rev bras hematol hemoter. 2015;37(5):302–305
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Impact
of
a
confirmatory
RhD
test
on
the
correct
serologic
typing
of
blood
donors
Luciana
Cayres
Schmidt
a,
Lilian
Castilho
b,
Otavio
Vinicius
Neves
Vieira
a,
Emília
Sippert
b,
Ane
Caroline
Gaspardi
b,
Marina
Lobato
Martins
a,
Maria
Clara
Fernandes
da
Silva
Malta
a,∗aFundac¸ãoCentrodeHematologiaeHemoterapiadeMinasGerais(Hemominas),BeloHorizonte,MG,Brazil
bUniversidadeEstadualdeCampinas(Unicamp),Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27February2015 Accepted3June2015 Availableonline9July2015
Keywords:
Bloodgroup
Moleculargeneticstransfusion Redbloodcellantigens
a
b
s
t
r
a
c
t
Background:TheRHDgeneishighlypolymorphic,whichresultsinalargenumberofRhD variantphenotypes.DiscrepanciesinRhDtypingarestillaprobleminbloodbanksand increasetheriskofalloimmunization.Inthisstudy,theRhDtypingstrategyatabloodbank inBrazilwasevaluated.
Methods:One-hundredandfifty-twosamplestypedasRhDnegativeandCorEpositiveby routinetests(automatedsystemandindirectantiglobulintestusingthetubetechnique) werereevaluatedforRhDstatusbythreemethods.Themethodwiththebestperformance wasimplementedandevaluatedforaperiodofoneyear(n=4897samples).Samplesthat wereDpositiveexclusivelyintheconfirmatorytestweresubmittedtomolecularanalysis.
Results:Thegeltestforindirectantiglobulintestingwithanti-DimmunoglobulinG(clone ESD1)presentedthebestresults.Seventysamples(1.43%)previouslytypedasRhDnegative showedreactivityinthegeltestforindirectantiglobulintestingandwerereclassifiedasD positive.Dvariantsthatmaycausealloimmunization,suchasweakDtype2andpartial DVI,weredetected.
Conclusion:TheconfirmatoryRhDtestusingthegeltestforindirectantiglobulintesting representsabreakthroughintransfusionsafetyinthisbloodcenter.Ourresultsemphasize theimportanceofassessingthebloodgrouptypingstrategyinbloodbanks.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
TheDblood groupantigenisthemostclinicallyimportant proteinoftheRhsystemduetoitsinvolvementinhemolytic
∗ Correspondingauthorat:Fundac¸ãoHemominas,Servic¸odePesquisa,AlamedaEzequielDias,321,SantaEfigênia,30130-110Belo
Hori-zonte,MG,Brazil.
E-mailaddress:maria.malta@hemominas.mg.gov.br(M.C.F.daSilvaMalta).
transfusionreactionsandhemolyticdiseasesofthefetusand newborns.1Despitemethodologicaladvances,discrepancies
inRhtypingarestillaproblemduringroutine immunohema-tologyservicetests.2–4
http://dx.doi.org/10.1016/j.bjhh.2015.06.001
revbrashematolhemoter.2015;37(5):302–305
303
TheRHDgeneishighlypolymorphicasithasmorethan 200 alleles.This results in a large number of RhDvariant phenotypes.5Rhdiscrepanciesmayarisewhenanindividual
hasavariantoftheDantigen,suchaspartialDorweakD,and maybemistypedasDnegative.Thesediscrepanciescancause incorrectbloodcomponenttransfusions,leadingtoincreased riskofalloimmunization.3
Inadditiontothegreatnumberofvariants,amajorcause ofdiscrepanciesinRhDtypingistheexistenceofseveralRhD typingmethodsandreagents withdifferentsensitivities.6–9
Bloodgrouptyping strategiesandpoliciesforthe selection ofmethodsand reagentsvary betweencountries.2 In
addi-tion,fewcenters intheworldroutinely performmolecular testsforthe Rh typing ofblood donors. Therefore, despite thebestefforts,thenumberofDvariantblooddonorstyped as D negative, even by an indirect antiglobulin test (IAT), isgreaterthan previouslyexpected.10 Thismisclassification
resultsinanincreasedriskofanti-Dalloimmunization,which hasimportanthealthimplications,especiallyforwomenof childbearingage.
Inthisstudy,theeffectivenessofserologicaltestsusedfor theRhDtypingofblooddonorswereevaluatedinalargeblood centerinBrazil(Fundac¸ãoHemominas).
Methods
Studypopulation
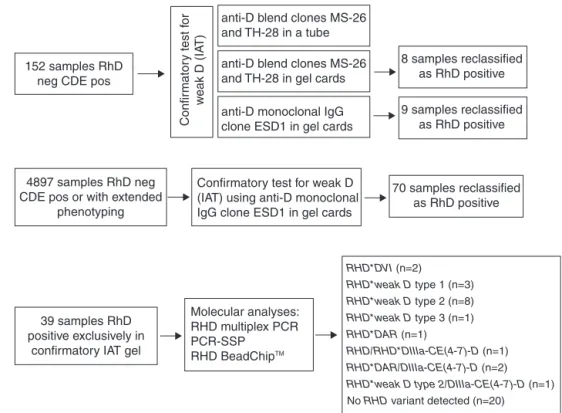
Inthefirststepofthisstudy,redbloodcell(RBC)samplesfrom 152blooddonorsphenotypedasDnegativewhowereCorE positiveinroutinetestsusinganautomatedsystem(Olympus PK72000)wereanalyzed.Thesesamplesweretestedforweak Dbythreedifferentserologicalmethodsandusingdifferent reagents.
Thesecond step consisted ofimplementing and evalu-ating aprotocol ofRhD phenotypingbased on an indirect antiglobulintechniqueusingLISS/Coombsgelcards(Diamed, Switzerland)andamonoclonalreagent,anti-D immunoglob-ulinG(IgG)(cloneESD1).Thisconfirmatorytestwasevaluated and comparedtothe IATtube test overone year toverify theRhDstatusofblooddonors(n=4897)who were pheno-typed as D negativeand C or E positive or with extended phenotyping.
Thelaststepofthisstudy consistedofusingmolecular methodstoconfirmtheRhDstatusofthesamplesthatwere typedasDpositiveexclusivelyintheconfirmatorygeltestfor indirectantiglobulintesting(n=39).
Serologicalstudies
RhDtypingwasfirstperformed withanautomatedsystem (OlympusPK7200)usingtwoanti-Dreagents:amonoclonal blendofanti-D clones(MS201 and MS26;Fresenius Kabi, Brazil)diluted1–32and1–64insalinesolution,anda mono-clonal antibody blend of RUM-1 and MS-26 clones (Lorne Reagents)diluted1–64insalinesolution.AlldonorRBCswere treatedwith0.2%bromelin.RBCsfoundtobeDnegativewere furthertested for weak D byIAT (tube technique)using a monoclonalanti-Dblend(FreseniusKabi,Brazil).
AllDnegativeRBCsamplesweretestedwithmonoclonal antisera(anti-CDE),andclonesP3X61,P3X25513G8andP3X234 (FreseniusKabi,Brazil)inatube,accordingtothe manufac-turer’sinstructions.
ThesamplestypedasDnegativeandCorEpositive,as described above, were tested for weak D (IAT) using three protocols: anti-D blend clones MS-26 and TH-28 (Diamed, Switzerland) inatube; anti-DblendclonesMS-26 and TH-28 in gel cards; and the anti-D IgG clone ESD1 in gel cards. The gel tests were performed on cards (ID LISS Coombs;Diamed,Switzerland)andwereincubatedat37◦Cfor
15min.
TheprotocolforweakDusinganti-DESD1ingelcardswas implementedintheblood centerasaroutineconfirmatory testandwasevaluatedforoneyear.
Molecularanalyses
Samples typedas Dpositive inthe confirmatory testwere submitted tomultiplex polymerasechainreaction (PCR) to amplifyfiveRHD-specificexons:3,4,5,7and9.11,12The
sam-pleswerealsoanalyzedforthepresenceofweakDTypes1, 2and3usingaPCR-sequencespecificprimer(PCR-SSP)13and
weretestedforthepresenceofotherDvariantsusingRHD BeadChipTManalysis(BioArraySolutions,Immucor)according
tothemanufacturer’sinstructions.
Ethicalconsiderations
TheEthicsCommitteeoftheFundac¸ãoHemominasapproved thestudy(CEPno.136.163).
Results
Samplesfrom152blooddonorstypedasDnegativeandCor Epositivebyanautomatedsystemwereevaluatedusingthe threeprotocols.Nine(5.9%)ofthesamplesphenotypedasD negativebythetubetestpresentedpositiveresultsingelcards (LISS/Coombs)usinganti-DIgG(cloneESD1).Theanti-Dblend reagent(clonesMS-26andTH-28)detectedpositivityofeight ofthesesamples.TheDpositivestatusoftheninesamples wasconfirmedbyRHDgenotyping.
Basedontheseresults,Fundac¸ãoHemominasdecidedto implementaprotocoltoconfirmRhDtypingusinganti-DIgG monoclonal(cloneESD1)ingelcardsofallblooddonors phe-notypedasDnegativeandCorEpositiveorwithextended phenotyping.
Duringoneyear,4897samplesofblooddonorsfrom dif-ferent regionsofMinasGerais(Brazil)were referredtothis bloodcentertoconfirmtheRhDtyping.AllRBCsampleshad originallybeentestedwithananti-Dblend(clonesMS-26and TH-28;Diamed-Biorad)bythetubetest.
Theresultsofthisstudyshowedthat70samples(1.43%) previouslytypedasRhDnegativebythetubetestpresented weakreactivityingelcardsusinganti-DIgG(cloneESD1)and werereclassifiedasDpositive.
304
revbrashematolhemoter.2015;37(5):302–305152 samples RhD neg CDE pos
8 samples reclassified as RhD positive
9 samples reclassified as RhD positive
70 samples reclassified as RhD positive
RHD*DVI (n=2)
RHD*weak D type 1 (n=3) RHD*weak D type 2 (n=8) RHD*weak D type 3 (n=1)
RHD*DAR (n=1)
RHD/RHD*DIIIa-CE(4-7)-D (n=1) RHD*DAR/DIIIa-CE(4-7)-D (n=2) RHD*weak D type 2/DIIIa-CE(4-7)-D (n=1) No RHD variant detected (n=20)
Confir
mator
y test f
or
w
eak D (IA
T)
4897 samples RhD neg CDE pos or with extended
phenotyping
39 samples RhD positive exclusively in
confirmatory IAT gel
Molecular analyses: RHD multiplex PCR PCR-SSP
RHD BeadChipTM
Confirmatory test for weak D (IAT) using anti-D monoclonal IgG clone ESD1 in gel cards
anti-D blend clones MS-26 and TH-28 in a tube
anti-D blend clones MS-26 and TH-28 in gel cards
anti-D monoclonal IgG clone ESD1 in gel cards
Figure1–FlowchartfortheserologicalandmolecularconfirmatoryRhDtestsperformedforblooddonors.
Twenty-sevenofthese39samplesamplifiedalloftheRHD -specificexonsbymultiplexPCR,and twelvesamplesfailed toamplifyatleastoneRHDexon.Thesampleswerefurther assessedbyPCR-SSPandRHDBeadChipTManalysisandwere
characterizedasfollows:RHD*DVI(n=2),RHD*weakDtype1
(n=3),RHD*weak D type 2(n=8), RHD*weak Dtype 3 (n=1),
RHD*DAR(n=1),heterozygousRHD/RHD*DIIIa-CE(4-7)-D(n=1),
RHD*DAR/DIIIa-CE(4-7)-D(n=2) andRHD*weakDtype2/ DIIIa-CE(4-7)-D (n=1). Intwenty samples,RHDvariants were not detectedbyRHDBeadChipanalysis.
Figure1summarizestheresults.
Discussion
ThecorrectdeterminationoftheRhDphenotypeisofgreat importanceintransfusionmedicine.Theexistenceofalarge numberofpolymorphismsoftheRHDgeneandtheplethora ofvariantRhDphenotypes,coupledwiththeavailabilityofa largenumber ofmethodsandreagents,makesRhD pheno-typingachallenge.3
Toevaluate RhDphenotypingin Fundac¸ão Hemominas, threedifferentmethodswerecomparedtoconfirmweakD tests(IAT) ofsamplesfrom 152Brazilianblood donors.Gel cardspresentedthebest resultstodetectanti-D IgG(clone ESD1);thistechniquecouldbeusedasaconfirmatorytest. ThismethodwasimplementedinthebloodCenterand eval-uated overthe courseofoneyear with4897blooddonors. Duringthis period,1.43% (n=70)ofthe samplespreviously typedasDnegativebythetubetestpresentedpositiveresults exclusivelyinthegeltestforindirectantiglobulintesting,and werereclassifiedasDpositive.
ThemoleculartestsusedtoconfirmtheRhDstatusof39 samplestypedasDnegativebyIATandasweakDpositivein theconfirmatorygeltestrevealedthepresenceofDvariants [RHD*DVI,RHD*weakDtype1,RHD*weakDtype2,RHD*weakD
type3,RHD*DAR,theheterozygousRHD/RHD*DIIIa-CE(4-7)-D, RHD*DAR/DIIIa-CE(4-7)-DandRHD*weakDtype2/ DIIIa-CE(4-7)-D)]in19samples(48.7%).WeakDtype2isamongthemost prevalentDvariantsinBrazil.9WhileweakDtype2presents
arelativelylowantigenicdensity,itisexpectedtoreactina tubeoringelwithoutanantiglobulintest.14However,some
studiesshowthatweakDtype2andDVI,aswellassomeother
Dvariants,maynotreactwithsomeanti-Dreagentsinadirect testandmayreactveryweaklyinthetubetest.9 Therefore,
thesesamplesmayoccasionallybemistypedasDnegativein routinetests.4,9,15
Therearemanyfactorsthatcanaffectthereproducibility and reliabilityofthetube test.These factors include prob-lems inthe washing step, variability inindividual reading techniques and thelevel ofexpertise neededtoaccurately graderesults.16Allofthesefactors,coupledwiththevariable
reactivitydisplayedbysomeanti-DreagentswithDvariant samplesandtheheterogeneousethniccompositionof Brazil-ianblooddonorswhichcangenerateacomplexscenarioof RhDvariants,mayexplaintheresultsobtainedherein.9,17
revbrashematolhemoter.2015;37(5):302–305
305
RHDgeneinBrazilian samplesphenotyped asCor E posi-tivewithaverylowdensityoftheDantigen,mainlyweak Dtype38,andindicatestheneedfortheimplementationof
RHDmolecularscreeninginserologicallyDnegativeandCorE positiveBraziliandonorstoreducetheriskofDimmunization associatedwiththeerroneoustransfusionofweakDRBCsto Dnegativerecipients.18
Conclusion
TheimplementationoftheconfirmatoryRhDtestusingthe geltestforindirectantiglobulintestingforRhDnegativeand CDEpositivesamplesinthebloodcenterrepresentsa break-throughintransfusionsafetytherebyallowingthecorrectRhD typingofdozensofsamplesthatwouldhavebeenerroneously classifiedasDnegativebydirectautomatedRhDphenotyping followedbythetubetest.Moreover,thisstrategydemonstrates the technical feasibility,even in alarge blood center such astheFundac¸ãoHemominas,whichtypes270,000 samples forABO/RhDandapproximately37,700IATtestsforweakD annually.
Theseresultsemphasizetheimportanceofassessingthe bloodgrouptypingstrategyinbloodbanks.Thisevaluationis importantbecauseRhDtypingdiscrepanciescanoccureven withtheuse ofstandardtechniquesand reagents thatthe manufacturersclaimareeffectivefordetectingweakD vari-ants.Thus,eachcentermustevaluatewhichprotocolisbest suitedtolocalconditions.Inaddition,RHDgenotypingagain provedtobeaveryusefultoolinidentifyinginconclusiveRhD typingcases.
Funding
FAPEMIG,Fundac¸ãoHemominas,FAPESP.
Conflicts
of
interest
Theauthorshavenoconflictsofinteresttodisclose.
Acknowledgments
ThisworkwassupportedbyFundac¸ãoHemominas;Fundac¸ão deAmparoàPesquisadoEstadodeMinasGerais(FAPEMIG), grants no. APQ-00485-12, BIP-00064-14, BIP-00009-14; and Fundac¸ão de Amparo à Pesquisa do Estado de São Paulo (FAPESP),grantno.2012/50927-0.
r
e
f
e
r
e
n
c
e
s
1. AventND,ReidME.TheRhbloodgroupsystem:areview. Blood.2000;95(2):375–87.
2.KulkarniS,KasiviswanathanV,GhoshK.Asimplediagnostic strategyforRhDtypingindiscrepantcasesintheIndian population.BloodTransfus.2013;11(1):37–42.
3.DenommeGA,DakeLR,VilenskyD,RamyarL,JuddWJ.Rh discrepanciescausedbyvariablereactivityofpartialand weakDtypeswithdifferentserologictechniques. Transfusion.2008;48(3):473–8.
4.PolinH,DanzerM,GasznerW,BrodaD,St-LouisM,ProllJ, etal.IdentificationofRHDalleleswiththepotentialofanti-D immunizationamongseeminglyD-blooddonorsinUpper Austria.Transfusion.2009;49(4):676–81.
5.FlegelWA.Moleculargeneticsandclinicalapplicationsfor RH.TransfusApherSci.2011;44(1):81–91.
6.JuddWJ,MouldsM,SchlanserG.ReactivityofFDA-approved anti-DreagentswithpartialDredbloodcells.
Immunohematology.2005;21(4):146–8.
7.JenkinsCM,JohnsonST,BellissimoDB,GottschallJL. IncidenceofweakDinblooddonorstypedasDpositiveby theOlympusPK7200.Immunohematology.2005;21(4): 152–4.
8.WesthoffCM.Review:theRhbloodgroupDantigen dominant,diverse,anddifficult.Immunohematology. 2005;21(4):155–63.
9.CredidioDC,PellegrinoJJr,CastilhoL.Serologicand
molecularcharacterizationofDvariantsinBrazilians:impact fortypingandtransfusionstrategy.Immunohematology. 2011;27(1):6–11.
10.EngelfrietCP,ReesinkHW,Fontao-WendelR,KormocziGF, MayrWR,PanzerS,etal.TestingforweakD.VoxSang. 2006;90(2):140–53.
11.MartinsML,CruzKV,SilvaMC,VieiraZM.Usoda
genotipagemdegrupossanguíneosnaelucidac¸ãodecasos inconclusivosnafenotipagemeritrocitáriadepacientes atendidosnaFundac¸ãoHemominas.RevBrasHematol Hemoter.2009;31(4):252–9.
12.Maaskant-VanWijkPA,FaasBH,DeRuijterJA,OverbeekeMA, VonDemBorneAE,VanRhenenDJ,etal.GenotypingofRHD bymultiplexpolymerasechainreactionanalysisofsix RHD-specificexons.Transfusion.1998;38(11-12): 1015–21.
13.MüllerTH,WagnerFF,TrockenbacherA,EicherNI,FlegelW, SchönitzerD,etal.PCRscreeningforcommonweakDtypes showsdifferentdistributionsinthreeCentralEuropeans populations.Transfusion.2001;41(1):45–52.
14.WagnerFF,FrohmajerA,LadewigB,EicherNI,LonicerCB, MüllerTH,etal.WeakDallelesexpressdistinctphenotypes. Blood.2000;95(8):2699–708.
15.WagnerFF,FrohmajerA,FlegelWA.RHDpositivehaplotypes inDnegativeEuropeans.BMCGenet.2001;2:10.
16.RumseyDH,CiesielskiDJ.Newprotocolsinserologictesting: areviewoftechniquestomeettoday’schallenges.
Immunohematology.2000;16(4):131–7.
17.DaSilvaMC,ZuccheratoL,LucenaF,Soares-SouzaGB,Vieira ZM,MartinsML,etal.ExtensiveadmixtureinBraziliansickle cellpatients:implicationsforthemappingofgenetic modifiers.Blood.2011;118(16):4493–5.