REVISTA
PAULISTA
DE
PEDIATRIA
www.rpped.com.br
ORIGINAL
ARTICLE
Use
of
honey
associated
with
Ananas
comosus
(Bromelin)
in
the
treatment
of
acute
irritative
cough
Décio
Medeiros
Peixoto
a,
José
Angelo
Rizzo
a,
Deborah
Schor
a,
Almerinda
Rêgo
Silva
a,
Dinaldo
Cavalcanti
de
Oliveira
a,
Dirceu
Solé
b,
Emanuel
Sarinho
a,∗aUniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil bUniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil
Received20October2015;accepted24March2016 Availableonline20August2016
KEYWORDS
Cough; Honey; Bromelin
Abstract
Objective: Toevaluatetheimmediateimprovementrateofirritativecoughinpatientstreated withthecombinationofAnanascomosusextractandhoney(Bromelin®)comparedwiththeuse
ofhoneyalone(placebogroup).
Methods: Pragmatic, double-blind, randomized, parallel-group study with children aged between2and15years,withirritativecough foratleast24hours.The double-blind assess-mentofcoughwasthroughthenumberofobservedcoughingepisodesandintensityscorefor aperiodof10minutesofobservation.Thedecreaseofonepointinthemeantotalscorewas consideredasatherapeuticeffect.
Results: Therewasareductionincoughingepisodesinbothgroups,aswellasinthecough scoreafter30minutesofdrugorhoneyadministration.Thechangeinclinicalscoreabovetwo points,whichcouldindicatemarkedimprovement,occurredinfivepatientsinthebromelin groupandonlyinoneintheplacebogroup,butwithoutsignificantdifference.Therewereno adverseevents.
Conclusions: The immediate improvement rate of irritative cough was similar in patients treatedwithcombinationofAnanascomosus extractandhoney(Bromelin®)compared with
theuseofhoneyalone(placebogroup).Itispossiblethathoneyhasatherapeuticeffecton mucusandcoughcharacteristics(ClinicalTrials:NCT01356693).
©2016SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
∗Correspondingauthor.
E-mail:emanuel.sarinho@gmail.com(E.Sarinho). http://dx.doi.org/10.1016/j.rppede.2016.04.002
PALAVRAS-CHAVE
Tosse; Mel; Bromelina
UsodomeldeabelhaassociadoaoAnanascomosus(Bromelin)notratamento datosseirritativaaguda
Resumo
Objetivo: Avaliarataxademelhoriaimediatadatosseirritativaempacientestratadoscom associac¸ãodoextratodoAnanascomosusemmeldeabelha(Bromelin®)ecompará-lacoma
deusodomelisolado(grupoplacebo).
Métodos: Estudopragmático davida real,duplo-cego, randômico, de grupos paralelos, em crianc¸as,entredoise15anos,comtosseirritativahaviapelomenos24horas.Aavaliac¸ãoda tossefoiduplo-cegafoifeitapormeiodaavaliac¸ãodonúmerodeepisódiosobserváveisedo escoredeintensidadedetosseduranteoperíodode10minutosdeobservac¸ão.Areduc¸ãode umpontonamédiadoescoretotalfoiconsideradacomoefeitoterapêutico.
Resultados: Emambososgruposhouvereduc¸ãodonúmerodeepisódiosdetosse,assimcomo doescoredetosseapós30minutosdeadministrac¸ãodomedicamentooudomel.Amudanc¸a deescoreclínicosuperioradois,quepoderiaindicarmelhoriaacentuada,ocorreuemcinco pacientesdogrupocombromelinaeemapenasumdoplacebo,massemdiferenc¸asignificante. Nãoocorrerameventosadversos.
Conclusões: Ataxademelhoriaimediatadatosseirritativafoisimilarentrepacientestratados comassociac¸ãodoextratodoAnanascomosusemmeldeabelha(Bromelin®)ecomousodomel
isolado(grupoplacebo).Épossívelquehajaumefeitoterapêuticodomelnascaracterísticas domucoedatosse(ClinicalTrials:NCT01356693).
©2016SociedadedePediatriadeS˜aoPaulo. PublicadoporElsevier EditoraLtda.Este ´eum artigoOpenAccesssobumalicenc¸aCCBY(http://creativecommons.org/licenses/by/4.0/).
Introduction
Viralprocessesof theairways areusuallyself-limitedand characterizedmainlybyfeverandcoughing,whicharetwo independentpredictors of the virus presenceand tend to disappear in approximately one week. However,coughing is themost common symptom inprimary care physicians’ officesandisafrequentcauseofvisitstoemergencycare; thissymptomcanbecomesointensetothepointofleading patientandrelativestoexhaustion.1---3
Insuchcases,symptomaticdrugsareoftenusedbythe patientsthatself-medicate,ofteninresponseto advertise-mentandthefactthattheydonotrequireaprescription. Availableinpharmaciesanddrugstores,withdifferent for-mulations, substances for cough relief can bring risks, especiallyinthepediatricagegroup.3,4
Dextromethorphananddiphenhydramineareoftenused asantitussiveagents,especiallyinchildren.5Aclinicalstudy compared these two therapeutic principles with honey, regardingthecontrolofnocturnalcoughandsleepqualityof childrenwithirritativecough,andfoundthathoney(2.5mL) administeredbeforesleep,showedbetterresultsregarding symptom relief when comparedwith these twodrugs.6 A recent Cochrane Library review, carried out with clinical studieswithacceptable methodologicalqualitystandards, did not report any evidence for or against the effective-nessof thesedrugs in thesymptomatic reliefof patients withcough.4 Therefore,an idealtherapeutic strategy for symptomaticcontrolofcoughremainsuncertaintodate.3---5 Consequently,theindividualandtherelativesdistressed bythecoughingseekmeasurestheyconsidersafeforcough relief,whenthissymptomisintense.Inthisaspect, empir-icaltherapybasedonnaturaland/or herbalproducts, has
beenincreasinglyemployedandmanypeopleuseitfreely, withorwithoutmedicalprescription,secondarytothe rela-tives’suggestionand/orthepatient’sownbeliefs.3
Inthis context,products derivedfromAnanas comosus
ThecombinationofA.comosusextractwithhoneymay represent a breakthrough in the symptomatic treatment, possiblybyblockingthetriggeringmechanismsofcoughdue toviralorirritativeconditions.Thus,thisstudywasaimed toevaluatetheeffectontheimmediateinhibition of irri-tative coughin patients treatedwitha combinationof A. comosus (pineapple) and honey (Bromelin®) and compare them withthosetreated with honeyalone (placebo) in a pediatricemergencycareenvironment.
Method
This pragmatic, real-life, double-blind, randomized study ofparallelgroups wascarriedoutin thepediatric service of Clinica Amaury Coutinho, linked to Recife City Hall. Patientsintheactivegroup(letterA)receivedthe combi-nationofhoneyandA.comosusextractHBS19820501(rich in Bromelin) as syrup formulation and the placebo group (letter B) received only honey. The quality of the honey wascertified in both groups and approved by the regula-toryagenciesandtheNational HealthSurveillanceAgency (Anvisa)forsale;thehoneyhadbeenrecentlyproducedand underwentstrictbacteriologicalcontrol.Randomizationwas carriedoutaccordingtoatablegeneratedbyExcelprogram. Thedouble-blindingwasreleasedandthetreatmentgroups wererevealedonlyaftertheanalysisofthestudyresults.
Children aged between two and 15 years, with irrita-tivecoughfor atleast 24hours,which ledtotheneed for medicalconsultation,participatedinthestudy.Weexcluded patients with a history of obstructive pulmonary dis-ease,cysticfibrosis,neuropathies,heartdisease,diabetes andidentifiableprimaryorsecondaryimmunodeficiencies. Patientsshouldhaveacutecoughduetoviralupperairway infection,thusconsideredduetothepresenceofmildfever or fever associated with hyaline or catarrhal rhinorrhea, lasting less than 72hours, without clinical manifestations of associated bronchospasm. After meeting the inclusion criteria, patients were randomly assigned to a treatment group(Bromelin® or honey).The patients’parents and/or
guardians agreed with their participation and signed the informedconsentform.Thetwotreatmentregimenswere administeredas follows:(a) children up to20kgreceived 5mL; (b) for those weighing>20kg, 1mL wasadministered for every five kilos of additional weight.Before the ran-domization, patients with detectable bronchospasm were prescribed-2adrenergic and, ifnecessary, asingle-dose of oral steroids and the patientwasconsidered ineligible forthestudy.TheorganolepticcharacteristicsofBromelin®
andhoneyaresimilar.
Patients’ evolution and clinical data after treatment werereportedinastandardizedclinicalreportform.Cough assessmentwascarriedoutindouble-blindfashionbyone of the investigators by counting the number of episodes observed and the cough intensity score during a 10-min observation period. The initial and final cough intensity scorewasclassifiedaccordingtoatoolas0=absent;1=mild, 2=moderate;3=intense;4=veryintense,accordingtoa pre-vious study.5 According to this tool, a decrease of one pointinthemeantotalscoreisconsideredastherapeutic effect.After waiting 30minutes post-drug administration, thetherapeuticresponsewasassessedduringa10-minute
observationperiod,whichwaschosenasitisfeasible and satisfactory inthe actualclinical setting ofan emergency careservice.
Therefore, after the coughing episodes observed by the researcher were quantified in the 10minutes prior to treatmentadministration,thestandardizedobservationwas repeated 30minutes after drug administration, again in a double-blind fashion.All evaluationsbeforeandafter the drugorplaceboadministrationwerecarriedoutbyasingle andthesameinvestigator(physician)inadouble-blind fash-ion(neithertheinvestigatornorthepatient,northefamily knew which product was used), in order to attain robust results.
Themedicationsafetywasassessedbythefrequencyof reportedadverseevents,suchasepisodesofvomiting, epi-gastricandabdominalpain,amongothers.The monitoring ofsubsequentadverseeventswasnotperformed.
Tocalculatesamplesize,thefollowingwasconsidered: power of test of 90%, rate of cough improvement in the active group of 40%, and 10% in the placebo group, two-tailed hypothesis test and 5% significance level. Thus, 60 patientswerenecessarytoassessthepossible therapeutic effectsoftheactivetreatment.Thiscalculationisaccording totheclinical trial byMuller etal.,whostudied approxi-mately30patientspergrouptoassessthepossibleeffectof Bromelinoninflammatorymarkers,butwiththeconsequent impactontheclinicalimprovementofcough.8
Statisticalanalysistookinto accountthenatureof the assessedvariables. Numericalvariableswereexpressedas meanandstandarddeviation or,whennecessary,in medi-ansand25---75percentiles,whereascategoricaloneswere expressedaspercentages. Numerical variableswere com-pared usingStudent’s t test, whereas the chi-squaretest was used for categorical ones. The value wasconsidered significantwhenp<5%.
ThestudywasapprovedbytheInstitutionalReviewBoard ofCentrodeCiênciasdaSaúdedaUniversidadeFederalde Pernambuco,withCAAEn.0311.0.172.000-10andthetrial wasregisteredat ClinicalTrialssobundern.NCT01356693 (ClinialTrials.govIdentifier).
Results
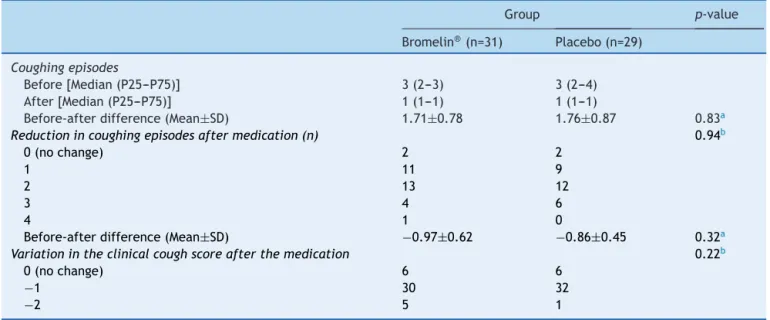
Table1demonstratesthatthetwogroupsofpatientswere similarinage,gender, daysofcoughingandmeanclinical scoreofcoughingepisodes.Table2showsthatbothgroups had a reduction in coughing episodes, as well as in the coughscore30minutesafterdrugorhoneyadministration. However,afteranalyzingthechangeinclinicalscorehigher thantwopoints,whichcouldindicateamoreaccentuated improvement,itwasseentohaveoccurredinfivepatients intheactivegroupandoneintheplacebogroup.Therewere noimmediateadverseevents.
Discussion
neurotransmit-Table1 GeneralbaselinecharacteristicsoftheBromelinandPlacebo(honey)studygroupsregardinggender,meanage,days ofsymptompersistenceandinitialcoughseverityscore.
Group p-value
Bromelin® (n=31) Placebo(n=29)
GenderM/F 13/18 13/16 0.97a
Ageinyears---mean(P25---P75) 5.3(3.0---7.5) 5.6(2.0---7.5) 0.82b
Daysofsymptoms 0.98a
1 6 6
2 10 9
3 15 14
Coughingepisodes---median(P25---P75) 3(2---3) 3(2---4) 0.98b
Coughscore---median 2 2 0.91b
Score>2,n(%) 26(84%) 26(90%) 0.87a
Daysof symptoms,numberofdayswithsymptoms ofcoughbefore consultation;coughing episodes---median(P25---P75),numberof coughingepisodesduringthe10minutesofinitialobservationbythedoctor;coughscore---median,initialcoughscoreobservedbythe doctor(0=absent;1=mild;2=moderate;3=intense;4=veryintense).
a Chi-square. b WilcoxonTest.
Table2 Changeinthenumberofcoughingepisodesandcoughscoresbetweentheobservationperiods(10minutes)beforeand 30minutesafteradministrationofthetreatmentregimenin60evaluatedpatients.
Group p-value
Bromelin®(n=31) Placebo(n=29)
Coughingepisodes
Before[Median(P25---P75)] 3(2---3) 3(2---4) After[Median(P25---P75)] 1(1---1) 1(1---1)
Before-afterdifference(Mean±SD) 1.71±0.78 1.76±0.87 0.83a
Reductionincoughingepisodesaftermedication(n) 0.94b
0(nochange) 2 2
1 11 9
2 13 12
3 4 6
4 1 0
Before-afterdifference(Mean±SD) −0.97±0.62 −0.86±0.45 0.32a
Variationintheclinicalcoughscoreafterthemedication 0.22b
0(nochange) 6 6
−1 30 32
−2 5 1
a Student’sttest. b Chi-square.
ters andleukotrienes, which induce an increase inneural receptorlevels,withtransientstimulationofafferentneural activity,mucushypersecretionand,possibly,exacerbation ofthe effectsoncholinergicmotor pathways.The feeling of irritation that precedes the cough motor action allows usto inferthe concept of cough syndromedue to hyper-sensitivity after acute respiratory viral infection, which, in somepatients, becomesa refractory,exhaustingcough duetoinflammationandexcessmucus.17 Although diphen-hydramine anddextromethorphanarethemostcommonly useddrugsinthemanagementofirritativecough,theyhave not been shown to be superior to placebo in controlling this symptom.18 On the other hand, a comparative study betweendextromethorphan andbuckwheat honeyshowed
theirequivalence, withhoneysuperiority when compared withplacebo-treatedpatients.15
Themucolyticactionofcertaindrugscanpromote reduc-tion in mucus viscosity and facilitate its elimination by coughingandthroughciliarybeatinginthebronchial epithe-lium.Thisispossibleduetothebreakdownofmucoproteins, whichsplitsthemintopolypeptides.Furthermore,the anti-inflammatoryactionofaspecificdrugcancontributetothe reductioninthickmucusproduction.7,17,19TheextractofA.
bonds by incorporating water molecules, thus facilitating thefluidizationofthickmucus.7,19
Invitrostudiesindicatethattheanti-inflammatory activ-ity of Bromelin, results in part from the inhibition of bradykininformationattheinflammationsite,throughthe depletionofplasmakallikreinlevels,aswellasthereduction of intermediates of the coagulation cascade, which lim-itsthe formation of fibrin.Additionally, Bromelinreduces leukocytemigration,aswellastheexpressionofadhesion molecules(CD128)tobloodvessels.7,19,20Thepossible ben-eficialeffectofBromelinmaybeduetoanintegratedseries of actions on cells and cytokines, such as: natural killer (NK)cellactivation,increasedproductionoftumor necro-sisalphafactor,aswellasInterferon-gamma(IFN-␥)several interleukins(IL-1,IL-2,IL-6)andofgranulocyte-macrophage colony-stimulating factor andseemscapable of interrupt-ingthecontinuous activationof CD4+ lymphocytes,which maintaintheinflammatoryprocess.21
Additionally, in healthy humans, oral ingestion of Bromelinpromotes achangeinthecircadian cycleof IFN-␥,IL-5andIL-10,suggesting an immunomodulatoryeffect onT lymphocytes.21 This observation has been the ratio-nalefortheuseofBromelin,eitheraloneorassociatedwith othernaturalproductsinthetreatmentofautoimmuneand inflammatorydiseases, suchasulcerativecolitis, multiple sclerosisandrespiratorytractdisorders.21,22Inchildrenwith acutesinusitis,Bromelinwasaddedtothestandardtherapy and there was a significant reduction in symptom dura-tionandtimeofrecovery,whencomparedwiththosewho receivedonly theconventional treatment; itis extremely safe,withonlyamildandself-limitingallergicreactionina patientallergictopineapple.13
Honeywasthecommonconstituentofthetwoformsof treatment,whichleadsustobelievethatthemucolyticand cough reliefpropertiesof honey wereresponsible for the reductioninthecoughscore,aswellasthereductioninthe number of coughing episodes observed in the two groups ofassessedpatients.15,23The literaturehassuggestedthat honeymaybeindicatedasarationalmeasureinthe treat-mentofcough,asitislowcost,hasrareadverseeventsand thereissomeevidenceofapossibletherapeuticaction.16,24 This clinical trial suggeststhat, considering thepatent improvementinbothgroups,thereprobablyisatherapeutic effectofhoneyonmucusandcoughcharacteristics,which may have made it difficult to identify differences when honey is associated with the possible additional pharma-cologicalandfavorableaction ofBromelin.The suggested hypothesis isthat the sweetnessof honeyitself promotes salivationandsecretionproductionintheairwaysandleads to mucus liquefaction, reducing cough due to the lower irritationinthelarynxandpharynxand,mostimportantly, withno side effects, soit can be safely used in children older than one year, when the intestinal microbiome is structured and defined and there is adequate immunity againstClostridium botulinum, a possible contaminantof honey.16,25,26 In a multicenter trial carried out in Europe, honeywasusedinchildrenaged1to18yearswithoutany sideeffectsandwithpossibletherapeuticbenefits.27
Thepresent study,asmentionedbefore,isapragmatic clinicaltrialcarriedoutinareal-lifescenarioofan emer-gencyservice, which mayhave created some limitations, suchasthe need touse more objectiveand reproducible
indicatorstoassesscoughimprovement,especiallyina pop-ulationofchildrenwithsuchawideagerangewideforthe analysisofclinicalsigncausedbysuchdiversepathologies. However,allthestudypatientshadacutecoughassociated withupperairwayinfectionand,additionally,allevaluations werecarriedoutin double-blindfashionandstandardized byasingleresearcher,allowinganinternalvalidationofthe study.Anevaluationmethodusingvideo, alongertimeof observationandabetter-definedoutlineofexistingdiseases wouldbeideal,butwouldhindertheoperationalassessment inarepresentativeclinicalscenarioofemergencycare.
The addition of Bromelin to honey does not result in an additionaleffect in the treatment of irritative cough. It seemsthat apossible effectof Bromelin,if any, would bebetterassessedbyusinganothersubstanceasplacebo, becausehoneycanbeapharmacologicallyactivesubstance incoughing.25---27 Similarly,newclinicaltrialswithalonger observationperiod,assessingotheroutcomesarenecessary toevaluatethispotentialtherapeuticeffectofhoney,either alone or associated, on irritative cough. In daily clinical practice,parents, relativesand patientsareinterested in thisexplanationwithsomeurgency,considering theircare and safetyand the possibilityof relieffor the annoyance thatirritatingcoughcausestopatients.16,25,26
Funding
HebronIndústriasQuímicas.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.KoolM,MontenyM,vanDoornumGJ,MollHA,BergerMY. Respi-ratoryvirusinfectionsinfebrilechildrenpresentingtoageneral practiceout-of-hoursservice.EurJGenPract.2014;21:1---7. 2.ThompsonM,VodickaTA,BlairPS,BuckleyDI,HeneghanC,Hay
AD,etal.Durationofsymptomsofrespiratorytractinfections inchildren:systematicreview.BMJ.2013;11:f7027.
3.AlbertRH.Diagnosisandtreatmentofacutebronchitis.AmFam Physician.2010;82:1345---9.
4.SmithSM,SchroederK,FaheyT.Over-the-counter(OTC) med-icationsforacutecoughinchildrenandadultsinambulatory settings.CochraneDatabaseSystRev.2008;23:CD001831. 5.ShadkamM,Mozaffari-KhosraviH,MozayanMR.Acomparisonof
theeffectofhoney,dextromethorphan,anddiphenhydramine onnightlycoughandsleepqualityinchildrenandtheirparents. JAlternComplementMed.2010;16:787---93.
6.PaulIM,YoderKE,CrowellRK,ShafferML,McMillanHS, Carl-sonMC,etal.Effect ofdextromethorphan,diphenhydramine andplaceboonnocturnalcoughandsleepqualityforcoughing childrenandtheirparents.Pediatrics.2004;114:e80---5. 7.FitzhughDJ, ShanS,DewhirstMW,Hale LP.Bromelain
treat-mentdecreasesneutrophil migration to sitesof inflamation. ClinImmunol.2008;128:66---74.
9.SeltzerAP.Adjunctiveuseofbromelainsinsinusitis:acontrolled study.EyeEarNoseThroatMon.1967;46:1281---8.
10.TaubSJ.Theuseofbromelainsinsinusitis:adouble-blind clin-icalevaluation.EyeEarNoseThroatMon.1967;46:361---2. 11.RimoldiR,GinesuF,GiuraR.Theuseofbromelainin
pneumo-logicaltherapy.DrugsExpClinRes.1978;4:55---66.
12.Ako H, Cheung AH, Matsuura PK. Isolation of a fibrinolysis enzymeactivatorfromcommercial bromelain.ArchInt Phar-macodynTher.1981;254:157---67.
13.BraunJM,SchneiderB,BeuthHJ.Therapeuticuse,efficiency and safety of the proteolytic pineapple enzyme Brome-lain in children with acute sinusitis in Germany. In Vivo. 2005;19:417---21.
14.CohenHA, RosenJ,Kristal H,Laks Y, BerkovitchM,UzielY, etal.Effect ofhoneyonnocturnal coughand sleepquality: a double-blind, randomized, placebo-controlled study. Pedi-atrics.2012;130:465---71.
15.PaulIM,BeilerJ,McMonagleA,ShafferML,DudaL,BerlinCM Jr.Effect ofhoney,dextromethorphan,and notreatmenton nocturnalcough and sleep qualityfor coughing childrenand theirparents.ArchPediatrAdolescMed.2007;161:1140---6. 16.EvansH,TuleuC,SutcliffeA.Ishoneyawell-evidenced
alter-native to over-the-counter cough medicines? J R Soc Med. 2010;103:164---5.
17.DicpinigaitisPV.Effect ofviralupperrespiratorytract infec-tionon cough reflex sensitivity. JThorac Dis. 2014;6Suppl. 7:S708---11.
18.WalmsleyJ,MarshallG.Overthecountercoughmedicinesfor acutecough.Thefactthatpeoplekeepbuyingthemedicinesis itselfevidence.BMJ.2002;324:1158.
19.SarmentoDM,MouraDP,LopesSL,SilvaSC.Bromelain mono-graph.AlternMedRev.2010;15:361---8.
20.SecorER,CarsonWF,SinghA,PensaM,GuernseyLA,Schramm CM, et al. Oral bromelain attenuates inflammation in an ovalbumine-inducedmurinemodelofasthma.EvidBased Com-plementAlternMed.2008;5:61---9.
21.Müller S, März R, Schmolz M, Drewelow B, Eschmann K, Meiser P.Placebo-controlled randomizedclinicaltrialonthe immunomodulatingactivities oflow-andhigh-dosebromelain afteroraladministration---newevidenceontheantiinflamatory modeofactionofbromelain.PhytotherRes.2013;27:199---204. 22.TaussigSJ,BatkinS.Bromelaintheenzymecomplexof pineap-ple(Ananascomosus)anditsclinicalapplication:anupdate.J Ethnopharmacol.1988;22:191---203.
23.Cohen HA,RozenJ,Kristal H,Laks Y,Berkovitch M,UzielY, et al.Effect ofhoneyonnocturnalcough andsleep quality: a double-blind, randomized, placebo-controlled study. Pedi-atrics.2012;130:465---71.
24.WarrenMD,PontSJ,BarkinSL,CallahanST,CaplesTL,Carroll KN,etal.Theeffectofhoneyonnocturnalcough andsleep qualityfor childrenandtheirparents.ArchPaediatrAdolesc Med.2007;161:1149---53.
25.PaulIM.Therapeuticoptionsforacutecoughduetoupper respi-ratoryinfectionsinchildren.Lung.2012;190:41---4.
26.AhmedN, Sutcliffe A, Tipper C.Feasibility study:honey for treatmentofcoughinchildren.PediatrRep.2013;5:31---4. 27.MiceliSopoS,GrecoM,MonacoS,VarrasiG,DiLorenzoG,