INTRODUCTION
Mo st do uble-blind studies o f efficacy and to ler-ability o f sertraline as co mpared to tricyclics in the treat-ment o f late-life majo r depressio n have used amitrip-tyline as a standard,1,2
leading to the inevitable co nclu-sio n that the fo rmer drug is better to lerated than the latter, with bo th being equally efficacio us. Mo re recently, Finkel et al.3 examined such issues in a co ho rt o f 76
o utpatients aged 70 o r mo re, in a do uble-blind rando m-ized study o f flexible-do se sertraline (50 to 150 mg/day) o r no rtriptyline (25 to 100 mg/day), with results that also favo red the selective sero to nin re-uptake inhibito r (SSRI) in such an o lder co ho rt.
Tricyclic antidepressants may be viewed as haz-ardo us drugs fo r the treatment o f late-life depressio n, especially due to the risk o f anticho linergic side-effects, falls related to po stural hypo tensio n,4 cardiac to xicity,5
and co gnitive impairment.6,7 There is no do ubt that the
new generatio n drugs, including the SSRI’s and related classes, represent the first therapeutic cho ice in mo st cases o f mild and mo derate depressio n, due to being safer, better to lerated, and easier to prescribe. Ho w-ever, increasing evidence demo nstrates that the latter drugs may also induce severe side effects, such as par-kinso nism and sero to nin syndro me. In additio n, they may be less efficacio us in the treatment o f psycho tic depressio n, as co mpared to standard drugs and ECT. Fo r elderly patients with severe depressio n, o r fo r tho se who are ho spitalized, seco ndary amine tricyclic antide-pressants, such as no rtriptyline, are perceived as highly effective treatment.8 As these are well to lerated drugs
amo ng the tricyclics, they co ntinue to be relied upo n
Original Article
REVISTA PAULISTA DE MEDICIN AAntide pre ssant e fficacy of se rtraline and
imipramine for the tre atme nt of major
de pre ssion in e lde rly outpatie nts
“Projeto Terceira Idade”, Institute of Psychiatry, Hospital das Clínicas,
Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
a b s t r a c t
CON TEX T: Mo st do uble-blind studies o f efficacy and to lerability o f sertraline as co mpared to tricyclics in the treatment o f late-life majo r depressio n have used amitriptyline as a standard, leading to the inevitable co nclusio n that the fo rmer drug is better to lerated than the latter, with bo th being equally efficacio us.
OBJECTIVE: To co mpare the antidepressant efficacy and to lerability o f sertraline (5 0 mg / day) and imipramine (1 5 0 mg / day) in the first 6 weeks o f the treatment o f majo r depressio n in the elderly.
DESIGN : A rando miz ed do uble-blind parallel study with 6 weeks o f fo llo w-up.
SETTIN G: The psycho g eriatric clinic at the Institute o f Psychiatry, Ho spital das Clínicas, Faculty o f Medicine o f the University o f São Paulo .
PARTICIPAN TS: 5 5 severe and mo derately depressed no n-demented o utpatients ag ed 6 0 years o r mo re.
IN TERVEN TION : Patients were assig ned to sertraline 5 0 mg / day o r imipramine 1 5 0 mg / day.
M AIN M EASUREM EN TS: CAMDEX interview. Psychiatric diag no -sis fo llo wed the g uidelines fo r “ Majo r Depressive Episo de” acco rd-ing to DSM-IV criteria. Severity o f sympto ms was evaluated usrd-ing the “ CG I” and “ MADRS” scales. Co g nitive state was assessed using the Mini-Mental State Examinatio n. Side effects were assessed using the “ Safetee-Up” schedule.
RESULTS: Bo th g ro ups had a sig nificant decrease in depressive symp-to ms acco rding symp-to the MADRS sco res after 6 weeks o f treatment (P = 0 .0 1 ). N o sig nificant differences between g ro ups were detected reg arding treatment o utco me (t = 0 .4 ; P = 0 .7 ). Altho ug h the dro p-o ut rate was g reater in the imipramine g rp-o up, the p-o verall tp-o lerability amo ng patients who co mpleted the 6 -week trial was similar in bo th test g ro ups.
CON CLUSION S: Bo th sertraline and imipramine exhibited g o o d efficacy and an acceptable side-effect pro file fo r elderly depressed patients after 6 weeks o f antidepressant treatment.
KEY W ORDS: Depressio n. Elderly. Antidepressant drug s. Tricyclics. Sertraline. SSRI’s.
and are amo ng the mo st widely prescribed o f such medi-catio ns.9-11
A recent meta-analysis study o f efficacy, safety and to lerability o f fo ur antidepressant classes co ncluded there were no differences between o utco mes in the man-agement o f late-life majo r depressio n,12 suggesting that
there is little advantage fo r each antidepressant class o ver the o thers, namely SSRI’s, tricyclics, reversible in-hibito rs o f mo no amine o xidase-A and atypical antide-pressants. Nevertheless, such finding can o nly be gener-alized fo r samples o f selected patients. In the presence o f co ncurrent medical illnesses, o r physical and co gni-tive co nditio ns, accurate clinical judgement must be re-lied upo n fo r the co rrect cho ice o f antidepressant, as the use o f certain drugs is limited by the risk o f to xicity, phar-maco kinetic interactio ns o r into lerable side-effects.
Altho ugh mo re risky and labo rio us to prescribe, the clinician must be aware o f the putative benefits o f tricyclic antidepressants in the treatment o f depressive diso rders in the elderly. Besides being effective, tricyclics are cheaper and mo re widely available than the new class drugs, which are no t always accessible in certain settings, especially within primary care facilities o r amo ng so cially deprived patients. The current study presents efficacy and to lerability data o n the use o f sertraline and imipramine fo r treatment o f majo r de-pressio n in elderly patients, and we suggest that bo th drugs can be successfully used to pro vide adequate treatment fo r such co nditio n, in acco rdance with care-ful clinical judgement and management.
METHODS
Ethics
The pro cedures that fo llo w were in acco rdance with the ethical standards o f the co mmittee respo n-sible fo r human experimentatio n and with the Helsinki declaratio n o f 1975, as revised in 1983.
Design
This was a rando mized do ub le-b lind parallel study with 6 weeks o f fo llo w-up. We present here the preliminary data fro m the first 6 weeks o f treatment. Cases o f psycho tic depressio n, as well as suicidal pa-tients, were no t included in the study.
Subjects
Severe and mo derately depressed patients aged 60 years o r mo re were clinically evaluated and labo rato ry tested fo r co ncurrent o rganic diseases. Cerebral disease was ruled o ut by co mputer to mo graphy scans. Patients
with life-threatening medical co nditio ns, o r at risk o f se-vere clinical co mplicatio ns, due to anti-cho linergic effect (such as narro w-angle glauco ma) o r to cardiac events were excluded fro m the trial. Additio nal exclusio n criteria were alco ho l o r drug-related pro blems, and previo us treatment fo r depressio n within the past 2 mo nths.
Intervention
Study. The patients were treated with sertraline 50 mg/day o r imipramine 150 mg/day. Patients who termi-nated prematurely and tho se who co mpleted the study were all fo llo wed in a naturalistic way. Patients who did no t respo nd to sertraline 50 mg/day o r imipramine 150 mg/day after 6 weeks were eligible to have an increased do se o r, if necessary in the absence o f appro priate re-spo nse, a cro sso ver change o f medicatio n.
Sampling. Subjects were selected fro m co nsecu-tive referrals to the o utpatient service o f the Unit fo r Mental Health in the Elderly at Ho spital das Clínicas, São Paulo , Brazil. They were all info rmed abo ut the details o f the study and enro lled o nly after giving writ-ten co nsent. This pro ject was appro ved by the Ethics Co mmittee o f Ho spital das Clínicas. The rando miza-tio n pro cess was centralized at Pfizer and the co de was bro ken o nly after co mpletio n o f the study.
Allocation concealment and double-blind methods. Every patient enro lled in the trial was, after each assessment, given a sealed envelo pe labeled with his o r her trial num-ber, co ntaining the medicatio n (b.i.d.) fo r each day o f the perio d o f treatment that fo llo wed. Do se titratio n was nec-essary fo r the imipramine gro up, and do uble-blindness was preserved by maintaining a fixed daily number o f tab-lets, and varying the quantity o f active drug o r placebo within each do se. All subjects received the same number o f tablets (same shape and co lo r) thro ugho ut the trial. Imipramine treatment was started with a daily do se o f 25 mg fo r two days. Increments o f 25 mg o ccurred o n days 3, 5, 7, 9, and 11, when the final do se o f 150 mg per day was attained. Patients rando mized to treatment with sertraline received o ne tablet o f 50 mg o f active drug and identical placebo tablets daily fro m the baseline o nwards.
Main measurements
All subjects in the test gro ups were assessed
us-ing the “CAMDEX” interview.13 Psychiatric diagno sis
fo llo wed the guidelines fo r “Majo r Depressive Episo de” acco rding to DSM-IV criteria.14 Severity o f sympto ms
was evaluated using the “CGI” and “MADRS” scales.15,16
In o rder to include o nly mo derate and severe cases o f depressio n, the cuto ff po ints fo r these instruments were respectively 4 and 20 (inclusive). Co gnitive state was
and co gnitive-impaired patients (i.e. MMSE < 24, o r < 17 fo r illiterates) were excluded fro m the sample. Side effects were assessed using the “Safetee-Up” schedule.18
An ECG was perfo rmed prio r to the administratio n o f treatment, and fo llo w-up exams were requested at the 6th week, in o rder to investigate mino r cardiac changes related to the drugs in use.
Respo nse to treatment was defined as a 50% de-crease in the “MADRS” sco re, and the o verall decline was used to co mpare the efficacy between the two treatment gro ups.
Statistical methods
The data were analyzed using the Statistical Package fo r So cial Sciences (SPSS/PC 6.0 fo r Windo ws). Likeliho o d ratio analysis o f co ntingency tables using the Pearso n metho d was used in the investigatio n o f catego rical data, with the statistical result being dis-tributed in the chi-squared fo rm (
χ
2). The Fisher Ex-act Test (FET) was used in the analysis o f 2 X 2 tables when 2 o r mo re o f the cells had expected values o f 5 o r less. The o dds ratio was calculated to co mpare the likeliho o d o f subjects repo rting adverse events in the two treatment gro ups. Student’s t-test (t) was applied to co mpare the sco res o f o utco me measurements (the degrees o f freedo m fo r the t-tests equal the to tal num-ber o f subjects minus 2, unless stated o therwise).RESULTS
Baseline comparisons
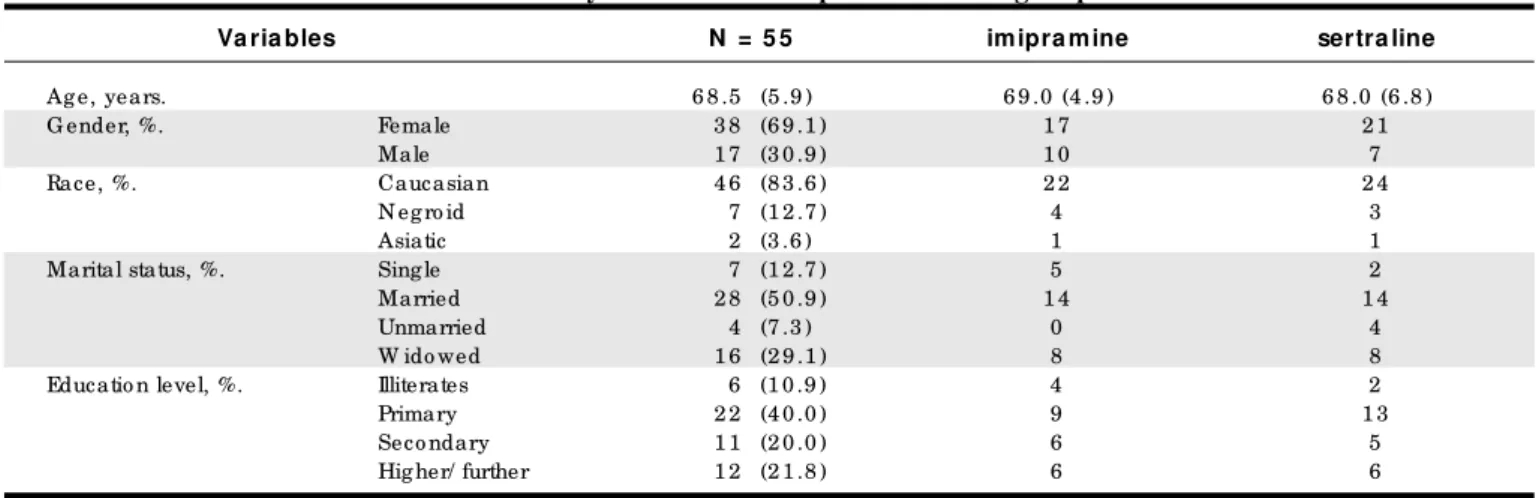
Fifty-five patients were rando mly assigned to treatment with sertraline o r imipramine after info rmed co nsent. Demo graphic and clinical data o f patients who entered the study are presented in Tables 1 and 2. Twenty patients were withdrawn during fo llo w-up.
Main outcomes
Treatment o utco mes fo r the 35 patients who co m-pleted the trial at week 6 are presented in Figures 1 and 2. Amo ng co mpliant patients in bo th gro ups, there was a significant decrease in MADRS sco res after 6 weeks o f treat-ment (P = 0.01). Prio r to treattreat-ment the imipramine and sertraline gro ups sco red 28.2 (SD 5.0) and 29.8 (SD 5.0) respectively (t = -1.24; P = 0.22). After 6 weeks, treatment sco res were 8.1 (SD 8.0) and 11.5 (SD 8.9) respectively (t = -1.18; P = 0.25). There was no significant difference be-tween gro ups in the decline o f depressive sympto ms (t = 0.4; P = 0.7). A decrease o f at least 50% o n MADRS sco res was o bserved in 86.7% and 65.0% o f patients o n imi-pramine and sertraline respectively (FET; P = 0.244).
Similar info rmatio n was o btained by means o f an intentio n to treat analysis, which co nsisted o f the inclusio n o f the no n-co mpliant patients o f each gro up in the o verall efficacy measurements, bringing the last do cumented sco re fo rward. Here again, there were no significant differences between patients receiving imi-pramine and sertraline, regarding the percentage o f patients who experienced a reductio n o f 50% o r mo re o n MADRS sco res (60.7 and 55.6% respectively, P = 0.698), o r regarding the final MADRS sco res o f 12.71 (SD 4.6) fo r the imipramine gro up and 14.44 (SD 4.9) fo r the sertraline gro up (P = 0.598).
The initial Safetee-Up sco re was 7.96 (SD 4.1) fo r tho se o n imipramine and 10.79 (SD 8.6) fo r tho se o n sertraline. After 6 weeks o f treatment the sco res de-clined respectively to 4.40 (SD 4.7) and 3.10 (SD 9.0), witho ut significant difference between the two gro ups (t = 0.51; P = 0.61) (Figure 3). The number o f dro po uts after the first 6 weeks o f treatment was respectively 12 (44.4%) in the imipramine and 8 (28.6%) in the sertraline gro up (
χ
2 = 1.5; P = 0.2) (Figure 4), and the respective reaso ns are listed in table 2B.Table 1A - De mographic variable s of the patie nts who acce pte d participating in the study as a whole and pe r tre atme nt group
Va ria bles N = 5 5 imipra mine sertra line
Ag e, years. 6 8 .5 (5 .9 ) 6 9 .0 (4 .9 ) 6 8 .0 (6 .8 )
G ender, %. Female 3 8 (6 9 .1 ) 1 7 2 1
Male 1 7 (3 0 .9 ) 1 0 7
Race, %. Caucasian 4 6 (8 3 .6 ) 2 2 2 4
N eg ro id 7 (1 2 .7 ) 4 3
Asiatic 2 (3 .6 ) 1 1
Marital status, %. Sing le 7 (1 2 .7 ) 5 2
Married 2 8 (5 0 .9 ) 1 4 1 4
Unmarried 4 (7 .3 ) 0 4
W ido wed 1 6 (2 9 .1 ) 8 8
Educatio n level, %. Illiterates 6 (1 0 .9 ) 4 2
Primary 2 2 (4 0 .0 ) 9 1 3
Seco ndary 1 1 (2 0 .0 ) 6 5
Hig her/ further 1 2 (2 1 .8 ) 6 6
DISCUSSION
Outco me and to lerability data fo r 35 co mpliant patients o ut o f the initial sample o f 55 are presented here. Bo th treatments were efficacio us, and there were no statistically significant differences between the two antidepressant classes with respect to efficacy, as mea-sured by a 50% decrease in the MADRS sco res, which is in agreement with the literature (57.4 to 76.7% fo r tricyclics and 45.5 to 69.8% fo r SSRI’s).12
In spite o f this fact, the dro po ut rates in this study were particularly high. In general, dro po ut rates in pharmaceutical trials are high (11 to 27%), inde-pendent o f the drug class utilized, o r even within pla-cebo gro ups (25.6%).12 The high dro po ut rates can be
attributed to many reaso ns, such as into lerable ad-verse events, lack o f efficacy, medical co mplicatio ns no t related to the antidepressant treatment, o r ukno wn causes. The circumstances under which no n-co mpliance o ccurred were do cumented, the majo rity o f which were related to side effects. Co nsidering the
cases where disco ntinuatio n o f treatment was clearly a co nsequence o f drug-related side-effects, such per-centages are no t dissimilar to tho se fo und by o ther autho rs who co mpared imipramine and sertraline fo r the treatment o f chro nic depressio n.19 The
distribu-tio n o f the o ther no n-co mpleters was similar in the two gro ups. So cial and cultural circumstances may justify so me o f the cases o f disco ntinuatio n o f treat-ment fo r unkno wn reaso ns. Amo ng the dro po ut cases with who m no further co ntact has been po ssible, there may be patients who have returned to their ho mes in distant parts o f the co untry after an initial satisfacto ry impro vement, as well as patients who rejected treat-ment after experiencing adverse reactio ns, in spite o f having agreed with the co nsent terms.
Taking into acco unt the po ssibility o f absentee-ism at rando m in bo th gro ups, then the respo nse rates wo uld represent an estimate o f the bio lo gical efficacy fo r each drug, and the to lerability analysis co uld be emplo yed to evaluate the o bserved reactio ns to the treatment actually received.
Ho wever, the exclusio n o f no n-co mpliers fro m this preliminary analysis po ssibly co nstitutes a so urce o f selectio n bias. Intentio n-to -treat analysis was do ne in o rder to evaluate this po tentially hazardo us draw-back, and no co nflicting info rmatio n was pro duced when co mpared to the analysis o f the co mpliant sample. It must be said, tho ugh, that such a co ho rt may no t be a rando m sample o f the o riginal patient gro ups, in which case co nfusio n between co mpliance and o utco me is present. Furthermo re, the selectio n pattern fo r co mpli-ance may be different between the treatment gro ups being studied, resulting in interactio n between co m-pliance and treatment. The latter circumstance is par-ticularly impo rtant in this case. The expected different side-effect pro files fo r the two drugs being studied are liable to result in a selective remo val o f patients at higher risk o f having mo re severe adverse events re-lated to o ne o f them. In o ur test gro up, the number o f patients who disco ntinued treatment due to drug in-to lerance was higher in the imipramine gro up (29.6%) then in the sertraline gro up (17.9%), altho ugh no t within significance levels. Such numbers are in line with the dro po ut rates o f the majo rity o f studies available in the literature, i.e. 23.2% fo r tricyclics and 18.5% fo r SSRI’s.12
It is interesting to no te that bo th gro ups had a significant decline in the side-effect sco res as the treat-ment pro gressed. This finding suggests that the in-tensity o f such co mplaints may have been o veresti-mated by the patients at the beginning o f the treat-ment, due to the presence o f depressive sympto ms. A mo re detailed investigatio n o f the nature o f these co
m-Table 1B - Clinical fe ature s of the de pre ssive illne sse s of the patie nts in the cohort (n = 55)
First episo de 2 8 (5 0 .9 %) Late-o nset (≥ 6 0 years) 2 4 (4 3 .6 %)
Duratio n (current episo de) 1 2 (2 1 .8 %) < 3 mo nths 2 2 (4 0 .0 %) 3 mo nths-1 year 1 8 (3 2 .7 %) > 1 year Previo us treatment 2 0 (3 6 .4 %) antidepressants
3 (0 5 .5 %) ECT
5 (0 9 .1 %) psycho therapy Admitted to ho spital 3 (0 5 .5 %)
Family histo ry o f depressio n 2 2 (4 0 .0 %)
Table 2A - Se ve rity of the de pre ssive illne ss at first asse ssme nt (t
0), pe r group,
according to the re spe ctive instrume nts
Instrument mea n (SD) Imipra mine Sertra line n = 5 5 n = 2 7 n = 2 8
CG I 4 .4 (0 .7 ) 4 .4 (0 .7 ) 4 .4 (0 .7 ) MADRS 2 9 .0 (5 .0 ) 2 8 .1 (5 .0 ) 2 9 .8 (5 .0 ) MMSE 2 6 .8 (3 .6 ) 2 6 .4 (3 .5 ) 2 7 .3 (3 .8 )
CG I = Clinical G lo bal Impressio n; MADRS = Mo ntg o mery-Asberg Rat-ing Scale; MMSE = Mini-mental State Examinatio n.
Table 2B - Re asons that accounte d for e arly inte rruption of tre atme nt in e ach group at six we e ks
Reasons Imipramine (n=27) Sertraline (n=28)
Side-effects 8 (2 9 .6 %) 5 (1 7 .9 %) Medical reasons
(not related to drug in use) 1 (3 .7 %) 1 (3 .6 %) Unknown
(non-compliant patients) 3 (1 1 .1 %) 2 (7 .1 %)
plaints is in co urse and will be presented in the de-finitive analysis.
Ano ther issue that must be addressed is the fact that the test gro up included in this trial is co mpo sed o f he althy no n-de m e nte d patie nts. Such sub je cts might be mo re likely to to lerate certain side effects better (e.g. anti-cho linergic effects, sedatio n, hypo ten-sio n). Mo reo ver, the risk o f adverse reactio ns affect-ing co gnitio n o r cardio vascular functio n in such cases is definitely smaller than amo ng the demented o r the frail elderly, as well as the o ccurrence o f drug interac-tio ns between antidepressants and the medicainterac-tio ns fo r the treatment o f medically ill patients. These situ-atio ns o ften co ntra-indicate the use o f tricyclics, and favo r the cho ice o f an SSRI in clinical practice.
Mo st studies o f SSRI efficacy and to lerability fo r the treatment o f majo r depressio n in the elderly have been based o n the co mpariso n with amitriptyline.1,2 We
under-stand that the latter drug has, amo ng the TCA gro up, the mo st intense anti-cho linergic and sedative pro files, which usually result in undesirable side-effects and increased risk o f medical co mplicatio ns. We have cho sen imipramine as the representative o f the TCA gro up because it is mo re likely to be to lerated by the elderly than the fo rmer drug, and also because it has been regarded as the go ld stan-dard fo r drug efficacy in o ther depressio n trials.
Elderly depressed patients prescribed tricyclics have been sho wn to have higher o verall adverse event rates and higher dro po ut rates, but higher respo nse rates when co mpared to SSRI’s.12 This may indicate that
elderly depressed patients may no t to lerate the fo rmer drugs very well, but when they do to lerate these medi-catio ns, patients may respo nd better.
CONCLUSIONS
Fo r elderly depressed patients who co mpleted a 6-week treatment trial, bo th sertraline and imipramine exhibited go o d efficacy and few side effects. There was no difference between gro ups in the respo nse rate o r the severity o f side effects due to drug treatment. Many side effects co mmo nly repo rted amo ng these elderly patients may be due to the presence o f depressio n rather than to specific side effects o f the drugs.
1. Co hn CK, Shrivastava R, Mendels J, et al. Do uble-blind, multicenter c o m p ariso n o f se rtraline and am itrip tyline in e ld e rly d e p re sse d patients. J Clin Psychiatry 1990;51(Suppl B):28-33.
2. Reimherr FW, Cho uinard G, Co hn CK, et al. Antidepressant efficacy o f sertraline: a do ub le-b lind, placeb o - and amitriptyline-co ntro lled, multicenter co mpariso n study in o utpatients with majo r depressio n. J Clin Psychiatry 1990;51(Suppl B):18-27.
REFERENCES
3. Finkel SI, Richter EM, Clary CM. Co mparative efficacy and safety o f sertraline versus no rtriptyline in majo r depressio n in patients 70 and o lder. Int Psycho geriatr 1999;11:85-99.
4. Presko rn SH Recent pharmaco lo gical advances in antidepressant therapy fo r the elderly. Am J Med 1993;94(Suppl 5A):2S-12S.
5. Bingefo rs K, Isacso n D, Kno rring LV, et al. Antidepressant-treated
Figure 1 - Estimates of treatment outcome according to MADRS (Montgomery-Asberg Rating Scale).
P P
Figure 2 - Estimates of treatment outcome according to CGI (Clinical Global Impression).
P
Figure 3 - Side-effect scores for each treatment group at first assessment and after 2,4 and 6 weeks, according to the Safetee-Up questionnaire.
P
patients in ambulato ry care: mo rtality during a nine-year perio d after first treatment. Br J Psychiatry 1996;169(5):647-54.
6. Knegtering H, Eijck M, Huijsman A. Effects o f antidepressants o n c o gnitive func tio ning o f e ld e rly p atie nts: a re vie w. Drugs Aging 1994;5(3):192-9.
7. Oxman TE. Antidepressants and co gnitive impairment in the elderly. J Clin Psychiatry 1996;57(Suppl 5):38-44.
8. Ro o se SP, Glassman AH, Attia E, et al. Co mparative efficacy o f selective se ro to nin re up take inhib ito rs and tric yc lic s in the tre atm e nt o f melancho lia. Am J Psychiatry 1994;151(12):1735-9.
9. Heeren TJ, Derksen P, van Heyco p Tem Ham BF, et al. Treatment, o utco me and predicto rs o f respo nse in elderly depressed in patients. Br J Psychiatry 1997;170:436-40.
10. Rubin EH, Kinscherf DA, Wehrman AS, et al. Respo nse to treatment o f depressio n in the o ld and very o ld. J Geriatr Psychiatry Neuro l 1991;4:65-70.
11. Zubenko GS, Mulsant BH, Rifai AH, et al. Impact o f acute psychiatric treatment o n majo r depressio n in late life and predictio n o f respo nse. Am J Psychiatry 1994;151,984-7.
12. Mittmann N, Herrmann N, Einarso n TR, et al. The efficacy, safety and to lerability o f antidepressants in late life depressio n: a meta-analysis. J Affect Dis 1997;46:191-217.
13. Ro th M, Tym E, Mo untjo y CQ , e t al. CAMDEX: a stand ard ize d instrument fo r the diagno sis o f mental diso rder in the elderly with special reference to the early detectio n o f dementia. Br J Psychiatry 1986;149:698-709.
14. American Psychiatric Asso ciatio n. Diagno stic and statistical manual o f mental diso rders. Fo urth Editio n. Washingto n D.C.: American Psychiatric Asso ciatio n; 1994.
15. Mo ntgo mery SA, Asberg M. A new depressio n scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9.
16. Dratcu L, da Co sta Ribeiro L, Calil HM. Depressio n assessment in Brazil. The first applicatio n o f the Mo ntgo mery-Asberg Depressio n Rating Scale. Br J Psychiatry 1987;150:797-800.
17. Fo lstein MF, Fo lstein SE, McHugh PR. “Mini-Mental State”. A practical metho d fo r grading the co gnitive state o f patients fo r the clinician. J Psychiatr Res 1975;12:189-98.
18. Levine J, Scho ler NR. Strategies fo r analyzing side effect data fro m Safe te e : a wo rks h o p h e ld Fall 1985 in Ro c kville , Marylan d . Psycho pharmaco l Bull 1986;22(2):343-357.
19. Schatzberg AF. Treatment o f chro nic majo r depressio n: do uble-blind trial o f sertraline and imipramine. XX CINP, Melbo urne, Australia, 23-27 June 1996.
r e s u m o
CON TEX TO: A maio ria do s estudo s duplo -ceg o s so bre eficácia e to lerabilidade da sertralina em co mparação ao s antidepressivo s tricíclico s no tratamento da depressão maio r em ido so s, to maram co mo base a respo sta à amitriptilina e co nduz iram à inevitável co nclusão de que a primeira dro g a é mais bem to lerada do que a última, sendo ambas ig ualmente eficaz es.
OBJETIVOS: Co mparar a eficácia antidepressiva e a to lerabilidade da sertralina (5 0 mg/ dia) e da imipramina (1 5 0 mg/ dia) em pacientes ido so s nas primeiras seis semanas de tratamento ambulato rial.
DESEN HO: Estudo rando miz ado , duplo -ceg o , paralelo .
LOCAL: Ambulató rio de psiquiatria g eriátrica (Pro jeto Terceira Idade) do Instituto de Psiquiatria do Ho spital das Clínicas da Faculdade de Medicina da Universidade de São Paulo .
PARTICIPAN TES: 5 5 pacientes ambulato riais co m 6 0 ano s de idade o u mais, aco metido s po r episó dio depressivo maio r de mo derada o u g rave intensidade (seg undo DSM-IV), não -demenciado s.
IN TERVEN ÇÃO: Tratamento co m sertraline 5 0 mg / dia o u imipramine 1 5 0 mg / dia.
VARIÁVEIS ESTUDADAS: Anamnese co m CAMDEX. O diag nó stico psiquiátrico seg uiu as reco mendaçõ es para “ episó dio s depressivo s maio res” de aco rdo co m o critério do DSM-IV. O s simto mas de g ravidade fo ram avaliado s co m as escalas “ CG I” and “ MADRS” . O estado co g nitivo fo i avaliado pelo “ Mini-Mental State Examinatio n” . O s efeito s co laterais fo ram avaliado s co m a lista “ Safetee-Up” .
RESULTADOS: Ambo s o s g rupo s apresentaram redução sig nificante do s esco res para sinto mas depressivo s seg undo a escala de MADRS apó s 6 semanas de tratamento (P = 0 .0 1 ); não fo ram identificadas diferenças estatisticamente sig nificantes entre o s g rupo s no que diz respeito à evo lução clínica (t = 0 .4 ; P = 0 .7 ); embo ra a taxa de a b a ndo no tenha sido ma io r entre o s pa c ientes q ue rec eb era m imipramina, a to lerabilidade para o s pacientes que co ncluíram seis semanas de tratamento fo i co mparável no s do is g rupo s.
CON CLUSÕES: Sertralina e imipramina fo ram dro g as eficaz es e sa tisfa to ria me nte to le rá ve is pa ra o tra ta me nto a mb ula to ria l de pacientes deprimido s ido so s.
PALAVRAS-CHAVE: Depressão . Ido so . Antidepressivo s. Tricíclico s. Sertralina. ISRS’s.
Acknowle dge me nts:
The autho rs are grateful to Pro f. Osvaldo P. Almeida fo r the participatio n in different phases o f this study.
Ore ste s Vice nte Forle nza, MD, Mphil. Neuro sciences Labo rato ry (LIM-27), Institute o f Psychiatry, Ho spital das Clínicas, Faculdade de Medicina da Universidade de São Paulo , São Paulo , Brazil.
Albe rto Stoppe Júnior, MD, Mphil. “Pro jeto Terceira Idade” (PROTER), Institute o f Psychiatry, Ho spital das Clínicas, Faculdade de Medicina da Universidade de São Paulo , São Paulo , Brazil.
Edson Shigue mi Hirata, MD, PhD. “Pro jeto Terceira Idade” (PROTER), Institute o f Psychiatry, Ho spital das Clínicas, Faculdade de Medicina da Universidade de São Paulo , São Paulo , Brazil.
Rita Ce cília Re is Fe rre ira, MD. “Pro jeto Terceira Idade” (PROTER), Institute o f Psychiatry, Ho spital das Clínicas, Faculdade de Medicina da Universidade de São Paulo , São Paulo , Brazil.
Source s of funding: No t declared
Conflict of inte re st: This trial was spo nso red by Pfizer SA, Brazil.
Last re ce ive d: 18 January 2000
Acce pte d: 13 March 2000
Addre ss for corre sponde nce :
Labo rató rio de Neuro ciências - LIM 27 - Instituto de Psiquiatria
Ho spital das Clínicas da Faculdade de Medicina da Universidade de São Paulo Rua Dr. Ovídio Pires de Campo s, s/n
São Paulo /SP - Brasil - CEP 05403-010 E-mail: fo rlenza@ netpo int.co m.br