www.jped.com.br
ORIGINAL
ARTICLE
A
double-blinded
randomized
trial
on
growth
and
feeding
tolerance
with
Saccharomyces
boulardii
CNCM
I-745
in
formula-fed
preterm
infants
夽
Lingfen
Xu
a,
Yun
Wang
b,
Yang
Wang
a,
Jianhua
Fu
a,
Mei
Sun
a,
Zhiqin
Mao
a,∗,
Yvan
Vandenplas
caDepartmentofPediatrics,ShengjingHospital,ChinaMedicalUniversity,Shenyang,China bDepartmentofPediatrics,QingdaoWomenandChildren’sHospital,Qingdao,China cUZBrussel,DepartmentofPediatrics,VrijeUniversiteitBrussel,Brussels,Belgium
Received21May2015;accepted10August2015 Availableonline3March2016
KEYWORDS
Feeding (in)tolerance; Growth; Necrotizing enterocolitis; Preterminfant; Probiotic; Sepsis
Abstract
Objective: Theuseofprobioticsisincreasinglypopularinpretermneonates,astheymay pre-ventnecrotizingenterocolitissepsisandimprovegrowthandfeedingtolerance.Thereisonly limitedliteratureonSaccharomycesboulardiiCNCMI-745(S.boulardii)inpreterminfants.
Method: A prospective, randomized, case-controlled trial with the probiotic S. boulardii
(50mg/kgtwicedaily)wasconductedinnewbornswithagestationalageof30---37weeksand abirthweightbetween1500and2500g.
Results: 125neonateswereenrolled;63inthetreatmentand62inthecontrolgroup.Weight gain(16.14±1.96vs.10.73±1.77g/kg/day,p<0.05)andformulaintakeatmaximalenteral feeding(128.4±6.7vs.112.3±7.2mL/kg/day,p<0.05)weresignificantlyhigherinthe inter-ventiongroup.Onceenteralfeedingwasstarted,thetimeneededtoreachfullenteralfeeding wassignificantlyshorterintheprobioticgroup(0.4±0.1vs.1.7±0.5days,p<0.05).Therewas nosignificantdifferenceinsepsis.Necrotizingenterocolitisdidnotoccur.Noadverseeffects relatedtoS.boulardiiwereobserved.
Conclusion: ProphylacticsupplementationofS.boulardiiatadoseof50mg/kg twiceaday improved weight gain, improved feedingtolerance,and hadno adverseeffectsin preterm infants>30weeksold.
©2016SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
夽
Pleasecitethisarticleas:XuL,WangY,WangY,FuJ,SunM,MaoZ,etal.Adouble-blindedrandomizedtrialongrowthandfeeding tolerancewithSaccharomycesboulardiiCNCMI-745informula-fedpreterminfants.JPediatr(RioJ).2016;92:296---301.
∗Correspondingauthor.
E-mail:maozq@sj-hospital.org(Z.Mao).
http://dx.doi.org/10.1016/j.jped.2015.08.013
PALAVRAS-CHAVE
(In)Tolerânciade alimentac¸ão; Crescimento; Enterocolite necrosante;
Neonatoprematuro; Probiótico;
Sepse
Ensaioduplo-cegorandomizadosobrecrescimentoetolerânciadealimentac¸ãocoma
SaccharomycesboulardiiCNCMI-745emneonatosprematurosalimentados comfórmula
Resumo
Objetivo: Ousodeprobióticosestácadavezmaispopularemneonatos prematuros,jáque podempreveniraenterocolitenecrosante(ECN)easepseeaumentarocrescimentoea tol-erânciadealimentac¸ão.HáapenasumaliteraturalimitadasobreaSaccharomycesboulardii
CNCMI-745(S.boulardii)emneonatosprematuros.
Método: Umensaiodecaso-controleprospectivorandomizadocomoprobióticoS.boulardii
(50mg/kgduasvezespordia)foirealizadocomrecém-nascidoscomidadegestacionalde30a 37semanasepesoaonascerentre1500e2500g.
Resultados: Foramincluídos125neonatos,63nogrupodetratamentoe62nodecontrole.O ganhodepeso(16,14±1,96emcomparac¸ãoa10,73±1,77g/kg/dia,p<0,05)eaingestãode fórmula comnutric¸ãoenteralmáxima (128,4±6,7emcomparac¸ãoa112,3±7,2mL/kg/dia,
p<0,05) foram significativamente maiores no grupo de intervenc¸ão. Assim que a nutric¸ão enteralfoiiniciada,otemponecessárioparaatingiranutric¸ãoenteralcompletafoi significa-tivamentemenornogrupoprobiótico(0,4±0,1emcomparac¸ãoa1,7±0,5dia,p<0,05).Não houvenenhumadiferenc¸asignificativaemsepse.NãoocorreuECN.Nãofoiobservadonenhum efeitocolateralrelacionadoàS.boulardii.
Conclusão: Asuplementac¸ãoprofiláticadeS.boulardiiaumadosede50mg/kgduasvezespor diamelhorouoganhodepeso,aumentouatolerânciadealimentac¸ãoenãotevenenhumefeito colateralemneonatosprematuros>30semanasdeidade.
©2016SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-NDlicense( http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The gastrointestinal (GI) barrier function, gut motility, mucosal immunity, and digestive/absorptive capacity are all significantly underdevelopedin the preterm neonate.1
Preterm infants have an increased risk of poor growth,
nosocomialinfections,andnecrotizingenterocolitis(NEC),
and of developing a different intestinal microbiota than
healthy breast fed infants.1,2 The latter is related to a
higher incidence of delivery through cesarean section,
decreased exposure to maternal microbiota, increased
exposuretoorganismsthatcolonizeneonatalintensivecare units (NICUs), antibiotics (multiple courses),and delay in enteralfeeding.3
Theroleforprobioticsinthecareofpretermnewborns
isdebated.Probioticsaredefinedas‘‘livemicroorganisms
which, whenadministeredin adequate amounts,confer a
health benefit to the host’’.4 While reports of improved
growthandadecreasedincidenceofNECareenticing,many aspects on the mechanisms of action are still unclear.5,6
Studieshave useddifferentstrainsanddosages,makingit difficulttodrawevidence-basedconclusions.5---7
Until now,researchers oftenselectedstrains belonging
tobacterialspeciesnaturallypresentintheintestinalflora,
such as lactobacilli and bifidobacteria.8 Saccharomyces
boulardii CNCM I-745 (S. boulardii) is a probiotic yeast
isolated from the peel of fruits such as lychees,
grow-ing in Indochina.9 S. boulardii hasbeen poorlystudied in
pretermandlowbirthweightinfants.Theobjectiveofthe present studywastoassesif S.boulardii administeredto
formula-fedpretermnewborns>30weeksofgestationalage
wouldimproveweightgainandclinicaloutcome.
Methods
Patientinclusion
StableformulafedpretermneonatesadmittedtotheNICU of the Shengjing Hospital of the ChinaMedical University inShenyang(China)wereincludedinthisprospective ran-domizedcontrolleddouble-blinded study,performed from April to July 2013. Informed consent was obtained from the infants’ parents/guardians. The study protocol was approvedbytheUniversityHospitalEthicalCommittee.
Thesamplesizewascalculatedpriortothestartofthe studyforasignificancelevelofp<0.05(two-sided),witha powerof80%(ˇ=0.2)toestimatetheneededsamplesize, andwithaweightgainstandarddeviationof9g/dayinboth groupsandaweightgaindifferencebetweenthetwogroups of 5g/day. This resulted in a sample size of 125 infants, consideringa20%dropoutrate.
Inclusionandexclusioncriteria
Inclusion criteria were hospital-born formula-fed infants witha gestationalageof 30---37weeksand abirth weight between1500and2500g.
chromosomal abnormalities, known immunodeficiency, hydropsfetalis,centralvenous catheter,antifungaldrugs, and probiotics. All included patients received parenteral nutrition and/or preterm formula. No neonates received mother’smilk.Minimalenteralnutritionortrophicfeeding was started as soon as possible at 1mL/kg/day. Minimal enteral feeding is the practice of feeding small volumes of enteralfeed in ordertostimulate the developmentof theimmature GItract of thepreterm infant; it improves GIenzymeactivity,hormonerelease,blood flow,motility, andmicrobialflora.Clinicalbenefitsincludeimprovedmilk tolerance, greater postnatal growth, reduced systemic sepsis, and shorter hospital stay.10 As soon as minimal
enteralfeeding was tolerated, the patient was randomly
allocatedtooneoftwogroupsat a1/1ratio(S.boulardii
orcontrolgroup).Randomizationwasconductedaccording
toarandomcomputer-determinedallocationorder
consid-eringbirth weight.Feeding volumewasincreasedwhenit
waswelltoleratedaccordingtothelocalprotocol.
Intervention
The intervention group receivedS. boulardiiCNCM I-745, administered two times per day as separate medication, notmixedwithformula,atadosageof50mg/kg(Bioflor®;
CMS Shenzhen Kangzhe Pharmaceutical Co. Ltd., Shen-zhen, China; manufactured by Biocodex, Paris, France); 50mgisapproximately109colonyformingunits(CFU).The
dosage of the probiotic was derived from previous stud-iesinneonates.11 Nothingwasadministeredtothecontrol
group.Thestudyperiodendedatthe28thdayafterbirth
orwhentheinfantwasdischargedfromthehospital,ifthis waspossibleearlier.However,theminimaldurationofthe
intervention was at least 7 days. Observational and
rou-tineclinicaldatawerecollectedfromallinfants.Blinding waspossiblebecausethenursingstaffwhoadministeredS. boulardiitotheinfants wasnotinvolved inthedailycare
andtheattendingneonatalteamwasunaware ofthe
ran-domizationassignments.
Outcome
Primary outcomes were short-term growth parameters: weightgain(g/kg/day) andlineargrowth(cm/week). Sec-ondary outcomes included: days of parenteral nutrition needed to reach full enteral feeding, maximal enteral feedingvolumetolerated(mL/kg/day),anddurationof hos-pitalization(days). Feeding intolerancewasdefinedwhen vomitingandgastricresiduals wereconsideredtoo impor-tant. Complications were defined as incidence of NEC (definedassuspectedorconfirmedpositiveBellstageIIor more)andsepsis(definedaspositivebloodculture).9
Statistics-registration
The data were collected and entered into a statistical database(SPSS,version16.0;IBM,Armonk,USA).Thedata are presented as mean±standard deviation. The demo-graphicdataandprocedure variableswereanalyzed using thet-testor thechi-squared test.A p-value of<0.05 was
consideredtoindicateastatisticallysignificantdifference. Thisstudydidnotreceiveexternalfunds,andwasregistered atthewebsitehttps://clinicaltrials.govunderthenumber NCT02310425.
Results
Patientdescription
Atotalof125formula-fedpretermneonateswereenrolled and randomly allocated. Sixty-three patients received S. boulardii assoon as they could tolerate minimal enteral feedingand62neonateswereincludedinthecontrolgroup. In total, 25 (20%) patients were considered dropouts (12 [19.1%]intheS.boulardiigroupand13[20.1%]inthe con-trolgroup) (Fig.1). Reasonsfor dropout werewithdrawal ofconsent(n=9),losstofollow-up(n=11),centralvenous catheter(n=1),congenitalsyphilis(n=1),and inappropri-ateinclusions(congenitalintestinalatresia[n=2],trisomy
21 [n=1]). Fifty-one subjects could be analyzed in the
interventiongroupand49inthecontrolgroup.The charac-teristicsofallneonatesatstudyentryarelistedinTable1, anddidnotshowanystatisticallysignificantdifference.
S.boulardiiwasadministeredfor thefirsttimeat 2.63 daysafterbirth(range:day1today6;in46infantswithin 3days,andinonlyfiveinfantsbetweenday4and6).The totalnumberofdaysofS.boulardiiadministrationaveraged 25.3days(range:9---28days).
Feedingtolerance
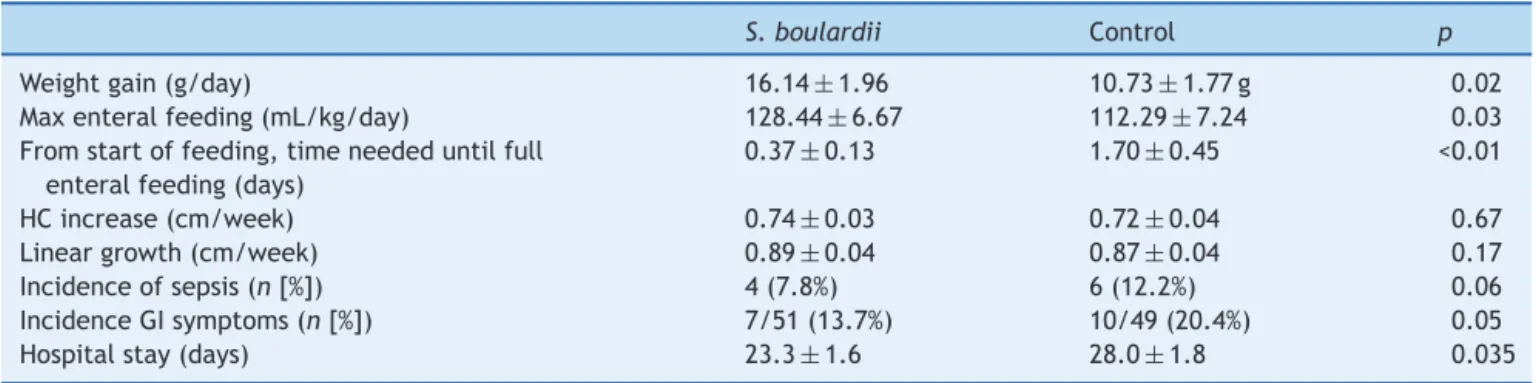
Formula intake at maximal enteral feeding (128.4±6.7
vs. 112.3±7.2mL/kg/day, p<0.05) was higher in the S. boulardii thanin thecontrol group,and thetimeneeded to reach full enteral feeding (0.4±0.1 vs. 1.7±0.5 day,
Randomized
S. Boulardii group
Control group: no probiotics
Drop out Drop out
Allocated to
S. boulardii group
Allocated to control group Premature and low birth weight
infants who met including criteria n=125
Table 1 Characteristics (mean+1 SD) of the included infants.
S.boulardii Control
Birthweight(g) 1947±54 1957±51 Gestationage(weeks) 33+0.72 33+1.04
Boys/girls 27/24 24/25
Respiratorydifficulties 5 6 Hyperbilirubinema(n[%]) 16(31.4%) 14(28%) Maximaltotalbilirubin
(mol/L)
18.5±2.2 19.4±2.8
Anemia(n[%]) 23(45.1%) 25(51.0%) Antibiotictreatment(n[%]) 11(21.6%) 9(18.4%)
Respiratorydifficulties:includesrespiratorydistresssyndrome andwetlung.
S,Saccharomyces;p>0.05(all).
p<0.05)wasshorterintheinterventionthaninthecontrol group(Table2).
Growthandhospitalstay
The weight gain in the S. boulardii group was 16.14±1.96g/kg/day versus 10.73±1.77g/kg/day (p<0.05) in the control group. There was no signifi-cant difference in linear growth, head circumference growth,incidence of abdominaldistension,and incidence ofsepsis (Table2).Hospitalstay inthe S.boulardii group wasshorter(p=0.035)(Table2).NoinfantsdevelopedNEC.
Adverseeffects
Nopretermsdevelopedfungemia,andnoadversereactions toS.boulardiiwerereported.
Discussion
This study demonstrated that S. boulardii can safely be administeredtopreterminfants,andthatitimprovesoral feeding tolerance and weight gain. In term infants, for-mulasupplemented withLactobacillus (L.)rhamnosus GG was shown to increase weight gain, but formulas sup-plemented withBifidobacterium (B.)longum, B.animalis
subsp.lactis,andL.reuterididnot.11---13Inpreterminfants,
administrationofB.brevealsoimprovedweightgain.14The
mechanismsby which weightgain is affected arenot yet
clear.
S.boulardiiiseffectiveinthetreatmentofanumberof GIdisordersrelatedtothepresenceofbacterialandviral pathogens.15 It competeswithpathogens for binding sites
andproduces a wide rangeof antimicrobial substances.16
S. boulardiihas theability toproduce polyamines, which
are substances essential for cell growth and
differentia-tionand enhance intestinalmaturation, whatis reflected
inincreasedlevelsofenzymeexpression.17S.boulardiiisa
yeastthatsignificantlyincreasestheactivity ofmetabolic enzymesintheintestinalmucosa,stimulatesthesecretion ofdisaccharideenzymes,participatesinthemetabolismand
absorptionofcarbohydrates, andstimulates secretory IgA
production as the result of a trophic effect onintestinal mucosa.18 In addition, S. boulardii promotes the stability
of the intestinal microbiome and reduces the possibility
of malabsorption caused by GI disorders.19 Translocation
ofS. boulardiihasnotbeen reported;onthecontrary, S. boulardiiwasreportedtoreducebacterialtranslocation.20
Based on these properties, it was hypothesized that S.
boulardii could improve growth and clinical outcomes in pretermorlowbirthweightinfants.
Although severalclinical trialsstronglysuggest a place for S. boulardii in the prevention and treatment of
sev-eral GI diseases in adults and children, data in preterm
infants are limited.18 S. boulardii supplemented formula
wasshown tobewelltolerated bypreterminfants andto
have beneficial effects onthe GI microbiome, bringing it
closertothatofbreastfedbabies.11Clinicaltrialsinpreterm
infants alsosuggested that S. boulardii improved feeding
tolerance and reduced the risk of sepsis.21,22 In order to
achieveoptimumgrowthfor apreterm infant,the goalis
tomimicintrauterinegrowthwhile obtaining afunctional
outcome comparable to term infants.23 A gain in weight
of 15---20g/kg/day, in length of 0.7---1.0cm/week, and in
head circumference of 0.7cm/week is recommended.24,25
In the S. boulardii group, the average weight gain was
16.14g/kg/day,lineargrowthwas0.9cm/week, andhead
circumference increase was 0.7cm/week. Weight gain in
thecontrol groupwas10.73g/kg/day,which is belowthe
recommendation.Thenumberofdaystoreachfullenteral
nutritionwasshorterintheS.boulardiithaninthecontrol
Table2 Comparisonofweightgain,growth(mean+1SD),feedingtolerance,adverseevents(sepsis,gastro-intestinal symp-toms),anddurationofhospitalizationbetweentheS.boulardiiandcontrolgroup.
S.boulardii Control p
Weightgain(g/day) 16.14±1.96 10.73±1.77g 0.02
Maxenteralfeeding(mL/kg/day) 128.44±6.67 112.29±7.24 0.03 Fromstartoffeeding,timeneededuntilfull
enteralfeeding(days)
0.37±0.13 1.70±0.45 <0.01
HCincrease(cm/week) 0.74±0.03 0.72±0.04 0.67
Lineargrowth(cm/week) 0.89±0.04 0.87±0.04 0.17
Incidenceofsepsis(n[%]) 4(7.8%) 6(12.2%) 0.06
IncidenceGIsymptoms(n[%]) 7/51(13.7%) 10/49(20.4%) 0.05
Hospitalstay(days) 23.3±1.6 28.0±1.8 0.035
group.Thebetterweightgainislikelytoberelatedtothe improvementoffeedingtolerance.Itwasobservedthatthe incidenceofvomiting,gastricresidualvolume,and abdomi-naldistension(‘‘GIsymptoms’’,Table2)weredecreasedin theinterventiongroupincomparisontothecontrolgroup, althoughtherewasnostatisticalsignificantdifference.The totalhospitalstayintheS.boulardiigroupwasshorterthan thatinthecontrolgroup.
Nosignificantdifferencewasobservedinlineargrowth
andheadcircumferenceevolution,whichcouldberelated
totherelativeshortinterventionperiodof1month.Other limitationsofthisstudyarethelackofinformationon
post-natal clinical characteristics of the neonates that could
have been factors influencing the outcome, such as the
ratioof patent ductus arteriosus, intraventricular
hemor-rhage, and others. Information on the number of infants
withpredisposingfactorsforNEC,sepsis,orotherproblems
such as pre-eclampsia, antenatal steroid use, premature
rupture of membranes, and caesarian birth are missing.
The absence of breastfeeding is anotherweakness of the
study.
ArecentCochranereview reportedon24trialson
pro-biotics in preterm infants and concluded that the trials
were highly variable with regard to enrollment
crite-ria (birth weight, gestational age), baseline risk of NEC,
timing, dose, formulation of the probiotics, and feeding
regimens.8Enteralsupplementationwithprobiotics
signifi-cantlyreducedtheincidenceofsevereNEC(stageIIormore) (typicalrelativerisk[RR]0.43,95%confidenceinterval[CI] 0.33---0.56; 20 studies, 5529 infants) and mortality (typi-calRR0.65,95% CI0.52---0.81; 17studies, 5112 infants).8
Accordingtothismeta-analysis,therewasnoevidencefor asignificantreductionofnosocomialsepsis(typicalRR0.91, 95%CI0.80---1.03;19studies,5338infants).8Inthepresent
trial,there werenopretermswhodeveloped NEC;this is
likelytoberelatedtothefactthatgestationalagefor inclu-sionwas30---37weeksandthatNECoccursmorefrequently ininfantsbornwithalessergestationalage.Previous clini-caltrialsshowedthatS.boulardiisupplementationdidnot reducetheincidenceofdeathorNECinverylowbirthweight infants,butimprovedfeedingtoleranceandreducedtherisk ofclinicalsepsis,whileadverseeffectsrelatedtotheintake ofS.boulardiiwerenotobserved.21,22
S. boulardii has a protective effect against various
enteric pathogens by two main mechanisms: production
of factors that neutralize bacterial toxins and
modula-tion of the host cell signaling pathway implicated in
proinflammatoryresponseduringbacterialinfection.18,19In
addition,S. boulardii can increase the activity of regula-tory T cells and secretion of IgA of intestinal epithelial
and crypt cells, improving intestinal protection through
immuneregulation.18Inthisstudy,therewasnostatistically
significant difference in the incidence of sepsis between
the two groups (4/51 vs. 6/49). This finding is in
agree-mentwiththeCochraneanalysis,showingthattheincluded
trialsreportednosystemic infectionwiththe
supplemen-tal probiotics organism.8 S. boulardii fungemia has been
reportedin patientswithdeepcentralvenousaccess.18 In
thisclinicaltrial,therewerenocasesoffungemia,andno
sideeffectsoccurred.TheauthorsoftherecentCochrane
reviewconcludedthattheupdatedreviewofavailable evi-dencestronglysupportsachangeinpractice,meaningthat
probioticsshould begiventopreterm infants todecrease
theriskforNECandmortality.8
Inconclusion,theresultsofthepresentstudyshowthat prophylacticuseofS.boulardiiinpreterminfants acceler-atesweightgainandimprovesfeedingtolerance.Thesedata confirmarecentretrospectiveanalysisconcludingthat pro-bioticsimprovefeedingtolerance,leadingtobetteroverall growthinpreterminfants.26 Itisthefirsttimethatbetter
weightgain of preterminfants provided withS. boulardii
has been demonstrated. Future double-blinded
placebo-controlledtrialsareneededtoconfirmthesedata.
Conflicts
of
interest
Y.VandenplasisaconsultantforUnitedPharmaceuticalsand Biocodex.Theothersauthorsdeclarenoconflictsof inter-est.
References
1.ColladoMC,CernadaM,NeuJ,Pérez-MartínezG,GormazM, VentoM.Factorsinfluencinggastrointestinaltractand micro-biota immune interaction in preterm infants. Pediatr Res. 2015;77:726---31.
2.SchwiertzA, GruhlB,Löbnitz M,MichelP,RadkeM,BlautM. Developmentoftheintestinalbacterialcompositionin hospital-izedpreterminfantsincomparisonwithbreast-fed,full-term infants.PediatrRes.2003;54:393---9.
3.ChauhanM,HendersonG,McGuireW.Enteralfeedingforvery lowbirthweightinfants:reducingtheriskofnecrotising ente-rocolitis.ArchDisChildFetalNeonatalEd.2008;93:F162---6. 4.FoodandAgricultureOrganizationoftheUnitedNations(FAO),
WorldHealthOrganization(WHO).Guidelinesfortheevaluation ofprobioticsinfood.ReportofaJointFAO/WHOWorkingGroup ondraftingguidelinesfortheevaluationofprobioticsinfood. London,Ontario,Canada:FAO,WHO;2002.
5.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight:asystematicreviewofrandomisedcontrolled tri-als.Lancet.2007;369:1614---20.
6.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weightinfants:anupdatedmeta-analysisof20 ran-domized,controlledtrials.JPediatrSurg.2012;47:241---8. 7.Deshpande GC, Rao SC, Keil AD, Patole SK. Evidence-based
guidelinesforuseofprobioticsinpretermneonates.BMCMed. 2011;9:92.
8.AlFalehK,AnabreesJ.Probioticsforpreventionofnecrotizing enterocolitisinpreterminfants.CochraneDatabaseSystRev. 2014;4:CD005496.
9.Vendt N, Grünberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rham-nosus GG: double-blind, randomizedtrial. JHum NutrDiet. 2006;19:51---8.
10.SenterreT.Practiceofenteralnutritioninverylowbirthweight andextremelylowbirthweightinfants.WorldRevNutrDiet. 2014;110:201---14.
13.WeizmanZ,AlsheikhA.Safetyandtoleranceofaprobiotic for-mulainearlyinfancycomparingtwoprobioticagents:apilot study.JAmCollNutr.2006;25:415---9.
14.KitajimaH,SumidaY,TanakaR,YukiN,TakayamaH,Fujimura M.EarlyadministrationofBifidobacterium breveto preterm infants:randomisedcontrolledtrial.ArchDisChildFetal Neona-talEd.1997;76:F101---7.
15.ElmerGW.Probiotics:‘‘livingdrugs’’.AmJHealthSystPharm. 2001;58:1101---9.
16.TalaricoTL,CasasIA,ChungTC,DobrogoszWJ.Productionand isolationofreuterin,agrowthinhibitorproducedby Lactobacil-lusreuteri.AntimicrobAgentsChemother.1988;32:1854---8. 17.ButsJP. Polyaminesinmilk,inbioactivefactorsinmilk.Ann
Nestle.1996;54:98---104.
18.VandenplasY,SalvatoreS,VieiraM,DevrekerT,HauserB. Pro-bioticsininfectiousdiarrhoeainchildren:aretheyindicated? EurJPediatr.2007;166:1211---8.
19.McFarlandLV.Systematicreviewandmeta-analysisof Saccha-romyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202---22.
20.Villar-GarcíaJ, HernándezJJ, Güerri-FernándezR, González A, Lerma E, Guelar A, et al. Effect of probiotics ( Saccha-romyces boulardii) on microbial translocation and inflam-mation inHIV-treated patients: a double-blind,randomized,
placebo-controlledtrial.JAcquirImmuneDeficSyndr.2015;68: 256---63.
21.SerceO,BenzerD,GursoyT,KaratekinG,OvaliF.Efficacyof
Saccharomycesboulardiionnecrotizingenterocolitisorsepsis inverylowbirthweightinfants:arandomisedcontrolledtrial. EarlyHumDev.2013;89:1033---6.
22.Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardiiforpreventionofnecrotizingenterocolitisinpreterm infants: a randomized, controlled study. Acta Paediatr. 2013;102:e560---5.
23.Agostoni C,BuonocoreG, CarnielliVP,DeCurtis M,Darmaun D,DecsiT,etal.Enteralnutrientsupplyforpreterminfants: commentaryfromtheEuropeanSocietyofPaediatric Gastroen-terology,HepatologyandNutritionCommitteeonNutrition.J PediatrGastroenterolNutr.2010;50:85---91.
24.GeorgieffMK.Nutrition.In:MacDonaldMG,SeshiaMM,Mullet MD,editors.Avery’sneonatologypathophysiologyand manage-mentofthenewborn.6thed.Philadelphia:LippincottWilliams andWilkins;2005.p.380---1.
25.UhingMR,Das UG.Optimizinggrowthinthepreterminfant. ClinPerinatol.2009;36:165---76.