w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Accelerated
phase
chronic
myeloid
leukemia:
evaluation
of
clinical
criteria
as
predictors
of
survival,
major
cytogenetic
response
and
progression
to
blast
phase
Vanessa
Fiorini
Furtado,
Gustavo
Rengel
Santos,
Denise
Siqueira
de
Carvalho,
Pedro
Vinícius
Staziaki,
Ricardo
Pasquini,
Vaneuza
Araújo
Moreira
Funke
∗UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6March2015
Accepted7July2015
Availableonline29July2015
Keywords:
Chronicmyeloidleukemia
Acceleratedphase
Imatinib
Prognosticfactor
Mortality
a
b
s
t
r
a
c
t
Background:Publishedcriteriadefiningtheacceleratedphaseinchronicmyeloidleukemia
areheterogeneousandlittleisknownaboutpredictorsofpooroutcome.
Methods:Thisisaretrospectivestudyof139subjectsintheacceleratedphaseofchronic
myeloid leukemiatreated withimatinibat asinglecenterin Brazil.The objectivewas
toidentifyriskfactorsforsurvival,majorcytogeneticresponseandprogressiontoblast
phase in this population. The factors analyzed were: blasts 10–29%, basophils≥20%,
platelets>1×106/Lor<1×105/Landwhitebloodcells>1×105/Lintheperipheralblood,
aswellasclonalevolution,splenomegaly,hemoglobin<10g/dL,timebetweendiagnosisof
chronicmyeloidleukemiaandimatinibtreatment,andhematologictoxicity.
Results:Risk factorsfor poor survival in multivariateanalysis were Grades 3–4
hema-tologictoxicity(p-value=0.001),blasts10–29%(p-value=0.023),andhemoglobin<10g/dL
(p-value=0.04).Riskfactorsfornotachievingmajorcytogeneticresponsewereblasts10–29%
(p-value=0.007),hemoglobin<10g/dL(p-value=0.001),andprevioususeofinterferon(p
-value=0.032).Riskfactorsforprogressionto theblast phasewerehemoglobin<10g/dL
(p-value=0.005), basophils≥20% (p-value=0.023), and time from diagnosis of chronic
myeloidleukemiatoimatinibtreatment>12months(p-value=0.030).
Conclusion: Thesedataindicatethatpatientswiththeaboveriskfactorshaveaworse
prog-nosis.Thisinformationcanguidethetherapytobeused.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published
byElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthorat:StemCellTransplant,UniversidadeFederaldoParaná(UFPR),RuaGeneralCarneiro,181,15◦Andar,80060-900
Curitiba,PR,Brazil.
E-mailaddress:vaneuza@ufpr.br(V.A.M.Funke).
http://dx.doi.org/10.1016/j.bjhh.2015.07.004
1516-8484/©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.PublishedbyElsevierEditoraLtda.Allrights
Introduction
Chronic myeloid leukemia (CML) is a clonal disorder of
hematopoietic stem cells characterized by the reciprocal
translocationt(9;22)(q34;q11)whichdeterminesthe
Philadel-phiachromosomeand constitutiveactivation ofthe
break-pointclusterregion-Abelson(BCR-ABL)tyrosinekinase.1,2At
thetimeofdiagnosis,90%ofpatientsareinthechronicphase
(CP).However,CMLcanprogressfromtheCPtoamore
aggres-siveclinicalpicture–theacceleratedphase(AP),whendisease
controlismoredifficult.APisasignalofprogressionand
trans-formationtotheusuallyfatalblastphase(BP).
Overthepastdecade,theintroductionofimatinib
mesy-latehasbeenconsideredthefirst-linetherapyforallphases
ofCML.3–7Clinicaltrialshaveestablishedtheefficacyof
imat-inib in targeting the pathophysiology of CML, resulting in
increasedsurvivalandfewersideeffectsthanwiththeuse
ofinterferon.8–10 InBrazil,thedelayedapprovalofimatinib
mesylateforfirstline therapyledmanypatientstoreceive
thistherapyonlyatadvancedphasesofthedisease.Intheera
oftyrosinekinaseinhibitors,itisimportanttodefine
progno-sticfactorsnotonlypriortotherapybutalsoduringthecourse
oftreatment.Thebiologicalcharacteristicsofthediseasecan
strongly influence the degree and duration of response to
imatinibandtheoverallsurvival(OS).
ThecriteriaoftheAPvaryintheliterature(Table1).While
somecriteriaareincludedinmostclassifications,suchas
per-centageofbasophilsandblastsintheperipheralblood(PB),
othersaresubjectiveandareincludedinonlysome
classifica-tions,e.g.persistentsplenomegaly.TheInternationalBlood
and MarrowTransplant (IBMTR) criteriahavebeen used in
studiesthatinvolvedbonemarrowtransplantation.11In2001
theWorldHealthOrganization(WHO)proposedanew
classi-ficationsysteminordertorefinethecriteriafortheAPand
BP.12 In2006, the MD Anderson Cancer Center reclassified
patientsandcomparedtheiroutcomeswithimatinibaswell,
basedonstandarddefinitionsandonthenewWHO
classifica-tionsystem.6TheEuropeanLeukemianet(ELN)criteriawere
revisedin2013.13
Objective
Themainpurposeofthisstudywastoidentifywhichrisk
fac-torswereassociatedwithpoorsurvival,withthelackofmajor
cytogeneticresponse(MCR),andwithprogressiontoBPina
BrazilianAP-CMLpopulationfromasinglereferralcenter.
Methods
This retrospective study, performed from January 2000 to
November 2011, comprised139 patientswith AP-CML who
were treated withimatinib atthe hematopoietic stem cell
transplant(HSCT)centerofHospitaldeClínicas ofthe
Uni-versidade Federal do Paraná, Brazil. The WHO criteria are
routinelyusedtoevaluatepatientswithAP-CMLatthiscenter.
However,astheobjectiveofthisstudywastodoanexploratory
analysisofpublishedriskfactors,subjectswerecategorized
withAP-CMLiftheyhadatleastoneoftheaforementioned
publishedcriteria.6,11–13 Allpatientsreceivedimatinibatan
initialdoseof600mgasfirsttherapyforAP-CML.Doseswere
incremented (maximum of 800mg) in casesof inadequate
responseorreduced(minimumof300mg)incasesof
hemato-logicalornon-hematologicaltoxicity,asnecessary.Thisstudy
wasapprovedbytheEthicsCommitteeofHospitaldeClínicas,
Universidade FederaldoParaná,which waivedthe
require-mentofinformedconsent,asthiswasaretrospectivestudy
withcollectionofdatafrommedicalrecords.
Thefollowingriskfactors,someofwhichwere selected
according to previously published criteria (Table 1), were
evaluated:basophils≥20%inPB,platelets>1000×109/L
unre-sponsivetotherapyor<100×109/LinPB, whiteblood cells
(WBC) >100×109/L in PB, blast 10–29% in PB, presence of
clonalevolution(CE),hemoglobin<10g/dL,andsplenomegaly.
Splenomegalywasconsideredwhenthespleenwaspalpable
≥10cmfromtheleftcostalmargindespitetheuseof
hydrox-yurea.Otherclinicalfactorsrelevanttothediseasewerealso
analyzed, including the Sokal score>0.8 (calculated at the
timeofdiagnosis),timebetweendiagnosisofCMLand
treat-mentwithimatinib>12months,previoususeofinterferon,
age>60years,andGrades3–4hematologictoxicity.
AsthePBblastscut-offpointvariesintheexistingcriteria,
thisstudyanalyzedPBblastsasacontinuumwithdeathas
theendpoint.Areceiveroperatingcharacteristic(ROC)curve
withdeathastheendpointwasdesignedtoidentifyacut-off
valueforthePBblastcount.
Cytogenetic analysis was performed by the G-banding
technique.Bonemarrowspecimenswereexaminedondirect
short-term(24-h)cultureswithatleast20metaphasesbeing
analyzed. BCR-ABL transcriptswere detected by analyzing
peripheralbloodwithquantitativereal-timepolymerasechain
reaction(PCR)accordingtotheInternationalScale.
Statisticanalyses were performedusing the STATA
pro-gram version 8.0. Bivariateand multivariate analyses were
performed using the Cox proportional hazards regression
model. Variables with p-values<0.20 in the bivariate
anal-ysis were included in the multivariate analysis model. A
p-value<0.05wasconsideredstatisticallysignificant.Disease
freesurvival(DFS)wasdefinedasthetimefromthebeginning
oftreatmenttolossofMCR.
Theprimaryendpointofthisstudywastheidentification
ofriskfactorsforsurvival.RiskfactorsforlackofMCRand
transformationtoBPwereevaluatedassecondaryendpoints.
TheBPconsideredPBormarrowblasts≥30%.
Results
Onehundredandsixty-threepatientsinAP-CMLwere
identi-fied.Twenty-fourpatientstreatedwithdasatinibornilotinib
wereexcluded.Thus,139AP-CMLpatientsweretreatedwith
imatinib.Ofthese139patients,60(43.2%)patientswho
pre-sentedatthiscenterwithAPhadonlyreceivedhydroxyurea
previously.Theremaining79(56.8%)patientsprogressedfrom
CPCMLtreatedmainlywithhydroxyureaorinterferonalpha
inisolationor withAra-C.Ofthe 139patientsincluded, 62
(44.6%)were female and 77(55.4%)were male. Medianage
was 43.6 years and 25 (18%) were >60 years ofage.
Table1–ListofthecriteriadefiningacceleratedphasechronicmyeloidleukemiaasrecommendedbyMDACC,6IBMTR,11 WHO12andELN.13
Criteria MDACC IBMTR WHO ELN
Blasts PBorBM10–29% PBorBM≥10% PBorBM10–19% PBorBM15–29%
Blastsandpromyelocytes ≥30% PBorBM≥20% NA ≥30%with
blasts<30%
Basophils PBorBM≥20% PB≥20% PB≥20% PB≥20%
Platelets >1000×109/L,or
<100×109/L,
unresponsiveto therapy
Persistent thrombocytosis
>1000×109/L,or
<100×109/L,
unresponsiveto therapy
Persistent throm-bocytopenia (<100×109/L)
unrelatedto therapy
WBC >10×109/L Difficultcontrol IncreasingWBC
count
unresponsiveto therapy
NA
Anemia NA Unresponsiveto
therapy
NA NA
Splenomegaly Persistent
splenomegaly unresponsiveto sustained therapy
Increasing spleensize
Increasing spleensize
NA
Cytogenetic NA CE CEnotpresentat
thetimeof diagnosis
Clonal chromosome abnormalitiesin Ph+cells
(CCA/Ph1),major route,on treatment
Others Myelofibrosis,
chloromas
Largefocior clustersofblasts inbonemarrow biopsy
MDACC:M.D.AndersonCancerCenter;IBMTR:InternationalBloodandMarrowTransplantRegistry;WHO:WorldHealthOrganization;ELN: EuropeanLeukemiaNet;NA:notapplicable;CE:clonalevolution;WBC:whitebloodcell;PB:peripheralblood;BM:bonemarrow.
(15.83%) died due todisease progression. Previoustherapy included interferon insixty-fourpatients (46.04%), hydrox-yureain131 patients(94.24%) and busulfaninonepatient (0.72%). Eighty-four patients (60.4%) had intervals between CML diagnosis and treatment with imatinib>12 months. Therewere128patientswithcytogenetictestsavailableand 79 patients with molecular tests available. Among them, 76patients (54.7%)achievedMCR,with 67(48%)attained a completecytogeneticresponseandnine(6.7%)partial cytoge-neticresponse;26patients(18.7%)achievedmajormolecular response(MMR).Thirtypatients(21.59%)progressedtoBPand fivepatients(3.6%)underwentHSCT.
Forty-onepatients(29.5%)hadCE.Amongthesepatients, 13had complex karyotypes,sevenhad trisomy of chromo-some8,threehad duplicationofthe Philadelphia chromo-some,fourhadchromosome7alterations,onehad isochro-mosome 17q and 11 had other minor route chromosomal aberrations.14 Thirty-eight (27.34%) had splenomegaly; 28
(20.14%)hadanemia;29(20.86%)hadplatelets>1000×109/L
or<100×109/Lunresponsivetotherapy; six(4.32%)had PB
basophils≥20%, ten (7.19%) had WBC>100×109/L and 13
(9.35%)hadPBblasts10–29%(Table2).
Risk factors for poor survival by bivariate analysis
includedWBC>100×109/L(p-value=0.1496),PBblasts10–29%
(p-value=0.009), PB basophils≥20% (p-value=0.04), Grades
3–4 hematologic toxicity (p-value=0.0001), hemoglobin<
10g/dL (p-value=0.033), age>60 (p-value=0.080), and time
fromCMLdiagnosistotreatmentwithimatinib>12months
(p-value=0.018).Inforwardmultivariateanalysis,onlyGrades
Table2–Characteristicsofthe139acceleratedphase chronicmyeloidleukemiapatients.
Variable
Gender,male/female,n(%) 77/62(55.4/44.6)
Age(years),median(range) 43.6(10–78)
Previoustherapy
Interferon,n(%) 64(46.0)
Hydroxyurea,n(%) 60(43.2)
Others,n(%) 15(10.8)
Hemoglobin<10g/dL,n(%) 28(20.14)
Platelets>1000×109/Lor<100×109/L,n(%) 29(21.86) Spleen≥10cmfromleftcostalmargin,n(%) 38(27.34)
PBblasts10–29%,n(%) 13(9.35)
PBbasophils≥20%,n(%) 6(4.32)
Clonalevolution(%) 41(29.5)
WBC≥100×109/L,n(%) 10(7.35)
Table3–Factorsassociatedwithlowersurvivalratesin139acceleratedphasechronicmyeloidleukemiapatientstreated withimatinib.
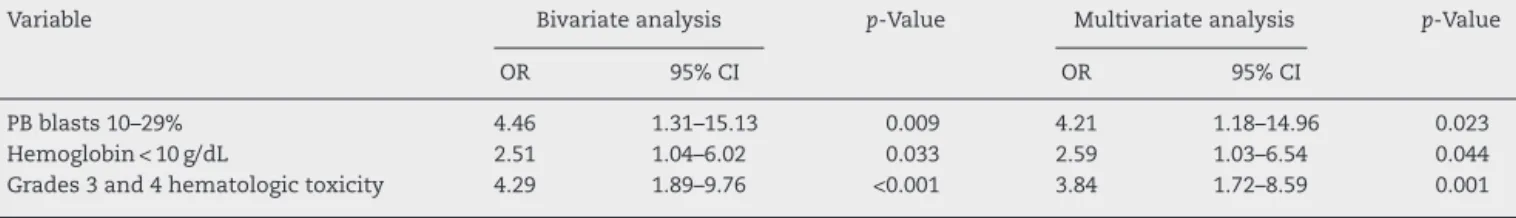
Variable Bivariateanalysis p-Value Multivariateanalysis p-Value
OR 95%CI OR 95%CI
PBblasts10–29% 4.46 1.31–15.13 0.009 4.21 1.18–14.96 0.023
Hemoglobin<10g/dL 2.51 1.04–6.02 0.033 2.59 1.03–6.54 0.044
Grades3and4hematologictoxicity 4.29 1.89–9.76 <0.001 3.84 1.72–8.59 0.001
OR:oddsratio;95%CI:95%confidenceinterval;PB:peripheralblood.
3–4 hematologic toxicity [p-value=0.001; odds ratio (OR) of 3.84; 95% confidence interval (95% CI) of 1.72–8.59], PB blasts10–29%(p-value=0.023;ORof4.21;95%CI,1.18–14.96) andhemoglobin<10g/dL(p-value=0.044;ORof2.59;95%CI, 1.03–6.54)remainedsignificant(Table3).
Risk factors for lack ofMCR bybivariate analysis were
hemoglobin<10g/dL (p-value=0.002), PB blasts 10–29%
(p-value=0.006), platelets>1000×109/L or <100×109/L (p
-value=0.088), splenomegaly (p-value=0.128), basophils
>20% (p-value=0.032), Grades 3–4 hematologic toxicity (p
-value=0.023),HighSokalscore(p-value=0.048),andprevious
use of interferon (p-value=0.041). In forward multivariate
analysis, only hemoglobin<10g/dL (p-value=0.001; OR of
5.27; 95% CI, 1.98–14.07), PB blasts 10–29% (p-value=0.007;
ORof6.84;95%CI,1.68–27.89)andprevioususeofinterferon
(p-value=0.032;ORof2.38;95%CI,1.08–5.24)wereidentified
assignificant(Table4).
Risk factors for progression to BP by bivariate
analy-sis were hemoglobin<10g/dL (p-value=0.003), high Sokal
score (p-value=0.064), PB blasts 10–29% (p-value=0.052),
WBC>100×109/L (p-value=0.024), time from CML
diagno-sistotreatmentwithimatinib>12 months(p-value=0.014),
and basophils>20% (p-value=0.007). In forward
multivari-ateanalysis,onlyhemoglobin<10g/dL(p-value=0.005;ORof
3.94;95%CI,1.53–10.15),basophils>20%(p-value=0.023;OR
of7.77;95%CI,1.32–45.62)andtimefromCMLdiagnosis to
treatmentwith imatinib>12months (p-value=0.030; ORof
3.12;95%CI,1.11–8.75)remainedsignificant(Table5).Patients
withone(p-value=0.040;hazardratio[HR]of2.60;95%CIof
1.04–6.49)andtwoormoreriskfactorsforprogressiontoBP(p
-value<0.001;HRof5.52;95%CI,2.14–14.25)hadlowersurvival
comparedwithpatientswhodidnothaveanyofthesefactors
(Figure1).
AsthePBblastscut-offpointvariesinexistingcriteria,this
studyanalyzedPBblastsasacontinuumwithdeathasthe
endpoint.ThePBblastcut-offpoint,basedontheROCcurve,
was3%withasensitivityof46.5%andspecificityof89.8%.
After a median follow-up of 39 months(range: 3.4–129
months),69.23%ofthepatientswerealive.TheOSwas66%at5
years(Figure2).ThefivepatientssubmittedtoHSCTwere
cen-soredintheOS.Ofthosefivepatients,fourremainaliveandin
profoundmolecularresponse.Onepatient,whorelapsedafter
transplantandreceivedtherapywithimatinib,alsoachieved
profoundmolecularresponse.Forthepatientswhoachieved
MCR, DFS was 83% at 5 years (Figure 3). MCR correlated
withbetterOS(p-value<0.001;HR=7.49;95%CI=3.62–15.52)
(Figure4).
Figure1–Survivalestimatesaccordingtothepresenceof prognosticfactorsforprogressiontoblastphase.Unbroken line:patientswithnoprognosticfactors(n=46);dashed
line:patientswithoneprognosticfactor(n=68,Cox
regression:p-value=0.04);dottedline:patientswithtwoor
moreprognosticfactors(n=25,Coxregression: p-value<0.001).
Twenty-six(18.71%)patientslostmolecularresponseand
17(12.23%)patientslostcytogeneticresponse.Patientswho
achievedMMRhadslightlybetterDFSratescomparedwith
those who did not reach MMR, but the difference did not
achievestatisticalsignificance(p-value=0.250;HZ=1.93;95%
CI=0.63–5.93)(Figure5).
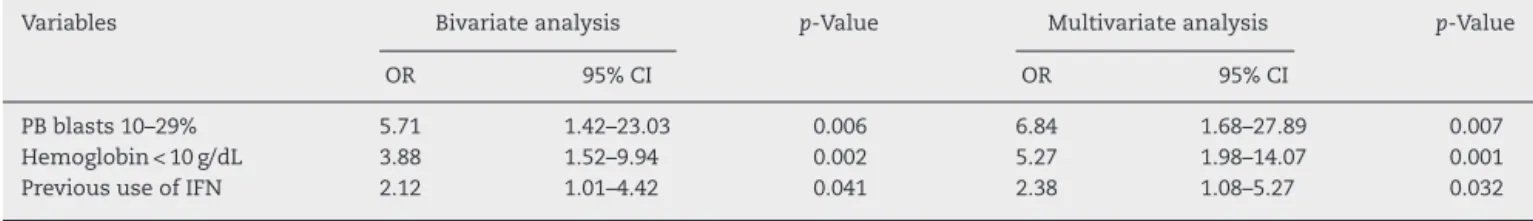
Table4–RiskFactorsfornotachievingmajorcytogeneticresponseof139acceleratedphasechronicmyeloidleukemia patientstreatedwithimatinib.
Variables Bivariateanalysis p-Value Multivariateanalysis p-Value
OR 95%CI OR 95%CI
PBblasts10–29% 5.71 1.42–23.03 0.006 6.84 1.68–27.89 0.007
Hemoglobin<10g/dL 3.88 1.52–9.94 0.002 5.27 1.98–14.07 0.001
PrevioususeofIFN 2.12 1.01–4.42 0.041 2.38 1.08–5.27 0.032
OR:oddsratio;95%CI:95%confidenceinterval;PB:peripheralblood;IFN:interferon.
Table5–Factorsassociatedwithprogressiontoblastphasein139acceleratedphasechronicmyeloidleukemiapatients treatedwithimatinib.
Variables Bivariateanalysis p-Value Multivariateanalysis p-Value
OR 95%CI OR 95%CI
TimebetweenCMLDxandRxwithimatinib>12months 3.27 1.21–8.85 0.014 3.12 1.11–8.75 0.03
PBbasophils≥20% 8.08 1.33–49.18 0.007 7.77 1.32–45.62 0.023
Hemoglobin<10g/dL 3.79 1.48–9.69 0.003 3.94 1.53–10.15 0.005
OR:oddsratio;95%CI:95%confidenceinterval;Dx:diagnosis;Rx:treatment;PB:peripheralblood.
Figure3–DiseaseFreeSurvival.
Figure4–Overallsurvivalaccordingtomajorcytogenetic response(Coxregression;p-value<0.001).Unbrokenline:
majorcytogeneticresponse(n=76);dashedline:failureto
achievemajorcytogeneticresponse(n=52).
Discussion
As AP-CML criteria vary inthe literature, the current pop-ulation wasstudiedtoevaluateprognosticfactors forpoor survival,lackofMCRandprogressiontoBP.Publishedcriteria aswellasotherclinicallyrelevantfactorswereconsidered.
Regardingtheprimaryendpoint,parametersofpoor sur-vivalwereGrades3–4hematologictoxicity,highblastcounts, andlowhemoglobinconcentration.Nostatisticalsignificance was found for platelets or CE. Kantarjian et al. reported pretreatmenthemoglobin<10g/dLandalackofcytogenetic response afterthree monthson imatinib therapyas nega-tivepredictors ofsurvivalinAP-CML.15 Similarrisk factors
wereidentifiedbyJiangetal.,whoreportedthatCML
dura-tionbeforetreatment>12months,hemoglobin<10g/dLand
PBblasts>5%wereindependentadverseprognosticfactorsfor
Figure5–DiseaseFreeSurvivalaccordingtomajor molecularresponse(Coxregression;p-value=0.250).
Unbrokenline:Majormolecularresponse(n=26);dashed
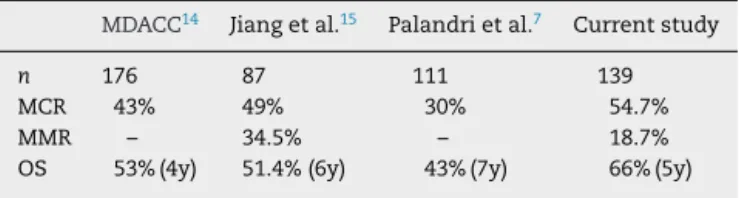
Table6–Publishedresultsofimatinibtherapyfor acceleratedphasechronicmyeloidleukemiapatients.
MDACC14 Jiangetal.15 Palandrietal.7 Currentstudy
n 176 87 111 139
MCR 43% 49% 30% 54.7%
MMR – 34.5% – 18.7%
OS 53%(4y) 51.4%(6y) 43%(7y) 66%(5y)
MDACC:M.D.AndersonCancerCenter;MCR:majorcytogenetic response;MMR:majormolecularresponse;OS:overallsurvival.
bothOS andprogression-freesurvival.16Interestinglywhen
theblastpercentagewasanalyzedasacontinuousvariablea
cut-offpointof3%inthePBwasidentifiedasapredictorof
poorsurvival,suggestingthatlowervaluesofPBblastscanbe
ofprognosticvalue.16
Hematologicaltoxicitywithimatinibismorefrequentin
thelatestagesofthedisease.Severepancytopeniadeveloped
bysomepatientsmayindicateanexhaustionofthenormal
clonewithdiseaseprogression.Someauthorshadpreviously
identifiedhematologictoxicityaftertyrosinekinaseinhibitor
therapyasariskfactorforsurvival.17,18
Risk factors for not achieving MCR were
hemoglobin<10g/dL, PB blasts>5%, and previous use of
interferon.Astreatment withimatinibcompared favorably
with the results of treatment using interferon and other
therapiesin AP-CML patients,15 it seems that delayingthe
most effective therapy was the explanation that previous
useofinterferonwasanegativefactorforMCR.TheGruppo
Italiano Malattie EMatologiche dell’Adulto (GIMEMA) CML
WorkingPartyfoundthatastableand confirmedcomplete
cytogenetic response to imatinib constitutes an affordable
surrogate marker of long-term OS and DFS, even among
AP-CMLpatients.7
PredictorsofprogressiontoBPwerehemoglobin<10g/dL,
basophils≥20%, and time from diagnosis to therapy>12
months.ThedefinitionofBPusedinthisstudy was30%or
moreblastsinordertoincludeeverypatientthatwouldbe
classifiedasinAPbyatleastonepublishedcriterion.Thelow
numberofpatientslimitedthepossibilityoffurther
categoriz-ingpatientsbyblastpercentageinmultivariateanalysisand
thatisalimitationofthepresentstudy.Anincreasedbasophil
countisoneoftheriskfactorsidentifiedassignificantforOS
andDFSbyHoffmannetal.,anditwasusedtocalculatethe
EuropeanTreatmentandOutcomeStudy(EUTOS)score.19
TheratesofMCRandMMRinthisstudywere54.7%and
18.7%,respectively,andthussimilartopreviousarticles6,7,15
(Table6).CMLduration>12monthsandhemoglobin<10g/dL
wereindependentadversepredictorsofDFS.16AsfortheMMR,
therewasasmallnumberofpatientswithavailablemolecular
tests,andthiscouldexplainthelackofsignificanceofMMR
ontheDFSrate,althoughatrendwasidentified.TheSokal
riskscorehasbeen reportedtopredictmolecular response
and OS.20 Moreover, Tripathiet al. identified a correlation
betweenanincreasedtotalleukocytecountandpoor
cytoge-neticresponse.21However,inthisstudy,theSokalscoreand
hyperleukocytosiswerenotassociatedwithprogressiontoBP.
CEandagewereclinicalfactorsnotsignificantlyassociated
witheither the primary or secondary endpoints. Although
some authors suggest that CE is not an important factor
for achieving MMR or complete cytogenetic response with
imatinibtherapy,22itisanindependentpoorprognostic
fac-tor for survival in both CP-CML and AP-CML according to
others.23Regardingage,olderpatientstreatedwithimatinib
werereportedbysomeauthorsashavingworsesurvival,but
notbyothers.24,25AccordingtoCortesetal.,agewasnotfound
tobeanindependentpoorprognosticfactorforachieving
cyto-geneticresponseandforsurvivalinAP-CMLpatients.26
Asmentionedbefore,Jiangetal.demonstratedthatCML
durationbeforetreatment≥12months,hemoglobin<100g/L,
and PB blasts≥5%were riskfactors forsurvivalamongAP
patientswithCML.Patientswereclassifiedintolowrisk(ifthey
hadnoneofthefactors),intermediaterisk(iftheyhadone
fac-tor),orhighrisk(forthosewithatleast2factors).16Allogeneic
HSCTgivessignificantsurvivaladvantagesonlyforhighand
intermediate-riskpatientswithAP-CML.Forlowriskpatients,
imatinibtherapyleadstoasimilaroutcomeasHSCT.16
This study provides long-term results of survival and
responseofacohortofAP-CMLpatientstreatedwith
imat-inib.Thefive-yearOSof60%andDFSof75%amongthose
whoachievedMCRarereassuringresults,whichindicatethat
patientswholackidentifiedriskfactorscanachievelong-term
responsewithimatinib.
Prospectivestudiesareveryimportanttodefinewhichis
thebesttherapyforthispopulation.Thus,identifyingrisk
fac-torscanallowfortailoredtherapywiththeaimofimproving
survival,whichisusuallypoorinadvancedstageCML.
Conclusion
These dataindicatethat patientswithGrades3–4
hemato-logictoxicityaftertherapywithimatinib,PBblasts10–29%,
andhemoglobin<10g/dL,hadworsesurvivalthansomeother
patients also classified as AP.Lackof MCR wasassociated
with hemoglobin<10g/dL, PB blasts 10–29%, and previous
useofinterferon.WhereasprogressiontoBPwascorrelated
withhemoglobin<10g/dL,PBbasophils≥20%,andtimefrom
CMLdiagnosistotreatmentwithimatinib>12months.This
informationcan allowfortailoredtherapy withthe aimof
improvingresults,whichareusuallypoorinadvancedstage
CML.
Conflicts
of
interest
TheauthorsFunkeVAMandPasquiniRarespeakersand/or
partoftheadvisoryboardofBMS-BrazilandNovartis-Brazil.
r
e
f
e
r
e
n
c
e
s
1.FaderlS,TalpazM,EstrovZ,O’BrienS,KurzrockR,Kantarjian HM.Thebiologyofchronicmyeloidleukemia.NEnglJMed. 1999;341(3):164–72.
2.SawyersCL.Chronicmyeloidleukemia.NEnglJMed. 1999;340(17):1330–40.
chronicmyeloidleukemia.NEnglJMed.2003;348(11): 994–1004.
4. DrukerBJ,SawyersCL,KantarjianH,RestaDJ,ReeseSF,Ford JM,etal.ActivityofaspecificinhibitoroftheBCR-ABL tyrosinekinaseintheblastcrisisofchronicmyeloidleukemia andacutelymphoblasticleukemiawiththePhiladelphia chromosome.NEnglJMed.2001;344(14):1038–42.
5. SawyersCL,HochhausA,FeldmanE,GoldmanJM,MillerCB, OttmannOG,etal.Imatinibinduceshematologicand cytogeneticresponsesinpatientswithchronicmyelogenous leukemiainmyeloidblastcrisis:resultsofaphaseIIstudy. Blood.2002;99(10):3530–9.
6. CortesJE,TalpazM,O’BrienS,FaderlS,Garcia-ManeroG, FerrajoliA,etal.Stagingofchronicmyeloidleukemiainthe imatinibera:anevaluationoftheWorldHealthOrganization proposal.Cancer.2006;106(6):1306–15.
7. PalandriF,CastagnettiF,AlimenaG,TestoniN,BrecciaM, LuattiS,etal.Thelong-termdurabilityofcytogenetic responsesinpatientswithacceleratedphasechronicmyeloid leukemiatreatedwithimatinib600mg:theGIMEMACML WorkingPartyexperienceaftera7-yearfollow-up. Haematologica.2009;94(2):205–12.
8. DrukerBJ,TalpazM,RestaDJ,PengB,BuchdungerE,FordJM, etal.EfficacyandsafetyofaspecificinhibitoroftheBCR-ABL tyrosinekinaseinchronicmyeloidleukemia.NEnglJMed. 2001;344(14):1031–7.
9. KantarjianH,SawyersC,HochhausA,GuilhotF,SchifferC, Gambacorti-PasseriniC,etal.Hematologicandcytogenetic responsestoimatinibmesylateinchronicmyelogenous leukemia.NEnglJMed.2002;346(9):645–52.
10.DrukerBJ,GuilhotF,O’BrienSG,GathmannI,KantarjianH, GattermannN,etal.Five-yearfollow-upofpatientsreceiving imatinibforchronicmyeloidleukemia.NEnglJMed. 2006;355(23):2408–17.
11.SpeckB,BortinMM,ChamplinR,GoldmanJM,HerzigRH, McGlavePB,etal.Allogeneicbone-marrowtransplantation forchronicmyelogenousleukaemia.Lancet.
1984;1(8378):665–8.
12.VardimanJW,HarrisNL,BrunningRD.TheWorldHealth Organization(WHO)classificationofthemyeloidneoplasms. Blood.2002;100(7):2292–302.
13.BaccaraniM,DeiningerMW,RostiG,HochhausA,SoveriniS, ApperleyJF,etal.EuropeanLeukemiaNetrecommendations forthemanagementofchronicmyeloidleukemia:2013. Blood.2013;122(6):872–84.
14.FabariusA,LeitnerA,HochhausA,MüllerMC,HanfsteinB, HaferlachC,etal.Impactofadditionalcytogenetic aberrationsatdiagnosisonprognosisofCML:long-term observationof1151patientsfromtherandomizedCMLStudy IV.Blood.2011;118(26):6760–8.
15.KantarjianH,TalpazM,O’BrienS,GilesF,FaderlS,Verstovsek S,etal.Survivalbenefitwithimatinibmesylatetherapyin
patientswithaccelerated-phasechronicmyelogenous leukemia–comparisonwithhistoricexperience.Cancer. 2005;103(10):2099–108.
16.JiangQ,XuLP,LiuDH,LiuKY,ChenSS,JiangB,etal.Imatinib mesylateversusallogeneichematopoieticstemcell
transplantationforpatientswithchronicmyelogenous leukemiaintheacceleratedphase.Blood.
2011;117(11):3032–40.
17.FunkeVA,MedeirosCR,LimaDH,SetúbalDC,BitencourtMA, BonfimCM,etal.Therapyofchronicmyeloidleukemiawith imatinibmesylateinBrazil:astudyof98cases.RevBras HematolHemoter.2005;27(3):159–65.
18.SneedTB,KantarjianHM,TalpazM,O’BrienS,RiosMB, BekeleBN,etal.Thesignificanceofmyelosuppressionduring therapywithimatinibmesylateinpatientswithchronic myelogenousleukemiainchronicphase.Cancer. 2004;100(1):116–21.
19.HoffmannVS,BaccaraniM,LindoerferD,CastagnettiF, TurkinaA,ZaritskyA,etal.TheEUTOSprognosticscore: reviewandvalidationin1288patientswithCMLtreated frontlinewithimatinib.Leukemia.2013;27(10):
2016–22.
20.HughesTP,KaedaJ,BranfordS,RudzkiZ,HochhausA, HensleyML,etal.Frequencyofmajormolecularresponsesto imatiniborinterferonalfapluscytarabineinnewlydiagnosed chronicmyeloidleukemia.NEnglJMed.2003;349(15): 1423–32.
21.TripathiAK,KumarA,RamaswamyA.Totalleukocytecounts andtherequirementofdosereductionduetocytopeniasas prognosticindicatorsaffectingresponsetoimatinibin chronicmyeloidleukemia.IndianJHematolBloodTransfus. 2011;27(1):7–13.
22.CortesJE,TalpazM,GilesF,O’BrienS,RiosMB,ShanJ,etal. Prognosticsignificanceofcytogeneticclonalevolutionin patientswithchronicmyelogenousleukemiaonimatinib mesylatetherapy.Blood.2003;101(10):3794–800.
23.O’DwyerME,MauroMJ,BlasdelC,FarnsworthM,KurilikG, HsiehYC,etal.Clonalevolutionandlackofcytogenetic responseareadverseprognosticfactorsforhematologic relapseofchronicphaseCMLpatientstreatedwithimatinib mesylate.Blood.2004;103(2):451–5.
24.BrennerH,GondosA,PulteD.Recenttrendsinlong-term survivalofpatientswithchronicmyelocyticleukemia: disclosingtheimpactofadvancesintherapyonthe populationlevel.Haematologica.2008;93(10):1544–9.
25.ThygesenLC,NielsenOJ,JohansenC.Trendsinadult leukemiaincidenceandsurvivalinDenmark,1943–2003. CancerCausesControl.2009;20(9):1671–80.