www.jped.com.br
ORIGINAL
ARTICLE
Antibody
persistence
following
meningococcal
C
conjugate
vaccination
in
children
and
adolescents
infected
with
human
immunodeficiency
virus
夽
Ana
Cristina
Cisne
Frota
a,
Lee
H.
Harrison
b,
Bianca
Ferreira
a,
Daniela
Menna-Barreto
a,
Raquel
Bernardo
Nana
de
Castro
c,
Giselle
Pereira
da
Silva
c,
Ricardo
Hugo
de
Oliveira
d,
Thalita
F.
Abreu
d,
Lucimar
G.
Milagres
c,
Cristina
B.
Hofer
a,∗aUniversidadeFederaldoRiodeJaneiro(UFRJ),DepartamentodeMedicinaPreventiva,RiodeJaneiro,RJ,Brazil bUniversityofPittsburgh,InfectiousDiseasesEpidemiologyResearchUnit,Pittsburgh,UnitedStates
cUniversidadedoEstadodoRiodeJaneiro(UERJ),DepartamentodeMicrobiologia,ImunologiaeParasitologia,Disciplinade
Microbiologia,RiodeJaneiro,RJ,Brazil
dUniversidadeFederaldoRiodeJaneiro(UFRJ),RiodeJaneiro,RJ,Brazil
Received25September2016;accepted25November2016 Availableonline22April2017
KEYWORDS
Meningococcal vaccine; Immunology;
Conjugatevaccines;
HIV; Children; Brazil
Abstract
Objective: HIV-infectedindividuals(HIVI)arethreatenedbymeningococcalinfectionand pre-sentedlowerresponsetovaccines.Dataarescarceonlong-termpersistenceofhumanserum bactericidalantibody(hSBA)afterameningococcalCconjugate(MCC)vaccineinHIVIyouth; theauthorsaimedtodescribethispersistenceinHIVI.
Methods: HIVI and HIV uninfected individuals (HIVU), aged 2---18 years, CD4 >15% were recruited. Seroprotection(hSBA ≥1:4)atbaselineandat 12---18months after immunization
wasevaluatedandtheassociationofthedifferentfactorswiththelong-termpersistencewas calculatedusinglogisticregression.
Results: Atotalof145HIVI,50HIVUwererecruitedandimmunized,andtheirmedianagewas 11years(medianageinHIVIgroupwas12years,and10yearsinHIVUgroup,p-value=0.02). 85HIVI(44%)hadundetectableviralload(UVL).Seroprotectionratewas27.2%:24.1%inHIVI and36%inHIVU12---18 monthsafter immunization(p=0.14).Baselineimmunity (oddsratio [OR]=70.70,95%CI:65.2---766.6);UVLatentry(OR:2.87,95%CI:0.96---8.62)andlowerfamily income(OR:0.09,95%CI:0.01---0.69)wereassociatedwithseroprotectionamongHIVI.
夽
Pleasecitethisarticleas:FrotaAC,HarrisonLH,FerreiraB,Menna-BarretoD,CastroRB,SilvaGP,etal.Antibodypersistence follow-ingmeningococcalCconjugatevaccinationinchildrenandadolescentsinfectedwithhumanimmunodeficiencyvirus.JPediatr(RioJ). 2017;93:532---7.
∗Correspondingauthor.
E-mail:cbhofer@hucff.ufrj.br(C.B.Hofer).
http://dx.doi.org/10.1016/j.jped.2017.01.003
Conclusion: Seroprotectionat12---18monthsaftersingledoseofMCCwaslowforbothgroups, andhigheramongindividualswhopresentedbaselineimmunity.AmongHIVI,vaccineshouldbe administeredafterUVLisachieved.
©2017PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradePediatria.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/
by-nc-nd/4.0/).
PALAVRAS-CHAVE
Vacina
meningocócica; Imunologia;
Vacinasconjugadas;
HIV; Crianc¸as; Brasil
PersistênciadeanticorposseguidadevacinaconjugadameningocócicaCemcrianc¸as eadolescentesinfectadosporvírusdaimunodeficiênciahumana
Resumo
Objetivo: AspessoasinfectadaspeloHIV(HIVI)estãosujeitasainfecc¸ãomeningocócicae apre-sentammenor respostaavacinas.Sãoescassososdadosarespeitodapersistênciadelongo prazodoanticorpobactericidanosorohumano(hSBA)apósvacinaconjugadameningocócicaC (MCC)emHIVIjovens,evisamosdescreveressapersistênciaemHIVI.
Métodos: ForamrecrutadaspessoasHIVIepessoasnãoinfectadasporHIV(HIVU),comidades entre 2 e 18 anos, CD4>15%. A seroprotec¸ão (hSBA ≥ 1:4)basal aos 12---18 meses após a
imunizac¸ãofoiavaliadaeaassociac¸ãodosdiferentesfatorescomapersistênciadelongoprazo foicalculadautilizandoaregressãologística.
Resultados: 145HIVIe50HIVUforamrecrutadoseimunizadosesuaidademédiafoi determi-nadaem11anos(aidademédianogrupoHIVIfoi12anosenogrupoHIVUfoi10anos,valor dep=0.02).85HIVI(44%)apresentaramcargaviralindetectável(CVI).Ataxadeseroprotec¸ão foi27.2%:24.1%nogrupoHIVIe36%nogrupoHIVU12-18mesesapósimunizac¸ão(p=0.14).A imunidadebasal[razãodechance(RC)=7070,IC:65,2---7666];CVInomomentodaparticipac¸ão (RC:2,87,ICde95%:0,96-8,62)erendafamiliarmaisbaixa(RC:0,09,ICde95%:0,01-0,69) foramassociadasaseroprotec¸ãoentreaspessoasHIVI.
Conclusão: Aseroprotec¸ãoaos12-18mesesapósúnicadosedeMCCmostrou-sebaixaemambos osgruposemaiselevadaentreaspessoasqueapresentaramimunidadebasal.Entreaspessoas HIVI,asvacinasdevemseradministradasapósaCVIseratingida.
©2017PublicadoporElsevierEditoraLtda.emnomedeSociedadeBrasileiradePediatria.Este ´
eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/
by-nc-nd/4.0/).
Introduction
Meningococcaldisease (MD)is an importantcause of
sep-ticemiaandmeningitis,andamajorpublichealthchallenge
worldwide.1,2 People living withhuman immunodeficiency
virus (HIV) infection have an increasedrisk for MD. Stud-ies have demonstrated a five to ten times higher risk of MD among HIV-infected (HIVI) children and almost ten-foldhigheramongadolescentsandadultswhencompared withHIV-uninfectedindividuals(HIVU).3,4Furthermore,HIVI children and adolescents have lower vaccine response rates despite being on combination antiretroviral ther-apy (cART).5---7 Vaccine-induced antibodies also may wane more quickly in persons with HIV than in healthy indi-viduals, indicating that immune reconstitution was not sufficient toensure long-term protection and highlighting theimportanceofevaluatinglong-termimmunogenicityto meningococcalvaccinesandfactorsthatmaybeassociated withabetterresponse.8,9
The persistence of antibodies after meningococcal serogroup C (MCC) conjugate vaccines has been studied in many high-risk populations with different degrees of immunosuppression,buttherearefewstudiesinHIVI.10---12
Theaimsofthisstudywere:(1)toassessthepersistence of serumbactericidal antibody againstMCC, usinghuman
complement(hSBA)inHIVIchildrenandadolescents,12---18 monthsafterasingledoseofMCC,andcomparedwithHIVU participants; and (2) to evaluate factors associated with antibodypersistence.
Materials
and
methods
Studydesignandpopulation
ThiswasaprospectivecohortstudyofHIVIandHIVUchildren
andadolescents followed at the Instituto de Puericultura
ePediatriaMartagãoGesteira(IPPMG),apediatrichospital
of theUniversidade Federal do Rio deJaneiro, reference
centerforHIVcareinRiodeJaneiro,Brazil,fromFebruary
2011toDecember2012.Theywererecruitedattheclinic’s
waitingroom.
Eligibilitycriteriawere:individualsaged2---18yearswho hadnotreceivedanypreviousMCCvaccine,hadnotreceived
alive vaccine within4 weeksbeforeentry, and werenot
scheduledtoreceiveothervaccineswithin2weeks.Forthe HIVIgroup,additionaleligibilitycriteriawereHIVinfection (definedastworeactiveanti-HIVtestsinchildren>2years old),absenceofWorldHealthOrganizationclinicalstages3 or4HIVclinicaldiseaseatentry,andCD4+T-lymphocytecell
entry.HIVU wererecruitedamonghealthychildren atthe clinicatthesamehospitalwhohadanegativeHIVserology
resultafter18monthsofage;thetest wasagainrequired
atentryforthosewhoweresexuallyactive.Individualsin bothgroupshadtoliveinthesamegeographicarea.
The exclusion criteria included pregnancy; presence
of any other immunosuppressive disease; use of systemic
immunosuppressivedrugs;useofantibioticsupto3weeks
orimmunoglobulintherapywithinthelast6monthsbefore
entry; bleeding disorders precluding intramuscular
injec-tion; adverse reactions to any vaccine components; and
psychiatricdisorders,includingillicitdrugoralcohol intoxi-cationatthetimeoftheinterview(patientorparent/legal
guardian).A negativepregnancytest wasrequired before
immunizationforparticipantsofreproductiveage.All
par-ticipants receivedone dose (0.5mL intramuscular deltoid
injection) of MCC oligosaccharide-CRM197, a toxoid of
Corynebacterium diphtheria (Chiron/Novartis Vaccines
---Siena,Italy) between February 2011 and December2012.
ForHIVIvolunteers,thevaccinewasprovidedbythe Brazil-ianNational MinistryofHealth;forHIVU,itwasfundedby theresearchgrant.
Blood samples for serogroup C Neisseria meningitidis
antibodies with hSBA, virological, and imunological
mea-surements,aswellasstandardizedquestionnaires,including
clinical, laboratory, and socioeconomic data were
col-lected at the entry, one to two, and 12---18 months
post-immunization visits. Demographic, clinical, virology,
andimmunologicdatawereobtainedfrommedicalrecords
(Table1).Viralload(VL)andCD4countresultsbefore,at beginning,andaftercART,aswellasnadirandzenithdata, wererecorded.
Short-termimmunogenicityandsafetyresultsafterfirst dose MCC immunization were previously reported.13 The hSBAassaywasperformedaspreviouslydescribed.13,14 Sero-protectionwasdefinedasanhSBAtiter≥1:4andindividuals wereclassified ashSBA persistenceaccordingtothe pres-enceoftiter≥1:412---18monthspost-immunization.
Statisticalanalysis
Continuous and categorical variables were compared
between HIVI and HIVU using Mann---Whitney and Fisher’s
exacttests,respectively.Continuousandcategorical
varia-bleswerecomparedbetweengroupsaccordingtoantibody
persistenceusinglogisticregressionanalysis.Variableswith p-values<0.15inunivariateanalysiswereincludedina
logis-ticregressionmodel.Thesamestrategywasusedtostudy
variablesinherenttoHIVinfectiontoassessamodeljustfor HIVI.Statisticalsignificancewasdefinedasp≤ 0.05.
All analyses were performed by using STATA software
(version13.0;StataCorpLP---CollegeStation,TX,USA).
Humansubjects
Ethical approval was obtained from the IPPMG
Institu-tional Review Board (IRB) and the Brazilian Ministry of
Health Ethics Commission (Comissão Nacional de Ética
em Pesquisa [CONEP]). Participants and/or their
par-ents/guardians signed an informed consent form before
participation;assentforchildrenaged7yearsorolderwas obtained,asrequiredbythelocalIRB.
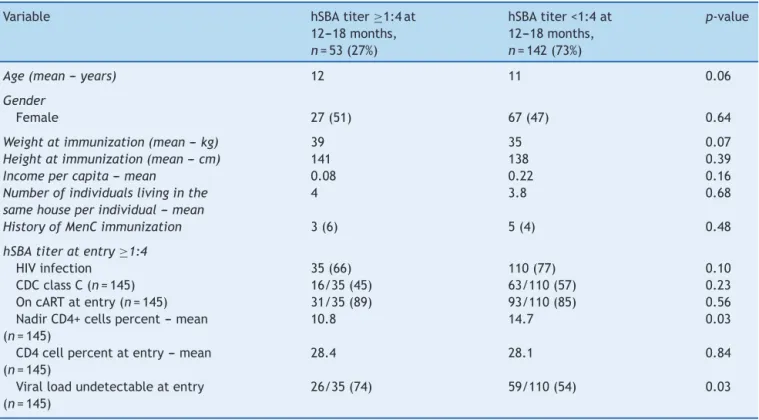
Table1 DifferencebetweenpatientswithhSBAtiter≥or<1:4at12---18months.
Variable hSBAtiter≥1:4at
12---18months, n=53(27%)
hSBAtiter<1:4at 12---18months, n=142(73%)
p-value
Age(mean--- years) 12 11 0.06
Gender
Female 27(51) 67(47) 0.64
Weightatimmunization(mean---kg) 39 35 0.07
Heightatimmunization(mean---cm) 141 138 0.39
Incomepercapita---mean 0.08 0.22 0.16
Numberofindividualslivinginthe samehouseperindividual---mean
4 3.8 0.68
HistoryofMenCimmunization 3(6) 5(4) 0.48
hSBAtiteratentry≥1:4
HIVinfection 35(66) 110(77) 0.10
CDCclassC(n=145) 16/35(45) 63/110(57) 0.23
OncARTatentry(n=145) 31/35(89) 93/110(85) 0.56
NadirCD4+cellspercent---mean (n=145)
10.8 14.7 0.03
CD4cellpercentatentry---mean (n=145)
28.4 28.1 0.84
Viralloadundetectableatentry (n=145)
26/35(74) 59/110(54) 0.03
Results
Atotalof154HIVIand50HIVUparticipantswereenrolledin thestudy.NineindividualsintheHIVIgroupwereexcluded at the 12---18 month visit (sixwere lost tofollow-up, one
wastransferredtoanotherstate,andonewasonprolonged
useofantibiotics);datawasavailablefor195participants.
Females comprised 52% of the participants, with no
dif-ferences between groups (p=0.11). The median age at
enrollmentwas11years:12yearsforHIVIand10yearsfor HIVUparticipants(p=0.02;Table1).
TherewerenodifferencesinmedianbodymassindexZ -scoresbetweenHIVIandHIVU,0.39and−0.14,respectively (p=0.17). The HIVU group had a higher median number of individuals living in the same household(five vs. four;
p<0.01).Althoughthemedianfamilyincomeinbothgroups waslessthanoneBrazilianminimumwage,whenthe distri-butionofthisvariablewasconsidered,theHIVUgrouphada significantlylowerfamilyincome(p=0.01).Atotalof89%of HIVIparticipantswerereceivingcART,124ofwhomstarted cARTbeforeentryandfive,afterentry.Themediantimeof cARTusewas7.5years(IQrange=5.9---9.9years).Atotalof 59(41%)hadahistoryof CentersforDiseasesControl and Prevention(CDC)HIVclinicalclassificationclassCconditions and85(44%)hadundetectableVLatentry.
Overall,14.9%(29/195)hadbaseline(before immuniza-tion)hSBA >1:4,witha higherproportionamongtheHIVU (30% vs. 9.7%; p=0.01). Among thosewith baseline hSBA >1:4,themeanfamilyincomewas0.17Brazilianminimum wage.
At12---18monthspost-immunization,53(27.2%)retained seroprotectiveSBAtiters:24.1%amongHIVIand36%among HIVU (p=0.14). Of the 29 individuals who had baseline immunity,21(72%)retainedprotectivehSBAtiterat12---18 months visit. Although the baseline immunity rate was higheramongHIVU,moreHIVIremainedwithhSBA>1:4,if theyhadpreviousimmunityatentry(8/15forHIVUvs.13/14 forHIVIgroup,p=0.04).
Therewasnodifferenceintheseroprotectiveratebyage atentry(p=0.08)orlengthoftimebetweenvaccinationand 12---18monthsvisits(p=0.57).
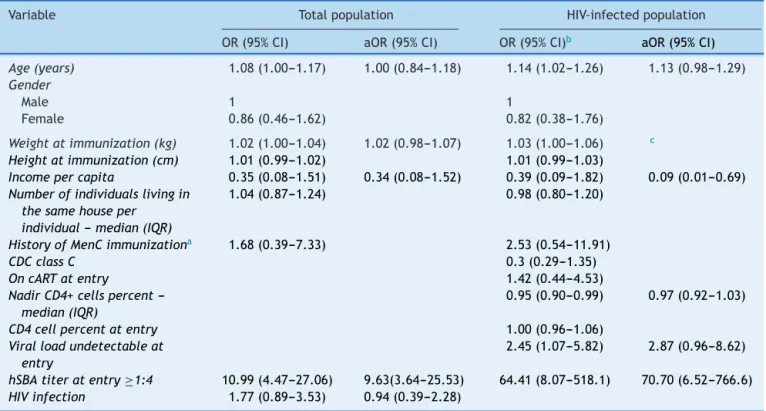
The main variable associated with hSBA persistence 12---18monthsafterMCCforthetotalpopulation,aswellas fortheHIVI,wasbaselinehSBA>1:4;however,olderageand higherbodymassindexwerealsoassociatedwithhSBA per-sistenceinthe totalpopulation. UndetectableVLatentry andincomewerealsoimportantamongHIVIgroup(Table2). Onthelogisticregressionmodel,baselinehSBA>1:4wasthe mainsignificantvariableonthemodelofthetotal popula-tion;thesamewasobservedonthemodeloftheHIVIgroup.
Table2 Unadjustedandadjustedoddsratio(ORandaOR),and95%confidenceinterval(95%CI)forhSBA≥1:4after12---18
monthsofimmunizationfortotalpopulation(n=195)andspecificallyforHIV-infectedindividuals(n=145).
Variable Totalpopulation HIV-infectedpopulation
OR(95%CI) aOR(95%CI) OR(95%CI)b aOR(95%CI)
Age(years) 1.08(1.00---1.17) 1.00(0.84---1.18) 1.14(1.02---1.26) 1.13(0.98---1.29) Gender
Male 1 1
Female 0.86(0.46---1.62) 0.82(0.38---1.76)
Weightatimmunization(kg) 1.02(1.00---1.04) 1.02(0.98---1.07) 1.03(1.00---1.06) c
Heightatimmunization(cm) 1.01(0.99---1.02) 1.01(0.99---1.03)
Incomepercapita 0.35(0.08---1.51) 0.34(0.08---1.52) 0.39(0.09---1.82) 0.09(0.01---0.69)
Numberofindividualslivingin thesamehouseper
individual---median(IQR)
1.04(0.87---1.24) 0.98(0.80---1.20)
HistoryofMenCimmunizationa 1.68(0.39---7.33) 2.53(0.54---11.91)
CDCclassC 0.3(0.29---1.35)
OncARTatentry 1.42(0.44---4.53)
NadirCD4+cellspercent ---median(IQR)
0.95(0.90---0.99) 0.97(0.92---1.03)
CD4cellpercentatentry 1.00(0.96---1.06)
Viralloadundetectableat entry
2.45(1.07---5.82) 2.87(0.96---8.62)
hSBAtiteratentry≥1:4 10.99(4.47---27.06) 9.63(3.64---25.53) 64.41(8.07---518.1) 70.70(6.52---766.6)
HIVinfection 1.77(0.89---3.53) 0.94(0.39---2.28)
hSBA,humanserumbactericidalassay;HIV,humanimmunodeficiencyvirus;IQR,interquartilerange;MenC,polysaccharidenon-conjugate meningococcalCvaccine;CDC,CentersforDiseasesControlandPrevention---HIVclinicalclassification;cART,combinedantiretroviral therapy.
a HistoryofpreviousmeningococcalpolysaccharideCvaccination.
Inthelattergroup,undetectableVLatentry(atthe immu-nization)alsopresentedatrendofsignificance(Table2).
Discussion
The persistence of MCC bactericidal antibodies 12---18
monthsafterimmunizationinBrazilianchildrenand
adoles-centswithandwithout HIVinfectionwaslow(27.2%),and
lowerinHIVI individuals, although notstatistically
signifi-cant.Theantibodypersistenceratewasinaccordancewith
thatobservedontheliterature(19---57%).12,15---17
The main variable associated with persistence of MCC antibodieswashSBAtiteratentry≥1:4.Forindividualswho hadbaselineimmunity,thesingledosevaccinewasatleast thesecond contactwiththisagent, andtheprime immu-nizationwouldbeconsideredabooster.18
To thebestof the authors’knowledge, thisis thefirst studyofMCCantibodypersistenceinHIVIchildrenand ado-lescents.Siberry etal.followed-up45childrenaged2---10 years, up to 72 weeks after two doses of meningococcal ACWY immunization.19 They observed a 45% persistence rateof theN.meningitidisCantibodies,definedasrabbit SBA≥1:128; Black patients hadhigher rates of long-term immunity.Indeed,inthemultivariateanalysis,a socioeco-nomicproxyvariable,income,wasassociatedwithimmune persistence. The authors hypothesize that patients with lower socioeconomic level were exposed to a crowded environment, and therefore had more chance to having beenexposedtoserogroupCN.meningitidis.Nonetheless, geneticfactorsmustalsoplayaroleontheassociationof lowersocioeconomic level and decreased immune persis-tence.
Basedonthepresent results,protectiveSBAtiter grad-uallywanedovertime,andatleasttwodosesofMCCare necessarytomaintainprotectionagainstN.meningitidisin participantsaged2---18years.Furthermore,optimalimmune responsetoMCCamongHIVImaycoincidewithundetectable VL,whichwouldhaveimplicationsforthetimingof immu-nizationinthisgroup.
In other pediatric studies, antibody persistence was higheramongolderthepatientsatimmunization.12,16,17,20---24 This phenomenon was not observed in the present study, followingadjustmentfor othervariables, suchasbaseline immunity.However,HIVinfectionwasnotsignificantly asso-ciatedwithlack of antibodyresponse after12---18months oftheimmunizationasexpected.ConsideringthattheHIVI grouphadaworseMCCimmuneresponse1---2monthsafter immunization,13 and since the HIVI group was older than theHIVUgroupandhadhighersocioeconomicstatus, per-hapsthesevariablesplayedaroleindeterminingthelack ofdifferencebetweenHIVIandHIVUgroupsorinage. Prob-ably,thestudysamplesizewasnotlargeenoughtodiscern differencesinsomegroups’covariates.
Inconclusion,itwasdemonstratedthatonedoseofMCC vaccinewasnotsufficienttomaintainimmuneprotectionat 12---18monthsamongbothHIVIandHIVU,evenamongHIVI withpreservedorrestoredimmunity.Studiesthatevaluate newapproaches tovaccinationmust bepursued.In order tomaintainimmune response amongtheHIVI individuals, itisprobablyimportanttoimmunizetheseindividualswith undetectableVL.
Funding
ThestudywasfundedbygrantsfromFogartyInternational
Centerof the National Institutes of Healthto CBH (Grant
No.5R01TW008397)andfromFoundationforResearch
Sup-port of the State of Rio de Janeiro (FAPERJ, Grant No.
#E-26/112.645/2012)toLGM.Thisworkwasalsosupported
in part by a grant from the Fogarty International
Cen-ter Global Infectious Diseases ResearchTraining Program,
NationalInstitutesofHealth,totheUniversityofPittsburgh
(GrantNo.D43TW006592).Thecontentissolelythe
respon-sibilityoftheauthorsanddoesnotnecessarilyrepresentthe officialviewsoftheNationalInstitutesofHealth.
Conflict
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
The authors would like thank the parents, children, and
adolescentswhoparticipatedinthisstudy.
References
1.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic menin-gitis,meningococcaemia, andNeisseriameningitidis.Lancet. 2007;369:2196---210.
2.MeningococcaldiseasecontrolincountriesoftheAfrican menin-gitisbelt,2014.WklyEpidemiolRec.2015;90:123---31. 3.CohenC,Singh E,Wu HM,MartinS, de GouveiaL, Klugman
KP,etal.Increasedincidenceofmeningococcaldiseasein HIV-infectedindividualsassociatedwithhighercase-fatalityratios inSouthAfrica.AIDS.2010;24:1351---60.
4.Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, KennedyJ,etal.Elevatedriskforinvasivemeningococcal dis-easeamongpersonswithHIV.AnnInternMed.2014;160:30---7. 5.Nair N, Moss WJ, Scott S, Mugala N, Ndhlovu ZM, Lilo K, et al. HIV-1 infection in Zambian children impairs the developmentand aviditymaturationofmeaslesvirus-specific immunoglobulinGaftervaccinationandinfection.JInfectDis. 2009;200:1031---8.
6.MossWJ,ClementsCJ,HalseyNA.Immunizationofchildrenat riskofinfectionwithhumanimmunodeficiencyvirus.BullWorld HealthOrgan.2003;81:61---70.
7.SutcliffeCG,MossWJ.DochildreninfectedwithHIV receiv-ing HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630---42.
8.GerettiAM,DoyleT.ImmunizationforHIV-positiveindividuals. CurrOpinInfectDis.2010;23:32---8.
9.KernéisS,LaunayO, TurbelinC,BatteuxF,Hanslik T, Boëlle PY.Long-termimmuneresponsestovaccinationinHIV-infected patients:asystematicreviewandmeta-analysis.ClinInfectDis. 2014;58:1130---9.
10.CollinsCL,RuggebergJU,BalfourG,TigheH,ArcherM, Bowen-MorrisJ, etal. Immunogenicityand immunologicmemory of meningococcalCconjugatevaccineinprematureinfants. Pedi-atrInfectDisJ.2005;24:966---8.
11.Zlamy M, Elias J, Vogel U, Frosch M, Jeller V, Cortina G, et al. Immunogenicity of conjugate meningococcus C vac-cine in pediatric solid organ transplant recipients. Vaccine. 2011;29:6163---6.
aftermeningococcal C vaccination in patients withjuvenile idiopathicarthritis:aretrospective cohortstudy.AnnRheum Dis.2014;73:728---34.
13.FrotaAC,MilagresLG,HarrisonLH,FerreiraB,MennaBarretoD, PereiraGS,etal.Immunogenicityandsafetyofmeningococcal Cconjugatevaccineinchildrenandadolescentsinfectedand uninfectedwithHIVinRiodeJaneiro,Brazil.PediatrInfectDis J.2015;34:e113---8.
14.MaslankaSE,GheeslingLL,LibuttiDE,DonaldsonKB,Harakeh HS, Dykes JK, et al. Standardization and a multilaboratory comparisonofNeisseriameningitidisserogroupAandCserum bactericidalassays.TheMultilaboratoryStudyGroup.ClinDiagn LabImmunol.1997;4:156---67.
15.GoldschneiderI,GotschlichEC,ArtensteinMS.Humanimmunity tothemeningococcus.I.Theroleofhumoralantibodies.JExp Med.1969;129:1307---26.
16.SakouII,TzanakakiG,TsoliaMN,SioumalaM,BarbouniA, Kypri-anouM, etal.Investigationofserum bactericidalactivity in childhoodandadolescence3---6yearsaftervaccinationwitha singledoseofserogroupCmeningococcalconjugatevaccine. Vaccine.2009;27:4408---11.
17.Perrett KP, Richmond PC, Borrow R, Nolan T, McVernon J. Antibody persistence in Australian adolescents following meningococcalCconjugate vaccination.PediatrInfect DisJ. 2015;34:279---85.
18.Vauloup-Fellous C, Grangeot-Keros L. Humoral immune response after primary rubella virus infection and after vaccination.ClinVaccineImmunol.2007;14:644---7.
19.SiberryGK,WarshawMG,WilliamsPL,SpectorSA,DeckerMD, Jean-Philippe P,etal. Safetyand immunogenicityof quadri-valent meningococcal conjugatevaccine in2-to 10-year-old humanimmunodeficiencyvirus-infectedchildren.PediatrInfect DisJ.2012;31:47---52.
20.Trotter CL, Borrow R, Findlow J, Holland A, Frankland S, Andrews NJ, et al. Seroprevalence of antibodies against serogroupCmeningococciinEnglandinthepostvaccinationera. ClinVaccineImmunol.2008;15:1694---8.
21.SnapeMD,KellyDF,LewisS,BannerC,KibwanaL,MooreCE, etal.SeroprotectionagainstserogroupCmeningococcaldisease inadolescentsintheUnitedKingdom:observationalstudy.BMJ. 2008;336:1487---91.
22.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasivebacteria with protein---polysaccharide conju-gatevaccines.NatRevImmunol.2009;9:213---20.
23.PerrettKP,WinterAP,KibwanaE,JinC,JohnTM,YuLM,etal. AntibodypersistenceafterserogroupCmeningococcal conju-gateimmunizationofUnitedKingdomprimary-schoolchildren in1999-2000andresponsetoabooster:aphase4clinicaltrial. ClinInfectDis.2010;50:1601---10.