8 Briefly, the amount of 8-OH gua and guanine
(Gua) was measured using a HPLC system equipped with an electrochemical detector, HP Agilent 1,100 module series and E.C.D. HP 1049 A. The amount of 8-OH gua and Gua was analyzed on a 250 4.6 mm Supelco LC-18-S reverse-phase column. The mobile phase was 50 mM potassium phosphate, pH 5.5, with acetonitrile, a 97 vol-ume acetonitrile and a 3 volvol-ume potassium phosphate, and the flow rate was 1.0 mL/min. The detector potential was set at 0.80 V for measuring the oxidized base. Gua and 8-OH Gua (25 pmol) were used as standards. The 8-OH gua levels were expressed as the number of 8-OH gua molecules/105 Gua molecules.
Statistical analysis
Experimental results were expressed as mean±SD. Statisti-cal analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Model assumptions were evaluated by examining the residual plot. Results were analyzed using repeated measures and a factorial ANOVA with two between-subject factors. Bonferroni test for pairwise comparisons was used to examine the effect of time and treatments. The differences were considered significant when p<0.05.
R
ESULTSHistopathological findings
The histologic design of the ovarian tissue in the SSG animals was normal. Ovarian tissues in the ischemia group showed condensed hemorrhage and severe vascular con-gestion along with degenerative and necrotic changes in many of the cells. The tissues in the I/R group showed histopathological changes of condensed hemorrhage, infiltration of inflammatory cells along with degenerative and apoptotic cells. Polymorphonuclear leukocytes (neu-trophils) were dominant cell types. In I/R/C group
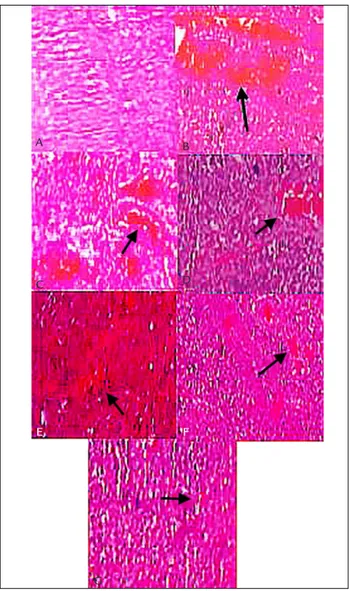
gen-eral histologic and cellular structures of the tissues were not normal in appearance; however, mild vascular conges-tion and edema were observed. In I/R/NC group only a slightly mild hemorrhage was around ovarian follicles. The general histologic structure of the ovarian tissue in this group was normal and no important pathologic findings in the structural level were observed except for only a slightly mild inflammation, vascular congestion and edema (Figure 1).
FIGURE 1 Histologic micrographs of ovarian tissue in SSG (A), I (B), I/R (C), I/C (D), I/R/C (E), I/NC (F) and I/R/NC (G) groups. Microgra-ph B shows condensed hemorrhage and severe vascular congestion (ar-row), as well as severe edema (arrowhead). Micrograph C shows con-densed hemorrhage and vascular congestion (arrow), as well as edema (arrowhead). Micrograph D shows moderate vascular congestion (ar-row) and moderate edema (arrowhead). Micrograph E shows mild vas-cular congestion and edema (arrow). Micrographs F and G show sli-ghtly mild vascular congestion and edema (arrows). Scale bar: 200 µm.
A B
C D
E F
The numerical densities of neutrophils were also estimated at 15 × 10-6/µm3 , 11 × 10-6/µm3, 17 × 10-6/µm3,
16 × 10-6/µm3 and 12 × 10-6/µm3, 15 × 10-6/µm3, 10 ×
10-6/µm3 in SSG, I, I/R, I/C, I/R/C, I/NC and I/R/NC
groups, respectively.
Biochmical findings
Superoxide dismutase (SOD) analysis
The value of SOD activity was 69.5±0.57 mmol/min/mg tissue in the SSG group. The values of SOD declined to 35.8±0.22 and 57.2±0.21 mmol/min/mg tissue in I and I/R groups, respectively. However, intraperitoneal admin-istration of 1 mg/kg of nanocurcumin reversed the trend and increased the activity of SOD to 78.2±0.31 mmol/ min/mg tissue in the ovarian tissue in I/R/NC group. The value of SOD activity in I/R/NC group was significantly higher than those of the other experimental groups (p<0.05) (Table 1).
Nitric oxide synthase (NOS) activity
The value of tNOS activities was increased in I and I/R groups and was significantly higher than those of SSG group (p<0.05). However, intraperitoneal administration of 1 mg/kg of nanocurcumin reversed the trend and decreased tNOS activity in the rats’ ovary. In I/R/NC group the value of tNOS activity was significantly lower than those of the other experimental groups (p<0.05) (Table 1).
Malondialdehyde (MDA) analysis
The results of the present study showed that concentra-tion of MDA in SSG group was 5.7±0.19 μmol/g protein in ovarian tissue. MDA level in I/R group was
signifi-cantly increased to 11.6±0.23 μmol/g protein (p<0.01). Intraperitoneal administration of nanocurcumin sig-nificantly decreased the levels of MDA in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
Myeloperoxidase (MPO) analysis
The level of MPO was significantly increased in I and I/R groups (p<0.05). Intraperitoneal administration of nano-curcumin reversed the trend and significantly decreased the levels of MPO in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
Total glutathione (tGSH) analysis
The values for tGSH levels were 9.8±0.33 and 4.9±0.31 nmol/g protein in SSG and I/R animals, respectively. In-traperitoneal administration of nanocurcumin signifi-cantly increased the levels of GSH in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
Glutathione peroxidase (GPO) analysis
The values for GPO levels were 38.6±2.64 and 17.6±1.41 u/g protein in SSG and I/R animals, respectively. Intra-peritoneal administration of nanocurcumin significant-ly increased the levels of GPO in ovarian tissues of I/R/ NC animals (p<0.05) (Table 1).
Glutathione reductase (GSHRd) analysis
The GSHRd activities in ovarian tissue in the SSG and I/R animals were 33.5±3.26 and 16.4±1.27 u/g protein, respectively. Intraperitoneal administration of nanocur-cumin significantly increased the levels of GSHRd in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
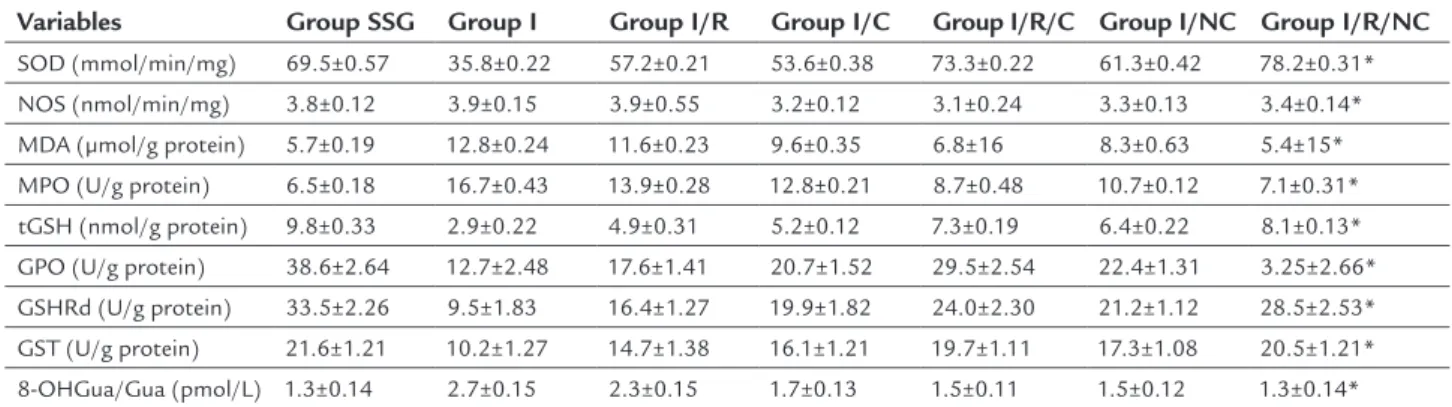
TABLE 1 Comparison of the activities of SOD, NOS, MDA, MPO, GSH, GPO, GSHRd, GST and a DNA damage product of 8-OHGua/Gua in the ovarian tissues of the animals of the all experimental groups. Data are expressed as mean±SD.
Variables Group SSG Group I Group I/R Group I/C Group I/R/C Group I/NC Group I/R/NC
SOD (mmol/min/mg) 69.5±0.57 35.8±0.22 57.2±0.21 53.6±0.38 73.3±0.22 61.3±0.42 78.2±0.31* NOS (nmol/min/mg) 3.8±0.12 3.9±0.15 3.9±0.55 3.2±0.12 3.1±0.24 3.3±0.13 3.4±0.14* MDA (μmol/g protein) 5.7±0.19 12.8±0.24 11.6±0.23 9.6±0.35 6.8±16 8.3±0.63 5.4±15* MPO (U/g protein) 6.5±0.18 16.7±0.43 13.9±0.28 12.8±0.21 8.7±0.48 10.7±0.12 7.1±0.31*
tGSH (nmol/g protein) 9.8±0.33 2.9±0.22 4.9±0.31 5.2±0.12 7.3±0.19 6.4±0.22 8.1±0.13* GPO (U/g protein) 38.6±2.64 12.7±2.48 17.6±1.41 20.7±1.52 29.5±2.54 22.4±1.31 3.25±2.66* GSHRd (U/g protein) 33.5±2.26 9.5±1.83 16.4±1.27 19.9±1.82 24.0±2.30 21.2±1.12 28.5±2.53*
GST (U/g protein) 21.6±1.21 10.2±1.27 14.7±1.38 16.1±1.21 19.7±1.11 17.3±1.08 20.5±1.21* 8-OHGua/Gua (pmol/L) 1.3±0.14 2.7±0.15 2.3±0.15 1.7±0.13 1.5±0.11 1.5±0.12 1.3±0.14*
I: ischemia; I/R: ischemia-reperfusion; I/Nimodipine: ischemia plus intraperitoneal administration of nimodipine; I/R/Nimodipine: ischemia plus reperfusion plus intraperitoneal administration of nimodipine; SOD: superoxide dismutase; NOS: nitric oxide synthase; MDA: malondialdehyde; MPO: myeloperoxidase; tGSH: total glutathione; GPO: glutathione peroxidase; GSHRd: glutathione reductase; GST: glutathione S-transferase; 8-OHGua/Gua: 8-hydroxy-2 deoxyguanine.
Glutathione S-transferase (GST) activity
The GST activities in ovarian tissue in the SSG and I/R animals were 21.6±1.21 and 14.7±1.38 u/g protein, re-spectively. Intraperitoneal administration of nanocur-cumin significantly increased the levels of GST in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
Measurement of 8-hydroxy-2 deoxyguanine (8-OH Gua)
The levels of 8-OHGua/Gua, a DNA damage product, were 1.3±0.14 and 2.3±0.15 pmol/L in SSG and I/R animals, respectively. Intraperitoneal administration of nanocur-cumin significantly decreased the levels of GSHRd in ovarian tissues of I/R/NC animals (p<0.05) (Table 1).
D
ISCUSSIONThe present study investigated whether intraperitoneal administration of nanocurcumin is useful in the prevention of ovarian damage in ischemia/reperfusion conditions in rat ovaries and its results revealed beneficial effects. Histo-pathological and biochemical assessments were performed in SSG, ischemia, ischemia-reperfusion, ischemia-controlled plus IP administration of nanocurcumin groups.
Histopathological, edema, vascular congestion, hem-orrhages and leukocyte infiltration parameters were used. Biochemically, the activities of SOD, NOS, MDA, MPO, GSH, GPO, GSHRd, GST and a DNA damage product of 8-OHGua/Gua were assessed in the ovarian tissues of the animals in all of the experimental groups.
Ischemia, ischemia-reperfusion and intraperitoneal nanocurcumin applied to tissues were analyzed histo-pathologically. Results showed that oxidative stress level followed a parallelism with tissue damage. Edema, vas-cular congestion, hemorrhages and leukocyte infiltration have been used as histopathological parameters in the evaluation of cell condition.36 Edema, vascular congestion,
hemorrhage and leukocyte infiltration in the I/R/NC animals were milder than in the I/R/C group.
In the present study, the levels of SOD in ovarian tissue were assessed and compared in all the experimental groups. SOD activity in SSG and IR/NC showed no sig-nificant difference. SOD is an antioxidant enzyme that catalyzes the conversion of superoxide free radical into hydrogen peroxide and molecular oxygen. SOD and en-dogenous antioxidant enzymes neutralize free radicals and protect tissues from the harmful effects of free radi-cals and active oxygen species.37 Our results showed that,
in the I/R/NC animals, SOD was increased compared to those in I/C and I/R/C groups and intraperitoneal ad-ministration of nanocurcumin protected the ovarian tissue against ischemia-reperfusion injury.
It has been demonstrated that hypoxia generates iNOSs, which plays an important damaging role in I/R injury.38 iNOS is increased after cellular stimulation via
cytokines in macrophages, neutrophils, and microglia and may also contribute to late-stage tissue injury.39 iNOS
derives primarily from the polymorphonuclear neutro-philic leukocytes during reperfusion, and down-regulation of iNOS could limit cell injury caused by hypoxia.40,41
Our findings showed that the iNOS levels in ovarian tis-sue of I and I/R rats were increased compared to those of the SSG animals. Down-regulation of iNOS could limit cell injury caused by hypoxia. Our results showed that, in the I/R/NC animals, iNOS was down-regulated com-pared to those in I/C and I/R/C. Thus, intraperitoneal administration of 1 mg/kg nanocurcumin protected ovarian tissue against ischemia-reperfusion injury to a greater extent than 100 mg curcumin.
MDA is a lipid peroxidation product and occurs as a result of the peroxidation of fatty acids that contain three or more double bonds. MDA causes cross-linking of membrane components and leads to negative conse-quences such as changes in ion permeability and enzyme activity by affecting ion exchange through the cell mem-branes.42,43 MDA levels in the present study were found
to be much lower in I/R/NC animals compared to those in the other experimental groups. This could protect the tissues against ischemia-reperfusion injury in nanocur-cumin-treated animals.
MPO is produced by neutrophils and macrophages, it catalyzes the reaction between hydrogen peroxide and chlorine and results in the toxic compound hypochlorous acid. Hypochlorous acid is involved in the formation of the hydroxyl radical.44,45 It has been demonstrated that
MPO activity is increased in ischemia-reperfusion induced ovarian tissue.46 This finding was in agreement with results
of our study. MPO activity was suppressed in nanocur-cumin-treated animals of our study.
GSH is an antioxidant used to measure oxidative stress. Reperfusion after ischemia is reported to cause severe damage to ovarian tissue and suppress the GSH levels.36 GSH plays a role in the protection of cells against
oxidative stress and toxic compounds as well as the meta-bolic processing of many endogenous compounds such as estrogen, prostaglandin and leukotrienes.47 GSH, as an
prevented in nanocurcumin-treated animals to a greater extent than seen in curcumin-treated animals.
GPO activity is significantly reduced in tissues un-dergoing oxidative stress-related conditions, as in isch-emia-reperfusion injury.48 GPO detoxifies the hydrogen
peroxide radical that forms in the cell by converting it to water and prevents the formation of more toxic products from hydrogen peroxide radical.49 In the present study, a
significant decrease in GPO activity was observed in ovar-ian tissues of I/R/NC animals.
GSH is oxidized during the detoxification of hydrogen peroxide radical. GSHRd is a NADPH-dependent enzyme that converts oxidized glutathione to reduced glutathi-one.50 GSHRd is reported to show higher activity in healthy
tissue and, in parallel with tissue damage, its activity is decreased.51 In our study, activity of GSHRd was
signifi-cantly increased in nanocurcumin-treated animals com-pared to those in I/C and I/R/C groups.
GST binds foreign substances to the –SH group of cysteine in glutathione, neutralizes the electrophilic regions and protects the cells from the harmful effects of foreign substance regions.52 Activity of GST has been reported to
be suppressed in oxidative tissue injury induced by isch-emia.52 Consistently, our findings showed that GST
activ-ity in ovarian tissue of nanocurcumin-treated animals was significantly lower than those in I/C and I/R/C groups.
DNA molecules are damaged if free radicals are in close proximity to the DNA molecules.53 Hydroxyl
radi-cal reacts very easily with deoxyribose and the bases, causing DNA damage through extracting hydrogen from nucleic acids or reacting with double bonds.54 8-OH Gua
is considered an important marker of DNA oxidation.55
Our findings showed that the ovarian tissues of I/C and I/R/C animals had higher levels of 8-OHGua than those of SSG animals. Nevertheless, our results showed that there were no significant difference between SSG and nanocurcumin-treated animals regarding the levels of DNA damage.
There are many studies in the literature about the im-provement of ischemia reperfusion injury. Studies dem-onstrated that the agents with antioxidant or anti-inflam-matory activities may be beneficial in reducing ovarian ischemia reperfusion injury. Also, studies revealed the beneficial effect of controlled reperfusion in the prevention of ovarian tissue damage. In spite of the profuse literature, ischemia/reperfusion damage continues to be a serious problem clinically. Essentially, early diagnosis and treat-ment of ovarian torsion plays an important role to provide urgent protection against life-threatening complications from ischemia and to prevent future infertility.56
Curcumin has been reported as a useful agent both for the prevention and treatment of I/R injury in many organs.57 These protective effects are mainly believed to
be based on inhibitory actions of curcumin on disease-mediated induction of inflammatory transcription factors, protein kinases, adhesion molecules, oxidative stress and inflammation.57 The administration of curcumin has
reported to reduce the generation of reactive oxygen spe-cies (ROS), monocyte adhesion, phosphorylation of c-Jun N-terminal kinase (JNK), p38 MAP kinase, and signal transducer and activator of transcription (STAT)-3 in TNF-a-stimulated cells.57 It has also been documented
that the administration of curcumin prior to conservative surgery (detorsion) provides a significant decrease in the oxidative stress markers in the ovarian tissues.21 The
com-parison between oxidative status and antioxidative status is clear enough to suggest that the administration of curcumin, as reported previously, leads to a decrease in the oxidative stress and an increase in antioxidation.21
Nano-sized particles ranging below several 10 nm are of great interest, because of the chemical and physical behavior of the particles arising from a quantum size effect, which is remarkably different from those in bulk and provides a great potential for use in practice.58 The
findings of the present study showed that nanocurcumin at very low concentrations, 1 mg/kg nanocurcumin versus 100 mg curcumin, produced significant improvements
compared to native curcumin.
Substances are administered by a wide variety of routes. A key factor determining the route selected is whether the agent is being administered for a local or systemic (either enteral or parenteral) effect. Parenteral administration methods typically produce the highest bioavailability of substances because these methods avoid the first-pass effect of hepatic metabolism, which occurs commonly with orally-administered drugs.58
Intraperito-neal administration seems more effective and may increase drug availability if oral administration poses any difficul-ties. It is clear that transperitoneal absorption of the agent is much faster than oral administration.25 Timely
treat-ment is very important in emergency conditions such as ovarian torsion.
ad-ministration of nanocurcumin seems useful when ovar-ian torsion takes place. This may help the patients preserve their future fertility. Our study demonstrated that intra-peritoneal administration of 1 mg/kg nanocurcumin can improve ischemia-reperfusion injury in ovarian tissue exposed to ischemia. Thus, dose-response studies should be conducted for nanocurcumin to determine its maximal efficacy in minimizing ischemia-reperfusion injury in ovarian tissue.
A
CKNOWLEDGMENTSThe authors would like to thank Dr. Rahim Moham-madi, Department of Surgery and Diagnostic Imaging for proofreading the manuscript.
R
EFERENCES1. Oelsner G, Shashar D. Adnexal torsion. Clin Obstet Gynecol. 2006; 49(3):459-63.
2. Geimanaite L, Trainavicius K. Ovarian torsion in children: management and outcomes. J Pediatr Surg. 2013; 48(9):1946-53.
3. Celik A, Ergün O, Aldemir H, Ozcan C, Ozok G, Erdener A, et al. Long-term results of conservative management of adnexal torsion in children. J Pediatr Surg. 2005; 40(4):704-8.
4. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000; 190(3):255-66.
5. Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992; 72(1):65-83.
6. Nakagiri A, Sunamoto M, Takeuchi K, Murakami M. Evidence for the involvement of NADPH oxidase in ischemia/reperfusion-induced gastric damage via angiotensin II. J Physiol Pharmacol. 2010; 61(2):171-9. 7. Halliwell B, Gutteridge JM. Free radicals in biology and medicine. London:
Oxford University Press; 1999.
8. Ingec M, Isaoglu U, Yilmaz M, Calik M, Polat B, Alp HH, et al. Prevention of ischemia-reperfusion injury in rat ovarian tissue with the on-off method. J Physiol Pharmacol. 2011; 62(5):575-82.
9. Wilhelm Filho D, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia- reperfusion injury. Mol Aspects Med. 2004; 25(1-2):199-210.
10. Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998; 39(8):1529-42.
11. Huang HY, Helzlsouer KJ, Appel LJ. The effects of vitamin C and vitamin E on oxidative DNA damage: results from a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2000; 9(7):647-52.
12. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993; 90(17):7915-22. 13. Oral A, Odabasoglu F, Halici Z, Keles ON, Unal B, Coskun AK, et al. Protective effects of montelukast on ischemia-reperfusion injury in rat ovaries subjected to torsion and detorsion: biochemical and histopathologic evaluation. Fertil Steril. 2011; 95(4):1360-6.
14. Mogilner JG, Lurie M, Coran AG, Nativ O, Shiloni E, Sukhotnik I. Effect of diclofenac on germ cell apoptosis following testicular ischemia-reperfusion injury in a rat. Pediatr Surg Int. 2006; 22(1):99-105.
15. Halici Z, Karaca M, Keles ON, Borekci B, Odabasoglu F, Suleyman H, et al. Protective effects of amlodipine on ischemia-reperfusion injury of rat ovary: biochemical and histopathologic evaluation. Fertil Steril. 2008; 90(6):2408-15. 16. Anderson AM, Mitchell MS, Mohan RS. Isolation of curcumin from turmeric.
J Chem Educ. 2000; 77(3):359-60.
17. Pizzo P, Scapin C, Vitadello M, Florean C, Gorza L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J Cell Mol Med. 2010; 14(4):970-81.
18. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009; 41(1):40-59.
19. Wang Y, Lu Z, Wu H, Lv F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int J Food Microbiol. 2009; 136(1):71-4. 20. Jovanovic SV, Boone CW, Steenken S, Trinoga M, Kaskey RB. How curcumin
works preferentially with water soluble antioxidants. J Am Chem Soc. 2001; 123(13):3064-8.
21. Sak ME, Soydinc HE, Sak S, Evsen MS, Alabalik U, Akdemir F, et al. The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int J Surg. 2013; 11(9):967-70.
22. Flora G, Gupta D, Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013; 30(4):331-68.
23. Cortés-Sanabria L, Paredes-Ceseña CA, Herrera-Llamas RM, Cruz-Bueno Y, Soto-Molina H, Pazarín L, et al. Comparison of cost-utility between automated peritoneal dialysis and continuous ambulatory peritoneal dialysis. Arch Med Res. 2013; 44(8):655-61.
24. Chaudhary K, Haddadin S, Nistala R, Papageorgio C. Intraperitoneal drug therapy: an advantage. Curr Clin Pharmacol. 2010; 5(2):82-8.
25. Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 2011; 59(5):2056-61.
26. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16(2):109-10.
27. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34(3):497-500.
28. Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987; 139(1):19-30.
29. Knowles RG, Merrett M, Salter M, Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990; 270(3):833-6.
30. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351-8.
31. Wei H, Frenkel K. In vivo formation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Res. 1991; 51(16):4443-9. 32. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein
sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968; 25(1):192-205.
33. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. 1976. Biochem Biophys Res Commun. 2012; 425(3):503-9. 34. Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;
113:484-90.
35. Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981; 77:398-405.
36. Celik O, Turkoz Y, Hascalik S, Hascalik M, Cigremis Y, Mizrak B, et al. The protective effect of caffeic acid phenethyl ester on ischemia-reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2004; 117(2):183-8. 37. Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, et
al. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000; 87(5):229-33. 38. Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, et
al. Inhibition of inducible nitric oxide synthase reduces renal ischemia/ reperfusion injury. Kidney Int. 2002; 61(3):862-71.
39. Jeddi S, Zaman J, Zadeh-Vakili A, Zarkesh M, Ghasemi A. Involvement of inducible nitric oxide synthase in the loss of cardioprotection by ischemic postconditioning in hypothyroid rats. Gene. 2016; 580(2):169-76. 40. Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in
myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003; 138(4):532-43.
41. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005; 12(10):1161-208.
42. Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005; 338(1):668-76.
43. Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res. 2005; 38(11):1575-83.
44. Van Antwerpen P, Boudjeltia KZ, Babar S, Legssyer I, Moreau P, Moguilevsky N, et al. Thiol-containing molecules interact with the myeloperoxidase/ H2O2/chloride system to inhibit LDL oxidation. Biochem Biophys Res Commun. 2005; 337(1):82-8.
ischemia-reperfusion injury and prevention by nimesulide. Lat Am J Pharm. 2012; 31(10):1481-8.
46. Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991; 51(2):155-94.
47. Celebi F, Akbas A, Saglam MB. Effect of sertraline in indomethacin-induced gastric mucosal damage. Asian J Chem. 2012; 24(5):1966-70.
48. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160(1):1-40.
49. Sharma H, Zhang X, Dwivedi C. The effect of ghee (clarified butter) on serum lipid levels and microsomal lipid peroxidation. Ayu. 2010; 31(2):134-40. 50. Polat B, Suleyman H, Alp HH. Adaptation of rat gastric tissue against
indomethacin toxicity. Chem Biol Interact. 2010; 186(1):82-9.
51. Shi HY, Li ZH, Zhang YX, Chen L, Xiang DY, Zhang YF. Two pear glutathione S-transferases genes are regulated during fruit development and involved in response to salicylic acid, auxin, and glucose signaling. PLoS One. 2014; 9(2):e89926.
52. Mansoorali KP, Prakash T, Kotresha D, Prabhu K, Rama Rao N. Cerebroprotective effect of Eclipta alba against global model of cerebral ischemia induced oxidative stress in rats. Phytomedicine. 2012; 19(12):1108-16.
53. Milligan JR, Aguilera JA, Nguyen TT, Ward JF, Kow YW, He B, et al. Yield of DNA strand breaks after base oxidation of plasmid DNA. Radiat Res. 1999; 151(3):334-42.
54. Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993; 9(7):246-9.
55. Aksoy AN. Ovarian ischemia-reperfusion injury: a brief review. SM J Gynecol Obstet. 2015; 1(2):1008-111.
56. Srivastava G, Mehta JL. Currying the heart: curcumin and cardioprotection. J Cardiovasc Pharmacol Ther. 2009; 14(1):22-7.
57. Okuyama K, Lenggoro IW. Nanoparticle Project in NEDO’s nanotechnology materials program: recent research reviews” PARTEC 2004, Nurnberg, Germany 16-18 3 2004.