w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Evaluation
of
the
effectiveness
of
packed
red
blood
cell
irradiation
by
a
linear
accelerator
Ricardo
Aparecido
Olivo
∗,
Marcus
Vinícius
da
Silva,
Fernanda
Bernadelli
Garcia,
Sheila
Soares,
Virmondes
Rodrigues
Junior,
Helio
Moraes-Souza
UniversidadeFederaldoTriânguloMineiro(UFTM),Uberaba,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8October2014 Accepted30December2014 Availableonline23April2015
Keywords: Bloodsafety Hemotherapyservice T-lymphocytes
a
b
s
t
r
a
c
t
Irradiationofbloodcomponentswithionizingradiationgeneratedbyaspecificdeviceis recommendedtopreventtransfusion-associatedgraft-versus-hostdisease.However,a lin-earacceleratorcanalsobeusedintheabsenceofsuchadevice,whichisthecaseofthe bloodbankfacilitystudiedherein.Inordertoevaluatethequalityoftheirradiatedpacked redbloodcells,thisstudyaimedtodeterminewhethertheprocedurecurrentlyemployed inthefacilityiseffectiveininhibitingtheproliferationofTlymphocyteswithoutdamaging bloodcomponents.
TheproliferationofTlymphocytes,plasmapotassiumlevels,andthedegreeofhemolysis wereevaluatedandcomparedtobloodbagsthatreceivednoirradiation.Packedredblood cellbagswereirradiatedatadoseof25Gyinalinearaccelerator.Forthispurpose,acontainer wasdesignedtoholdthebagsandtoensureevendistributionofirradiationasevaluatedby computedtomographyanddose-volumehistogram.
Irradiationwasobservedtoinhibittheproliferationoflymphocytes.Thepercentageof hemolysisinirradiatedbagswasslightlyhigherthaninnon-irradiatedbags(p-value>0.05), butitwasalwayslessthan0.4%oftheredcellmass.Althoughpotassiumincreasedinboth groups,itwasmorepronouncedinirradiatedredbloodcells,especiallyaftersevendaysof storage,withalinearincreaseoverstoragetime.
Thefindingsshowedthat,atanappropriatedosageandundervalidatedconditions,the irradiationofpackedredbloodcellsinalinearacceleratoriseffective,inhibitinglymphocyte proliferationbutwithoutcompromisingtheviabilityoftheredcells.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
Transfusion-associatedgraft-versus-host disease(TA-GvHD) isarareandacutedelayedtransfusionreactionwhichoccurs
∗ Correspondingauthorat:AvenidaFreiPaulino,30,Abadia,38025-180Uberaba,MG,Brazil.
E-mailaddress:raolivo@terra.com.br(R.A.Olivo).
afterthetransfusionofbloodcomponents;thiscomplication iscorrelatedtoahighmortalityrate.Themainmechanism for the occurrence of TA-GVHD is the transfer of T lym-phocytes from the blood donor that damage and promote aresponseinthetissuesoftherecipient.Afterrecognizing
http://dx.doi.org/10.1016/j.bjhh.2015.03.001
host tissues as foreign, the cytokines released by trans-fusedTlymphocytes,suchasinterleukin-1(IL-1)andtumor necrosisfactor(TNF),driveaninflammatoryresponse.These cytokinesactivateinflammatorycells,includingnaturalkiller (NK)cells,macrophages,andotherTlymphocytes,resulting inthedestructionofhosttissuestherebycausingTA-GVHD.1,2 AnothermechanismfortheoccurrenceofTA-GVHDoccurs throughdonor-recipientHLAincompatibility.3The develop-ment of this transfusion reaction appears to relateto the numberand viabilityofTlymphocytestransfusedinblood components(althoughthisnumberisnotyetknown),tothe levelofimmunosuppressionofthepatient,andtotheextent thatantigensoftheHLAsystemarecommontoboththedonor andrecipient.4,5 Therefore,thegreaterthenumberofblood components received, the greater the chanceof TA-GVHD occurringinat-riskpatients.
Insusceptiblepatients,TA-GVHDoccurswhenthenumber ofviabletransfusedlymphocytesismorethan1×104cells/kg
ofbodyweight.3,5Itisknownthatnon-leukodepletedpacked redbloodcell(PRBC)bagshaveapproximately2–3×109
leuko-cytes,whereasleukodepletedPRBCbagshavefrom2–3×106
leukocytes.Therefore,evenleukodepletionisnotableto pro-tectthesepatientsfromTA-GVHD.3,6 Insituationsinwhich thepatienthasahealthyimmunesystem,lymphocytesare destroyed. However, in immunosuppressed patients, these cellsarenotdestroyedbytherecipient’simmunesystemand soafterproliferation andproducingcytokines,the lympho-cytesmaycauseaTA-GvHD-relatedinflammatoryresponse. Thus,theonlyeffectivemethodtopreventthisdisease com-pletelyistoinactivatedonorlymphocytesbyirradiatingblood components.
Determination No. 34 of the Ministry of Health of Brazil/NationalHealth SurveillanceAgency (ANVISA) states thattheirradiation ofblood and bloodcomponentsshould beperformedinaspecificcellirradiator,andthatwhenthis equipmentisnotavailable,irradiationcanbecarriedoutin alinearacceleratorusedforradiationtherapy.7Usinglinear acceleratorsinsteadofcellirradiatorshasbeenthesubject ofdiscussionamongauthors.AlthoughVetterandDodd con-sidertheperformanceofbothmethodstobesimilar,8dose uniformitymayfailtomeetthequalitystandards inlinear acceleratorsifthemethodisnotstandardized.Thiswasalso reportedbyJanatpouretal.oncomparingtheirradiationof bloodcomponentswithX-raysandgamma-rays.9Bashiretal. demonstratedsimilareffectsinthedosageofpotassiumand inthedegreeofhemolysisinredbloodcellssubjectedtoX-rays andgammaradiation,andsuggestedemployinglinear accel-eratorsintheplaceofradioactiveequipmentduetothelower maintenancecostandpersonneltraining,andthedangersof theinappropriateuseofcesium.10
Radiationprotocolsforlinearacceleratorsdescribeawide rangeoftypesandsizesofcontainerstobeusedduring radi-ation.Protocolsalsodifferinrespecttothedistancebetween thecontainerandtheradiationsource,thequantityofbagsto beirradiatedbyeachprocedure,andtherangeofresults.11,12 Properirradiationshouldprovidehomogeneousdistribution ofradiationinsidethecontainerused,resultingininhibition oflymphocyteproliferationandlowerlevelsofhemolysisthan establishedbyregulatorybodies.13ThePRBCunitsproduced intheRegionalBloodCenterofUberaba(HRU)areirradiated
in aclinical linear acceleratorin the Radiotherapy Depart-mentoftheHospitaldeClínicasoftheUniversidadeFederal doTriânguloMineiro(UFTM).Thisstudyaimedtoevaluatethe efficiencyofirradiationofPRBCsandpossibledamagetored bloodcellsusingthismethod.
Methods
Standardizationofthemethodandirradiationofpacked redbloodcells
A 30cm×30cm×15cm polycarbonate container was
designed with a 0.5cm wall thickness big enough to hold upto18PRBCbags(Figure1).Whenfewerthan18bagsare beingprocessed,aspeciallidwascreatedtoreducethesize inside the container in order to better control irradiation. A 0.6-mL TN30013 waterproof Farmer ionization chamber coupled toa T10010 Unidos Eelectrometer (PTW Freiburg) jointly calibrated by the Brazilian Institute of Energy and NuclearResearch(IPEN)wereusedtomeasurethedoseinthe middleofthecontainer.Acomputedtomography(CT)ofthe container wasperformed toprovideimages fortheEclipse three-dimensionaltreatmentplanning system.The analyti-calanisotropicalgorithm(AAA) wasusedtocalculatedose distribution intheirradiatedmaterialincluding identifying regionsofunevenirradiation.Radiationindicators(RadTag® RTG15,RadTagTechnologies,Alberta,Canada)wereplaced oneachbagtodetectirradiationdosesoffrom15to50Gy.
Fourirradiationproceduresinvolvingtenor12PRBCbags eachwereperformedatatotaldoseof25Gyintheparallel opposed fields ofaClinac600TM linearaccelerator(Varian) withanominalpowerof6MV.
Uponconfirmationoftheirradiationofthebagsbythe radi-ationindicators,tenbagsfromthetotalof42irradiatedPRBC
bagswererandomlytakenfortesting.Tennon-irradiatedbags wereusedascontrol.Samplesof20mLwerecollectedfrom eachirradiatedandnon-irradiatedbagtomeasurepotassium levelsandtoevaluatethedegreeofhemolysisonDays1,7, 14,21and28.Day1(D1)wasdefinedasthedaypreviousto bagirradiation.Another10mLsamplewascollectedto com-parethelymphocyteproliferationandapoptosisbeforeand 1haftertheirradiationprocess.
Cultureofmononuclearcellsofthepackedredbloodcell sampletoevaluatecellproliferation
Theproliferationrateofperipheralbloodmononuclearcells (PBMCs) was assessed by labeling with carboxyfluorescein diacetatesuccinimidylester (CFSE) andsubsequent culture stimulatedbyphytohemagglutinin.PBMCswereisolatedfrom PRBCbyFicoll-Hypaqueseparation.Thecellswereincubated with 5M CFSE at room temperature in the dark for five
minutesand after5%fetal bovineserum (FBS)was added. Subsequently,they were washed in complete Roswell Park MemorialInstitute(RPMI) mediumwith10%FBS, andthen 4×105 cells were transferred to a 96-well plate to which
5g/mLphytohemagglutinin(SIGMAChemicalCo.,St.Louis,
USA)was added. Some cells were maintained without the addition ofthe stimulus in order to determinebasal fluo-rescence. The sampleswere incubated for 72h at 37◦C in 5% carbon dioxide, and then the cells were collected and resuspendedin Dulbecco’sPhosphate-Buffered Saline solu-tion(DPBS)forflowcytometryanalysis(25,000 events/tube) usingaFACSCaliburflowcytometer(BD,USA).The percent-ageofcell proliferationwas determinedbysubtracting the stimulatedCFSE-labeledcellcountfromcontrolbasal fluores-cence(cellslabeledwithCFSE,butwithoutstimulation).All procedureswereperformedundersterileconditions.
Statisticalanalysis
Atfirst,thevariables weresubjectedtodescriptiveanalysis usingmeasuresofcentralityanddispersion.TheShapiro–Wilk test was used to test all variables for normality, whereas Bartlett’stest wasused totesthomogeneity ofvariance of independentgroups(irradiated and non-irradiated).Due to non-normalityandnon-homogeneityofvariancesforsome variablesinthegroups,nonparametric analysisofvariance was performed using the Mann–Whitney test to compare groups, whereas the Friedmantest, Kendall’s coefficient of concordanceandsimplelinearregressionwereusedto ana-lyzepotassiumlevelsandhemolysisovertime.Lymphocyte proliferation was evaluated by comparing the proliferation ratesobtainedbeforeand afterirradiation usingthepaired Wilcoxontest.Analphaerrorof5%wasconsideredacceptable (p-value<0.05)foralltests.
Results
Tenirradiatedbags and ten non-irradiated bags were ana-lyzedbycomparingtheproliferationrates,potassiumlevels, andthedegreeofserumhemolysisbetweenthetwogroups.
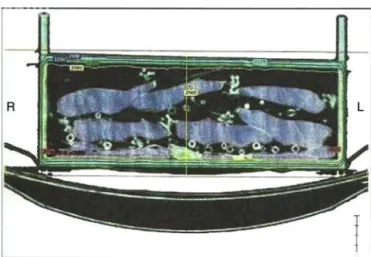
Figure2–Tomographicimageofthecontainerafterthe irradiationprocess.Dosedistributionwashomogenousin thecentralsectionofthetomography(yellowline).
Distributionofionizingradiationwasalsoevaluatedforthe irradiatedbags.
Evaluationoftheirradiationprocedure
Dosimetryshowedthatthecalibrationfactorofthedevicemet therequiredfactor,thatis,1cGy/MU.Dosedistributionwas homogenous inthe centralsection oftheCT (Dose=25Gy) (Figure2).Aregionwherethedosereached26.25Gywasalso observed;this isavariationof5%abovetherecommended dose. Analysis of the histogram (Figure 3) shows that the averagedosewas28.12Gy.Thefigureclearlyshowsthatthe minimum dose was 24.37Gy and the maximum dose was 29.32Gy.Irradiationofalltheten irradiatedPRBCbags was confirmedbyRadTag®RTG15labels.
Efficiencyoftheirradiationprocess
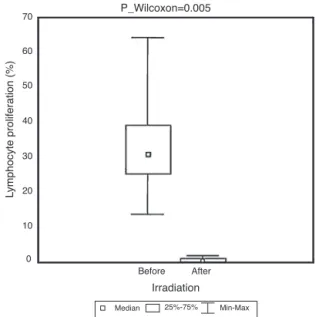
Regardingtheproliferationoflymphocytes,themedian pro-liferationindexwas29.8%beforeirradiation,and0.5%after irradiation(p-value=0.005;Figure4).
Storagelesions
The potassium levels (mmol/L) for irradiated and non-irradiatedbagsduringthestoragetimeareshowninTable1. Overstoragedays,asignificantincreaseinpotassiumlevels wasobserved(p-value<0.0001;Friedmantest)bothinthe non-irradiatedandirradiatedgroups,withmediansof61.5mmol/L and89.2mmol/Lafter28daysofstorage,respectively.Astrong linearcorrelationwiththestoragetime(r=1inbothcases) wasalsoobserved.Afteradjustingthelinearregressionmodel, therewasanaverageincreaseof12.96mmol/Linpotassium levels in the non-irradiated group for every week of stor-age.For theirradiatedgroup,theincreasewasevenhigher: 18.69mmol/Lforeachweekofstorage.OnDay7,potassium levels of the non-irradiated group were significantlylower thaninandirradiatedgroup(p-value=0.0002).
100
80
60
40
20
0 0
0 20 40 60 80 100
500 1000
Dose [cGy]
Some structure are unapproved or rejected Cumulative dose volume histogram
Relative dose [%]
Ratio of total structure volume [%]
1500 2000 2500
Figure3–Cumulativedosehistogram.
Table1–Potassiumlevels(mmol/L)inrespecttostoragedayandbagirradiation.
Bagirradiation Storageday n Median Mean(min–max) Standarddeviation
Non-irradiated D1 10 5.05 6.85(4.80–15.10) 3.66
D7 10 26.85 28.28(24.30–34.30) 3.65
D14 10 39.05 40.89(35.40–49.80) 5.21
D21 10 51.70 50.97(44.40–58.60) 4.68
D28 10 61.50 60.31(52.90–67.90) 5.50
Irradiated D1 10 5.85 11.05(4.60–29.90) 8.59
D7 10 44.80 47.59(38.00–59.70) 7.97
D14 10 71.90 71.94(54.70–81.10) 7.82
D21 10 82.60 80.79(63.50–92.50) 8.49
D28 10 89.20 87.89(70.60–99.00) 7.53
significantincreaseinhemolysis(p-value<0.0001;Friedman test) over time both for the non-irradiated and irradiated
groups with a strong linear correlation with storage time
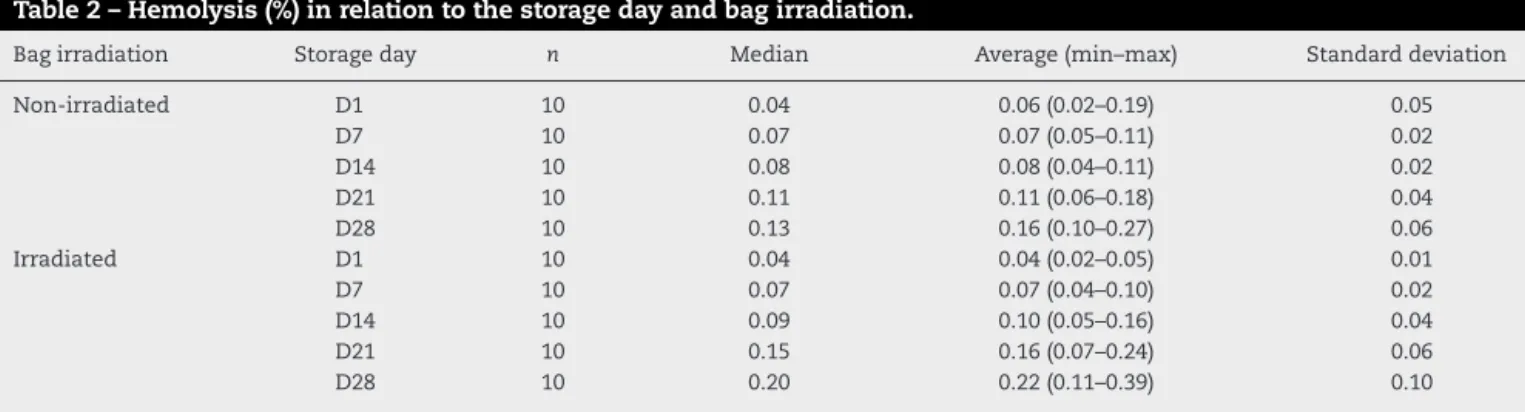
(r=0.74 and r=1, respectively). After adjusting the linear regressionmodel,anaverageincreaseof0.0246%inhemolysis perweekofstoragewasobservedinthenon-irradiatedgroup; thiswashigherintheirradiatedgroup(0.044%).Howeverthe differencesbetweenthenon-irradiatedandirradiatedgroups werenotsignificant(p-value>0.05)atanystoragetime,andat notimehemolysiswashigherthan0.4%.
Discussion
DuetounderreportingoftransfusionreactionsinBrazil,the incidenceandprevalenceofTA-GVHDarenotyetfullyknown. IntheUK,accordingtotheAnnualSHOTReportof2011,no
casesofTA-GVHD werereportedfrom 2001to2011,unlike
whatoccurredbetween1996and2001,when13caseswere
recorded.14 In Japan, a country considered to have a high prevalenceofthistransfusionreaction, therehavebeenno
Table2–Hemolysis(%)inrelationtothestoragedayandbagirradiation.
Bagirradiation Storageday n Median Average(min–max) Standarddeviation
Non-irradiated D1 10 0.04 0.06(0.02–0.19) 0.05
D7 10 0.07 0.07(0.05–0.11) 0.02
D14 10 0.08 0.08(0.04–0.11) 0.02
D21 10 0.11 0.11(0.06–0.18) 0.04
D28 10 0.13 0.16(0.10–0.27) 0.06
Irradiated D1 10 0.04 0.04(0.02–0.05) 0.01
D7 10 0.07 0.07(0.04–0.10) 0.02
D14 10 0.09 0.10(0.05–0.16) 0.04
D21 10 0.15 0.16(0.07–0.24) 0.06
70
60
50
40
30
20
10
0
Before After
Median 25%-75% Min-Max Irradiation P_Wilcoxon=0.005
L
ymphocyte proliferation (%)
Figure4–Pre-andpost-irradiationpatternoflymphocyte proliferation.Cellproliferationwasdeterminedby
subtractingcarboxyfluoresceindiacetatesuccinimidylester labelingfromcontrolbasalfluorescence–cellslabeledwith CFSE,butwithoutstimulation.
casessince2000accordingtodatapublishedbytheSafety Vig-ilanceDivision,BloodServiceHeadquartersoftheJapanese RedCross Society.15 However,57 caseswere confirmedout of232suspectedcasesfrom 1993to1999. Thedecrease in the frequency of this transfusion reaction is attributed to the implantation of blood component irradiation between 1998and1999,therebydemonstratingtheimportanceofthis procedure.15ThemaingoaloftheMinistryofHealththrough ANVISAhas been tounderstand the mechanismsofthese transfusionreactions inorder toproposemeasuresto pre-venttheiroccurrence,aswellastoensuretheeffectiveness of hemotherapy and the safety of both blood donor and recipient.7
Thereasontopreventthistransfusionreactionusing irra-diated PRBCs in susceptible patients is the ineffectiveness of treating this complication. Medications such as corti-costeroids, immunoglobulins, anti-thymocyte globulin, and granulocyte-macrophage colony-stimulating factor are not effectiveand sotheonlyoptionispreventionbythe irradi-ation ofblood components.16,17 Ionizingradiation destroys theDNAoflymphocytes,preventingtheirproliferationand, thus,reducingtheproductionofcytokinesproducedinthis process.2Theequipmentrecommendedfortheirradiationof cellularbloodcomponentsisacompactgamma-rayirradiator specificallydesignedforthispurpose.Nonetheless,itshigh costandtheuseofradioactivematerialhinderitsuseinBrazil, soithasbeensuccessfullyreplacedbythelinearaccelerator,a procedurewhichisacceptedbyBrazilianregulations.7 Regard-lessofthemethod,whatisreallycrucialisthattheprocedure isvalidated.Therecommendedradiationdoseis25Gy,adose which isconsidered adequate toinactivate lymphocytes.18 Thisdoseshouldnotbelowerthan15Gy,becausethiswould notinhibitlymphocyteproliferation,norshoulditbehigher than50Gy,asthereisariskofsevereredbloodcelldamage.7
BothgammaraysandX-rayshavebeenstudiedovertheyears, andthereseemstobenodifferencebetweentheresults.9,10
Oneofthekeyrequirementsforeffectiveirradiationisthe homogeneousdistributionofradiation insidethe container thatcontainsthebagsbeingirradiated.Thus,thefirststageof thisstudyaimedtostandardizetheprocedure.Firstly,a con-tainer,whichwouldeliminateasfar aspossibleairaround thePRBCbags,wasdeveloped,asaircancauseanuneven dis-tributionofphotonsleadingtoaheterogeneous,anisotropic distributionoftheradiation.
Thecontainerusedinthisstudyminimizedthepresenceof air,whichmay,inpart,explainthehomogeneousdistribution ofthedose.Otherstudiesfounddifferentkindsofcontainers were effective.11,12 Another factorwhichwas importantfor theevendosedistributioninthisstudy,asobservedbyPatton andSkowronski,11wasthatirradiationusedparallel oppos-ing fields.Two studiesemployingthesingle-fieldtechnique reporteddifferentresults;onehadhomogeneousdistribution ofradiation12andtheotheraheterogeneousdistribution.19
Regardingprocedure validation,severalstudiesused dif-ferent methodologiesthereby impedingcomparisonsofthe effectivenessofprotocols.Pinnaròetal.validatedtheir radi-ation procedurebasedonlyondosimetrymeasurementsof irradiated blood components.12 On the other hand, Patton and Skowronski and Bashir etal. assessedonlythe potas-siumlevelsandthedegreeofhemolysistodemonstratethe effectiveness of radiation in a linear accelerator.10,11 Goes etal.studiedtheproliferationoflymphocytesirradiatedwith Cobalt-60,anddidnotfindproliferationofthesecellsatadose of25Gy usingalimitingdilutionassay.20Furthermore, Pel-szynskietal.didaseriesofexperimentsusingredcellunits irradiatedwithin24haftercollection.Theyfoundthat15Gy inactivated>4log10ofTcells,butviableTcellsweredetected inallexperiments.However,whentheyused25or30Gy,no T-cellgrowth(>5log10depletion)wasdetected.21
Inthisstudy,weusedCTconvertedintoadosevolume his-togramtoevaluatedosehomogeneity.Thedifferencebetween thesemethodsisthatCTproducescross-sectionalimagesof tissueforanalysis,whereasthehistogramevaluatesthetotal irradiatedvolume.Thedosedistributionintheirradiated ves-selwasfoundtobehomogeneousinthecentraltomographic sectionofthecontainer,witharegionwherethedosereached 26.25Gy–morethantherecommendedminimumof25Gy– andavariationof5%oftheprescribeddose;thisisin accor-dancewiththeplanningrecommendedbytheInternational CommissiononRadiationUnitsandMeasurements,22which recommendsdosesrangingfrom−5%to+7%oftheprescribed
doseassatisfactoryplanning.
Theefficacyofradiation wasdemonstrated bya signifi-cantreductioninlymphocyteproliferationinirradiatedPRBCs (29.8%pre-irradiationversus0.5%post-irradiation).The fluo-rescenceemissionobservedinthecytometryoflymphocytes intheirradiatedbags(0.5%)mayhavebeencausedbythe pres-enceofdoubletsoraggregatesorevenDNAfragmentsfrom these cells, but not by transformed lymphocytes.24 There-fore,thisandtheproofthataneffectiveradiationdosewas delivered7,18showsthatthismethodpreventstheonsetof TA-GVHDinpatientstransfusedwiththesebloodcomponents.
Inthis study, twostorage lesions,potassiumlevels and hemolysis were analyzed in irradiated and non-irradiated PRBCsinordertodeterminetheinfluenceofirradiation.The increaseinpotassiumlevelsobservedinbothgroups,but sig-nificantlymorepronouncedinirradiatedPRBCsfromDay7of storage,wasgradualandlinearovertimeashasbeenreported intheliterature.25Giventhelowdegreeofhemolysis(lower than0.4%),webelievethatincreasedpotassiumlevelsmay notbeassociatedwithhemolysis,butwiththeincreaseinthe permeabilityoftheplasmamembranetoions,anoccurrence thatwasreportedbyMoreiraetal.26
The increase in potassium after irradiation was also observed by Janatpour et al.,9 who found a value of 108.7mequiv./LinPRBCunitsirradiatedbyalinear accelera-toratadoseof25GyonDay28.Similarresultswerefound byBashiret al.10 Transientlyincreasedpotassiumlevels in transfusedpatientsoccur duetotheirredistributioninthe body.Thenumberoftransfusions,hypovolemia,PRBCstorage time,PRBCirradiation,andtheinfusiontimearecriticalrisk factorsforhyperkalemia.25 Asthereisnospecificreference valueforpotassiumlevelstocomparethequalityinirradiated PRBCbags7,13andgiventhatthevaluesfoundinthisstudydid notdifferfromthoseobservedbyotherauthors,7,10,25we con-cludedthatirradiatedPRBCsaresuitablefortransfusionup tothe28thdayafterirradiation,whichisinaccordancewith currentlegislationinBrazil.
Therewasagradualandlinearincreaseinhemolysis dur-ingstorage,however,withnosignificantdifferencebetween irradiatedandnon-irradiatedPRBCs.Wealsofoundthat,even inirradiatedPRBCs,thedegreeofhemolysiswassignificantly lowerthantherecommendedlimitof0.8%.7,13
Conclusions
Insummary,theprocessofradiationinalinearaccelerator, atanappropriatedosageandundervalidatedconditions,is effective to irradiate PRBCs,thereby inhibiting lymphocyte proliferation withoutcompromising theviability ofthered bloodcellstobetransfused.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. DwyreDM,HollandPV.Transfusionassociatedgraftversus hostdisease.VoxSanguinis.2008;95(2):85–93.
2.RuhlH,BeinG,SachsUJ.Transfusionassociatedgraftversus hostdisease.TransfusMedRev.2009;23(1):62–71.
3.SchroederML.Transfusionassociatedgraftversushost disease.BrJHaematol.2002;117(2):275–87.
4.LandiEP,OliveiraJS.Doenc¸adoenxertocontrahospedeiro pós-transfusionalguiaparairradiac¸ãogamade
hemocomponentes.RevAssocMedBras.1999;45(3): 261–72.
5.MathaiJ.Irradiatedbloodcomponents.IndianJMedRes. 2005;122(5):371–3.
6.AsaiT,InabaS,OhtoH,OsadaK,SuzukiG,TakahashiK,etal. Guidelinesforirradiationofbloodandbloodcomponentsto preventpost-transfusiongraft-vs.-hostdiseaseinJapan. TransfusMed.2000;10(4):315–20.
7.BrasilMinistériodaSaúde,AgênciaNacionaldeVigilância Sanitária.Resoluc¸ãodaDiretoriaColegiada–RDCn◦34,de11 dejunhode2014.DispõesobreasBoasPráticasnoCiclodo Sangue.Availableat:http://bvsms.saude.gov.br/bvs/ saudelegis/anvisa/2014/rdc003411062014.pdf
8.DoddB,VetterRJ.Replacementof137Csirradiatorswithx-ray
irradiators.HealthPhys.2009;962Suppl.:S27–30.
9.JanatpourK,DenningL,NelsonK,BetlachB,MackenzieM, HollandP.ComparisonofX-rayvs.gammairradiationof CPDA1redcells.VoxSanguinis.2005;89(4):215–9.
10.BashirS,NaikF,CardiganR,ThomasS.EffectofX-irradiation onthequalityofredcellconcentrates.VoxSanguinis. 2011;101(3):200–7.
11.PattonGA,SkowronskiMG.Implementationofablood irradiationprogramatacommunitycancercenter. Transfusion.2001;41(12):1610–6.
12.PinnaròP,SorianiA,D’AlessioD,GiordanoC,FoddaiML,Pinzi V,etal.Implementationofanewcostefficacyforblood irradiationusinganondedicateddevice.JExpClinCancer Res.2011;30:7.
13.Guidetothepreparationuseandqualityassuranceofblood components:RecommendationNoR.(95)15.16thed. Strasbourg:EDQM;2011.
14.Bolton-MaggsPHB,CohenH,onbehalfoftheSHOTSteering Group.TheannualSHOTreport2011;2012.p.22.Available from:http://www.shotuk.org/wp-content/uploads/2012/07/ SHOT-ANNUAL-REPORTFinalWebVersionBookmarked 20120622.pdf
15.SafetyVigilanceDivision,BloodServiceHeadquarters, JapaneseRedCrossSociety.HaemovigilancebyJRCS2008. Tokyo.SVD/BSH/JRCS;2011.Availablefrom:
http://www.jrc.or.jp/vcmslf/anzenHVreport2008en.pdf
16.OtoOA,PaydasS,BaslamisliF,TuncerI,ErginM,KalakocE, etal.Transfusion-associatedgraft-versus-hostdisease.EurJ InternMed.2006;17(3):151–6.
17.AgbahtK,AltintasND,TopeliA,GokozO,OzcebeO. Transfusion-associatedgraft-versus-hostdiseasein immunocompetentpatients:caseseriesandreviewofthe literature.Transfusion.2007;47(8):1405–11.
18.RosenNR,WeidnerJG,BoldtHD,RosenDS.Preventionof transfusionassociatedgraftversushostdisease:selectionof anadequatedoseofgammaradiation.Transfusion. 1993;33(2):125–7.
19.MergenC,KunzelR,GóesEG,CasEV,AlvesNM,BotelhoMZ. Dosimetriadosangueirradiadocomequipamentode cobaltoterapia.DiscScientiaSérie:CiênciasNatTecnol,S Maria.2005;6(1):67–77.
20.GóesEG,BorgesJC,CovasDT,OrellanaMD,PalmaPV,Morais FR,etal.Qualitycontrolofbloodirradiation:determinationT cellsradiosensitivitytocobalt60gammarays.Transfusion. 2006;46(1):34–40.
implicationsforpreventingtransfusion-associated graft-versus-hostdisease.Blood.1994;83(6):1683–9.
22.Morgan-FletcherSL.Prescribing,recordingandreporting photonbeamtherapy(SupplementtoICRUReport50).ICRU 62(1999).BrJRadiol.2001;74(879):294.
23.MoroffG,LubanNLC.Theirradiationofbloodandblood componentstopreventgraftversushostdisease:technical issuesandguidelines.TransfusMedRev.1997;11(1):15–26.
24.Flowcytometry.Corefacility.Celldeathandapoptosis. Dundee:UniversityofDundee;2012.Availablefrom:
http://www.lifesci.dundee.ac.uk/services/flowCytometry/ ca/celldeath
25.VraetsA,LinY,CallumJL.Transfusionassociated hyperkalemia.TransfusMedRev.2011;25(3):184–96.