w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Update
article
Resistance
of
dialyzed
patients
to
erythropoietin
Michelle
Teodoro
Alves
a,
Sandra
Simone
Vilac¸a
b,
Maria
das
Grac¸as
Carvalho
a,
Ana
Paula
Fernandes
a,
Luci
Maria
Sant’Ana
Dusse
a,
Karina
Braga
Gomes
a,∗aUniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,Brazil bHospitalFelícioRocho,BeloHorizonte,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23July2014 Accepted24November2014 Availableonline17February2015
Keywords:
Dialysis Erythropoietin Iron
a
b
s
t
r
a
c
t
Resistanceto recombinant human erythropoietin is a common conditionin dialyzed patientswithchronickidneydiseaseandisassociatedwithmorehospitalizations,increased mortalityandfrequentbloodtransfusions.Themaincauseofhyporesponsivenessto recom-binanthumanerythropoietininthesepatientsisirondeficiency.However,ahighproportion ofpatientsdoesnotrespondtotreatment,eventotheuseofintravenousiron,which indi-catesthepresenceofotherimportantcausesofresistance.Inadditiontotheirondeficiency, themostcommoncausesofresistanceincludeinflammation,infection,malnutrition, inad-equatedialysis,andhyperparathyroidism,althoughotherfactorsmaybeassociated.Inthe presenceofadequateironstores,othercausesshouldbeinvestigatedandtreated appropri-ately.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
Chronickidney disease(CKD) isconsideredapublic health problemworldwidewithhighincidenceandprevalencerates.1 In end-stage renal disease (ESRD), renal function must be replacedbydialysis orrenaltransplantation.2 InBrazil,the numberofpatientsondialysishasincreasedgraduallyover theyears.AccordingtotheSociedadeBrasileiradeNefrologia (SBN),42,695and100,397patientswereunderdialysisin2000 and2013,respectively.3
Anemiaisoneofthemostfrequentearlycomplications ofCKD.4 Themaincause iserythropoietin(EPO)deficiency due to impaired kidney function. However, other causes
∗ Correspondingauthorat:Av.AntônioCarlos,6627,Pampulha,31270-901BeloHorizonte,MG,Brazil. E-mailaddress:karinabgb@gmail.com(K.B.Gomes).
shouldbeconsideredwhentheseverityofanemiais incon-sistent with the decrease in renal function; when there is evidence of iron deficiency or matching decreases in hemoglobin, leukopenia and/or thrombocytopenia are also found.5
ThetreatmentofanemiainCKDpatientsusuallyinvolves theuseofrecombinanthumanerythropoietin(rHuEPO).The main cause ofrHuEPOtreatment failure isthe lossor low iron availability.6 The prevalenceof irondeficiency is very commoninCKD,affectingasmanyas50%ofpatients.7 How-ever,despiterHuEPOandintravenousironinthemajorityof patients,theprevalenceofanemiareaches34%inBrazil.8This indicatestheexistenceofotherimportantfactorsrelatedto rHuEPOresistance.
http://dx.doi.org/10.1016/j.bjhh.2015.02.001
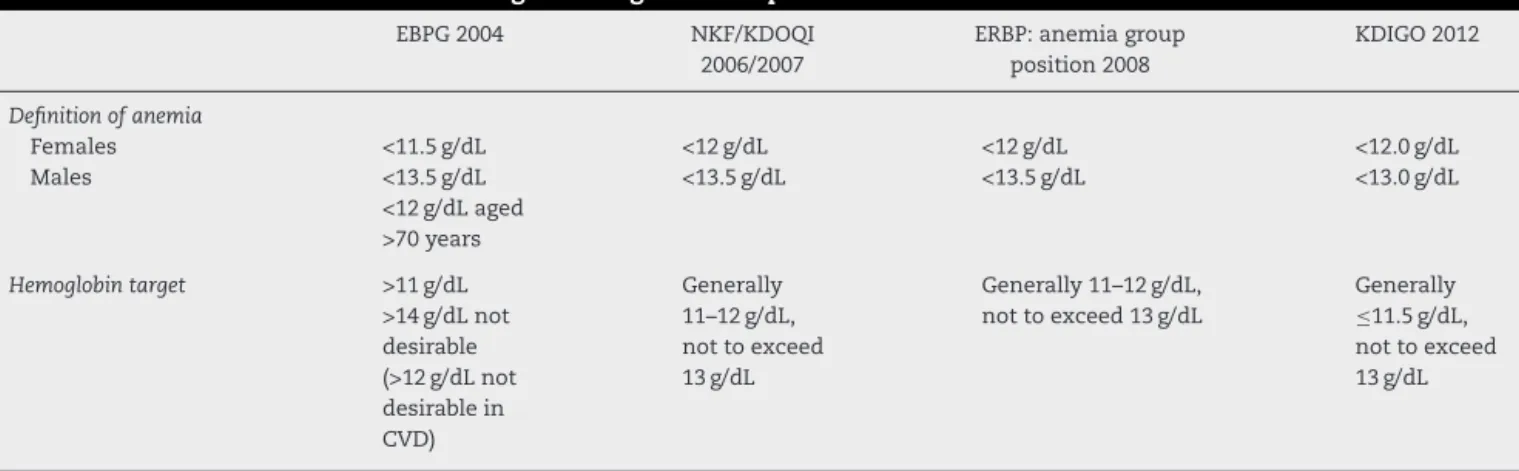
Table1–DefinitionofanemiaandhemoglobintargetinCKDpatients.
EBPG2004 NKF/KDOQI
2006/2007
ERBP:anemiagroup position2008
KDIGO2012
Definitionofanemia
Females <11.5g/dL <12g/dL <12g/dL <12.0g/dL
Males <13.5g/dL
<12g/dLaged >70years
<13.5g/dL <13.5g/dL <13.0g/dL
Hemoglobintarget >11g/dL >14g/dLnot desirable (>12g/dLnot desirablein CVD)
Generally 11–12g/dL, nottoexceed 13g/dL
Generally11–12g/dL, nottoexceed13g/dL
Generally ≤11.5g/dL, nottoexceed 13g/dL
EBPG:EuropeanBestPracticeGuidelines;NKF/KDOQI:NationalKidneyFoundationKidneyDiseaseOutcomesQualityInitiative;ERBP:European RenalBestPractice;KidneyDisease:KDIGO:ImprovingGlobalOutcome;CVD:cardiovasculardisease.
ThedefinitionofanemiainCKDpatientshaschangedwith someguidelines beingproduced overthelast fewyears.In 2004theRevisedEuropeanBestPracticeGuidelines(EBPG)on Anemiadefinedlowhemoglobinlevels asvalues<11.5g/dL inadultfemales and <13.5g/dL inadultmales (<12g/dL in
over 70-year olds). Patients with CKD should maintain a
hemoglobinlevel>11g/dL(hematocrit>33%).Inaddition, lev-els>12g/dLare notrecommended forpatientswithsevere cardiovasculardisease.9
Anupdateofthe2006NationalKidneyFoundationKidney DiseaseOutcomesQualityInitiative(NKF/KDOQI)guidelines in2007suggestedthatanemiaisassociatedwithhemoglobin levels<13.5g/dLinadultmalesand<12.0g/dLinadultfemales. In patients with CKD, hemoglobin should be between 11 and12g/dL,howeverhemoglobintargetsgreaterthan13g/dL mayincreasetheriskforseriousadverseeffectsandarenot recommended.5,10
TheKDOQImodifiedtheEBPGdefinitiondefininganemia inadultmalesashemoglobin<13.5g/dLregardlessofagesince the decrease in hemoglobin levels amongover 60-year-old malesisfrequentlyrelatedtoconcurrentdiseases.In addi-tion,inadultfemalesthe hemoglobintarget is12g/dL.The EuropeanRenalBestPractice(ERBP)WorkGroupagreeswith theKDOQIdefinitions.11
Recently,theKidneyDisease:ImprovingGlobalOutcome (KDIGO)groupdefinedanemiainadultsandchildrenaged>15 yearswithCKDwhenthehemoglobinlevelsare<13.0g/dLin malesand<12.0g/dLinfemales.12Table1showsthe defini-tionsofanemiaandhemoglobintargetsinCKDpatients.
Althoughthereisnoconsensusabout the definitionfor rHuEPO resistance, the evaluation of resistance is recom-mendedifthereisanincrease≥25%inerythropoietindose or <1mg/dL gain in hemoglobin levels after 2–4 weeks of treatment.13
Accordingto the BrazilianMinistry ofHealth,14 rHuEPO resistance is defined as a persistent anemia (hemoglobin <10–12g/dL) or the necessity of very high erythropoietin doses of epoetin alfa (300IU/kg/week subcutaneously or 450IU/kg/weekintravenously).Epoetinalfashouldbeinitiated atadoseof50–100IU/kgsubcutaneously,onetothreetimesa week.Theinitialgoaloftreatmentistoachievearateofweekly increaseinhemoglobinlevelsof0.3g/dL.Ifafterfourweeksof
treatment,thisresponseisnotobservedandthehemoglobin remainsbelow11g/dL,thedoseshouldbeincreasedby25%. However,afterfourweeksifthehemoglobinlevelisgreater than13g/dL,thedrugshouldbesuspendedtemporarily,since the maintenanceofhigherhemoglobin levelsisassociated withincreasedmorbidityandmortality.Therecommended therapeutictargetistopreservehemoglobinlevelsfrom11to 12g/dLorhematocritfrom33%to36%.5
AnemiainCKDisusuallynormocyticandnormochromic. Thecharacteristicsoferythrocytesasdeterminedby hema-timetric indices, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) can characterize the etiology of anemia.15 In addition to hematimetric indices, the laboratory investigation includes complete blood cell count,reticulocytecount, serumiron,determinationofthe transferrin saturation and serum ferritin,as well asoccult bloodinstoolsandthelevelsoffolicacidandvitaminB12.12,16 Thebiochemicalmarkersofirondeficiency (serumiron, ferritin,transferrinsaturationandsolubletransferrin recep-tor–sTfR)havelimitedvalueinfunctionalirondeficiencyas theyare changedinseveral clinicalconditions suchasthe onesthat evolvewithrHuEPOtherapy.17 However, reticulo-cytehemoglobincontent(CHrorRet-He)isasensitiveindirect markerofiron deficiency,which reflectsrecent changesin erythropoiesis.18ThemeasurementofCHrinperipheralblood samplesisusefulforassessingtheamountoffunctionaliron that was availablein the bone marrow fornew red blood cell productionover theprevious3–4days.19CHrmaybea moresensitivemarkeroffunctionalirondeficiencyinpatients receivingerythropoietintherapy.20Itmayalsobeanearly indi-catoroftheeffectivenessofironreplacementtherapy.21
Table2–Riskfactorsofresistancetorecombinant humanerythropoietin.
Absoluteorfunctionalirondeficiency Gastrointestinalbloodloss
Hemolysis Inflammation Infection
Neoplasticdiseases Malnutrition
FolicacidandvitaminB12deficiencies Inadequatedialysis
Hyperparathyroidism ACEinhibitorsandARBs Anti-erythropoietinantibodies Geneticpolymorphisms
ACE:angiotensin-convertingenzyme;ARBs:angiotensinIItype1 receptorblockers.
sTfR-Findexwithinappropriatereferencevalues);(2)reduced ironsupplywithstill-normalerythropoiesis(CHrwithin ref-erencevalues;increasedsTfR-Findex);(3)depletionofstores andfunctionalirondeficiency(reducedCHr;increased sTfR-Findex);and(4)functionalirondeficiencyinastateofiron repletion(reducedCHr;sTfR-Findexwithinreferencevalues). Thestrongassociationbetweenanemiaandcardiovascular complicationsshouldbehighlighted.Thedecreaseintissue oxygenationcauses tachycardia,vasodilationandincreased cardiacwork andmay cause leftventricular hypertrophy.23 Thedevelopmentandpersistenceofanemiainpatientswith CKDarealsoassociatedwithworse qualityoflife,reduced exercisecapacity,decreasedmentalagilityandrenalfunction andincreasetheprevalenceofhospitalizationandmortality.13 ThemaincausesofresistancetotreatmentwithrHuEPO
(Table2)indialysispatientsarediscussedinthisreview.
Causes
of
resistance
to
treatment
with
recombinant
human
erythropoietin
in
patients
under
dialysis
Irondeficiency
Irondeficiencyorimpairmentofironavailabilityisthemost frequentcause ofrHuEPO treatment resistanceinpatients underdialysis.24,25
Inthesepatients,thedeficiencyorreductionoftotaliron storescanoccurduetoanincreaseindemandofthisnutrient duringtheproductionofredbloodcellsinthebonemarrow. Thisabsoluteirondeficiencymayalsoberelatedtothe dial-ysisprocedure,whichpromotesprematuredestructionofred bloodcells(hemolysis),butalsoduetogastrointestinal bleed-ing,orfrequentlaboratorybloodtestsandsurgeries,which patientscanbesubmittedto.26
Infunctionalirondeficiency,suitablestoresofthisnutrient canbeobserved,but themobilizationofirontothe blood-streamisinsufficienttoreachthedemandoftheerythroid marrow.Thisconditioniscommonininflammatorystatesdue tothecytokinesthatblockthereleaseofironfromdeposits.27 Iron deficiency anemia is characterized bymicrocytosis andhypochromiaandacarefulmicroscopicexaminationof
erythrocytes may leadto suspicion ofiron deficiency. Fur-thermore,transferrinsaturationandserumferritinlevelsmay helptodistinguishbetweenconditionsassociatedwith defi-ciencyorimpairmentintheavailabilityofiron.5
Hepcidin, a small peptide synthesized mainly in hepatocytes, is the central regulator of systemic iron homeostasis.28,29 Hepcidin binds to ferroportin, an iron transporter present on cells of the intestinal duodenum, macrophages, and cellsofthe placenta,and regulatesiron release to the plasma. When hepcidin concentrations are low, moleculesofferroportin areexposed ontheplasmatic membraneandreleaseiron.Whenhepcidinlevelsincrease, hepcidinbindstoferroportininducingitsinternalizationand degradation,therebyleadingtoreductionsinironrelease.30,31 Hepcidin concentration in turn is regulated by iron, erythropoieticactivityandinflammation.32IL-6induces hypo-ferremia duringinflammationbyinducingthesynthesisof hepcidincausedbydecreasesinserumironandtransferrin saturation.Inaddition,thiscytokinebyitselfrapidlyinduces hypoferremia.Sincetheprocessoferythropoiesisisthelargest consumer ofiron inthe body,the decrease iniron supply reduceshemoglobinsynthesisandcanleadtoanemia.33
Hepcidin deficiency or resistance to hepcidin is asso-ciated with iron overload in hereditary hemochromatosis, iron-loadinganemia,andhepatitisC.Hepcidinexcessor ferro-portindeficiencyisthecauseofiron-refractoryirondeficiency anemia,ferroportindisease,anemiaofinflammation,CKDs, andcancer.34
Chronicinflammationandinfection
Therole ofinflammationinthedevelopmentofanemiain patientswithCKDshouldbehighlighted.Itisknownthatthe releaseofcytokinessuchasinterferon-gamma(IFN-␥),tumor necrosisfactoralpha(TNF-␣),interleukin1(IL-1)and inter-leukin 6(IL-6)caninduce rHuEPOerythroid progenitorcell resistanceorimpairthereleaseofstoredironinthe reticu-loendothelialsystemfortheproductionofhemoglobin.35–37 Infectiousdiseasesmayalsoberelatedtoanemiaresultingin chronicinflammation.38
Arelationship betweencytomegalovirus(CMV) infection and lack of response to rHuEPO has been associated to increasedproductionofproinflammatorycytokinessuchas IFN-␥ and TNF-␣.39,40 Betjes et al.41 showedan association betweenCMVandlowhemoglobinlevelsinpatientswithCKD undergoinghemodialysisandhighdosesofrHuEPO.
Besideschronicinflammatoryprocess,humanparvovirus B19(B19) canalsocause anemiaduetoinfection and lysis oferythroidprecursorsinthebonemarrow.42,43 This infec-tion mayworseninpatientswho haveincreasedred blood cell destruction,as observed in patients undergoing dialy-sistreatment.Moreover,thesepatientsareunderincreased riskofcontractingHIVinfectionthroughbloodtransfusionor hemodialysis.44–47
studieshavereportedtheoccurrenceofacquiredpureredcell aplasia(PRCA)andseveretransfusion-dependentanemiain kidneytransplantpatientsinfectedbyB19.48,52–55 PRCAisa disorder characterizedby anemia that leadsto the almost complete absenceoferythroid cells fromprecursors inthe bonemarrowbutwithnormalproductionofgranulocytesand megakaryocytes.BesidesB19infection,whichhastropismfor erythroidprecursorsinthebonemarrow,otherconditionsthat resultinPRCAincludethepresenceofautoantibodiesdirected againstredlineageprogenitors,transienterythroblastopenia inchildhood,pregnancy,leukemia,infectiousprocesses, tox-ins,andtheuseofsomedrugs.56
Cofactordeficiencyandmalnutrition
Inpatientsunderdialysis,themaincausesofproteinenergy malnutritionincludelowintakeofnutrients;musclelossdue toincreasedproteincatabolism and decrease intheir syn-thesis; insulin resistance; lossof nutrients by dialysis and oxidativestress.57Theinflammatoryprocessisalsoamajor cause of proteinenergy malnutrition, which may occur in 13–51%ofpatientsunderhemodialysis.58
Malnutritionhasbeenassociatedwithresistanceto treat-mentwithrHuEPOinpatientsunderdialysis.Laboratorytests show low percentages of transferrin saturation index, low serumalbuminconcentrationsandbodymassindex(BMI),but highlevelsofC-reactiveprotein(CRP)inthesepatients.59,60In additiontobeamarkerofironstores,ferritinmay alsobe increasedinmalnutrition.61
DeficienciesoffolicacidandvitaminB12maybe associ-atedwithanemiaandresistancetotreatmentwithrHuEPO. Thus, when macrocytosis is detected, the levels of these nutrientsshouldbeevaluated.62Besidesthechangesin ery-thropoiesis,folicacidandvitaminB12deficienciescanlead toincreasesinhomocysteinelevels,whichinturnis associ-atedwithanincreasedriskofcardiovascularcomplicationsin renalpatients.63,64
Inadequatedialysis
InpatientswithCKD,damagetoerythrocytescanoccurinthe presenceofuremictoxins,whichalsoinhibittheproduction ofEPOanderythropoiesis.Furthermore,thedialysisprocedure causesmechanicaldamagetoerythrocytes,andleadstoblood loss.65,66
Theinadequacyofthedialysisdoseisanimportantcause of anemia in patients under dialysis. In order to evaluate whetherthe dialysisprocedureisremovingenoughuremic toxins, the patient’s blood is sampled at the start and at the end of dialysis. The levels of urea in the two blood samples are then compared. In the Kt/V method, the dia-lyzerureaclearance(K)ismultipliedbydialysistime(t),and the product divided bythe patient’s urea distribution vol-ume(V).AccordingtotheNKF/KDOQIguidelinesforpatients underhemodialysis,theKt/Vtargetis≥1.3,andinpatients underperitonealdialysisthetarget is≥1.7/week.67 Astudy byGawedaetal.61showedthatpatientswithadequate dialy-sisassessedbyKt/V,requirelowerdosesofrHuEPO.Although thepathophysiologicalmechanismthatlinksinadequate dial-ysistothelackofresponsetorHuEPOisstillnotcompletely
understood,otherfactorssuchasinflammationandvascular accesscomplicationsmaybeassociatedwithpoorerresponse totreatment.
Theadequacyofthe doseofdialysis isalsorelatedtoa decreaseincosts,sincepatientswiththebestvaluesofKt/V
requiresmallerdosesofrHuEPO.68
Hyperparathyroidism
Hyperparathyroidism, characterized by increased parathy-roid hormone(PTH), isassociatedwithlackofresponseto treatment withrHuEPO duetoendogenous EPO inhibition, reduction oferythroid precursors inthe bone marrow and erythrocytesurvival.Thishormoneisalsoassociatedtothe inductionofbonemarrowfibrosis.60,69,70
According to the NKF/KDOQI,71 PTH levels between 150 and 300pg/mL are desirable in patients undergoing dial-ysis. However, the threshold at which PTH levels could affectthe responsetorHuEPOremainsunclear.Raoetal.72 demonstratedthatpatientswhorespondedtotreatmentwith rHuEPOhadlowerPTHlevels(around266±322pg/mL) com-pared withthose who did not respondto treatment, with mean levels of 800±248pg/mL. Another study by Gaweda etal.61demonstratedthatPTHlevelsof300,600and900pg/mL wereassociatedwithapproximately90%,79%and67%ofthe maximumresponsetotreatmentwithrHuEPO,respectively.
Angiotensin-convertingenzymeinhibitorsand angiotensinIItype1receptorblockers
Therenin–angiotensinsystemwas previouslyonlythought to affect the cardiovascular system. However, this system playsalsoanimportantroleinhematopoiesiswhichexplains thereductioninhematocritlevelsoranemiaasasideeffect oftreatmentusingangiotensin-convertingenzymeinhibitors (ACE inhibitors)andangiotensin IItype1receptorblockers (ARBs).73,74
The ACE, which plays a central role in blood pressure control system,75 is also responsible for the hydrolysis of acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), a tetrapeptide which naturally occurs in many body tissues. The phys-iological AcSDKP is a negative regulator of erythropoiesis thatinhibitstheentryofhematopoieticstemcellsintheS phaseofthecellcycle,keepingtheminphaseG0.76,77 Stud-ieshaveshownthattheuseofACEinhibitorsisassociated with increasedplasma concentrations of this tetrapeptide. Thus, patients taking antihypertensive ACE inhibitors may be resistant to treatment with rHuEPO.78,79 The lack of angiotensin II production, due to an interruption of the renin–angiotensinsystem,isadirectcauseofanemia, indicat-ingthatangiotensinIIregulateshematopoiesis.80Angiotensin II actsasagrowth factor anddirectly stimulates prolifera-tionoferythroidprogenitorsinthebonemarrow.Additionally, angiotensin II enhances EPO secretion, which results in increasedredbloodcellmass.73
illnesses including renal transplantation, decreased kidney functionandheartfailure.Sincethiseffectcanbereversible, thedecisiontodecreasethedoseordiscontinueACEinhibitors orARBstherapyshouldconsidertheseverityoftheclinical conditionandavailabilityofalternativetherapies.83
Anti-erythropoietinantibodies
AlthoughtreatmentwithrHuEPO iswell toleratedbymost patients,asmallnumber produceantibodiesthatcan neu-tralizeeitherendogenous EPOand recombinant proteins.84 Most cases of antibody production have been associated with the formulation of epoetin alfa when administered subcutaneously.85
In some cases, the anti-erythropoietin (anti-EPO) anti-body productioncan lead todevelopment ofserious PRCA andtransfusion-dependentanemia.86–88Recentstudieshave shownthat anti-EPOantibody-mediatedPRCAisarare but important adverse effect in patients with CKD who take rHuEPO.89–91
AccordingtotheNationalGuidelinespublishedby Brazil-ianMinistryofHealth,PRCAshouldbeevaluatedinpatients receivingepoetinalfaoveratleastfourweeksthatdevelop:(1) adropinhemoglobinlevelsequaltoorgreaterthan0.5g/dL perweek,intheabsenceoftransfusions,andrequirementofat leastoneredbloodcellunitperweektomaintainhemoglobin levels;(2)normalleukocyteandplateletcounts;(3)absolute reticulocyte count <10×103/L.92 Treatment recommenda-tions for patients with PRCA induced by erythropoiesis stimulatingagents(ESA)are:(1)discontinuationofESA;(2) cor-rectionofanemiabybloodtransfusion,ifnecessary;(3)kidney transplantand(4)introductionofimmunosuppressive ther-apystartingwithcyclosporineAaloneorincombinationwith corticosteroidsorcorticosteroidswithcyclophosphamide.85,86 DiagnosticconfirmationofPRCAinducedbyanti-EPO anti-bodiesshouldincludetheantibodylaboratorydetectionand abonemarrowexaminationthatshowstheabsenceof ery-throid lineage precursors.93 However, to date, there is no consensusaboutthe methodofchoiceforthe detectionof theseantibodies,sincethedifferentmethodshaveadvantages anddisadvantages.87Moreover,nocommerciallaboratorykit isavailableforthedetectionofanti-EPOantibodiesinthe clin-icalpractice.89
GeneticpolymorphismsandtheEPOreceptor
Some genetic polymorphisms may result in individual response variations to rHuEPO. Jeong et al.94 investigated theassociationbetweentheinterleukin1B(IL-1B)geneand ACEgenepolymorphismsanderythropoietinresistanceindex (RI-EPO) inpatients undergoing hemodialysis. Associations betweenthepresenceoftheIL-1B-511C/CandACED/D geno-typeswithlowerRI-EPOwereidentified,indicatingthatthese polymorphismsmaybeusefulgeneticmarkersinassessing therequireddoseofrHuEPOinpatientsundergoing hemodial-ysis.
ItisknownthatendogenousandrecombinantEPO stimu-lateserythropoiesisbybindingtotheEpoRreceptor.95,96 An mRNAalternativesplicingcangiverisetothesolubleformof thereceptor(sEpoR)whichlacksthetransmembranedomain.
sEpoRhasahigheraffinityforEPOandactsasapotent antago-nistofthehormone,whichcanleadtoresistancetotreatment withrHuEPO.Therefore,highlevelsofsEpoRmaybe associ-atedwithadministrationofhighdosesofrHuEPO.97,98
Conclusion
Themain causeofinadequate responseto treatmentwith rHuEPOisirondeficiency.However,severalotherfactorsmay beassociatedwiththis resistanceinpatientswithCKDon dialysisinthepresenceofadequateironstores,andmustbe investigatedincluding:inflammation,infection,malnutrition, inadequatedialysisandhyperparathyroidism.Inaddition,B19 infection,anti-EPOantibodyproduction,andthepresenceof polymorphisms have been identified as possible causes of resistancetorHuEPOindialysispatients.However,other stud-ies inthe Brazilian population, which has its own genetic characteristics,withalargersamplesizeshouldbeperformed tovalidatethesefactors.
Finally,asthemainreasonforpoorresponsetotheuseof rHuEPOisirondeficiency,whichcauseschangesinthesize and color ofred blood cells,the importanceofmonitoring patients underdialysis througha simpleblood testshould be emphasized, asthis may reveal morphological changes suchasmicrocytosisandhypochromiaconsequenttothe defi-ciencyofthisnutrient.Ontheotherhand,deficienciesoffolic acidand/orvitaminB12alsoleadtoclinicallysignificant mor-phologicalchangesinredbloodcells,suchasmacrocytosis. Detectionofmorphologicalchanges ofredblood cells may influencepropersupplementationwiththeadequatenutrient bringingbenefitstopatientsunderhemodialysisbyproperly correctingtheanemia.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthankFAPEMIG,CNPq/Brazil,andPRPq/UFMGfor thefinancialsupport.MGC,APF,LMSDandKBGaregrateful forCNPqResearchgrants(PQ).
r
e
f
e
r
e
n
c
e
s
1.RomãoJuniorJE.Adoenc¸arenalcrônica:dodiagnósticoao
tratamento.PráticaHospitalar.2007;52:183–7.
2.ManualdeTransplanteRenal.Associac¸ãoBrasileirade TransplantesdeÓrgãos(ABTO).Availablefrom:http://www.
abto.org.br/abtov03/Upload/file/ProfissionalManual/manual
transplanterim.pdf[cited31.10.14].
3.CensodaSociedadeBrasileiradeNefrologia2013.SãoPaulo: SociedadeBrasileiradeNefrologia(SBN).Availablefrom:
http://www.sbn.org.br/pdf/censo2013-14-05.pdf[cited
31.10.14].
4.KazmiWH,KauszAT,KhanS,AbichandaniR,RuthazerR,
ObradorGT,etal.Anemia:anearlycomplicationofchronic
5. NationalKidneyFoundation.K/DOQIclinicalpractice
guidelinesandclinicalpracticerecommendationsforanemia
inchronickidneydisease.AmJKidneyDis.2006;26Suppl.
3:1–145.
6. MacdougallIC,HuttonRD,ColesGA,WilliamJD.Theuseof
erythropoietininrenalfailure.PostgradMedJ.
1991;67(783):9–15.
7. HutchinsonFN,JonesWJ.Acost-effectivenessanalysisof
anemiascreeningbeforeerythropoietininpatientswith
endstagerenaldisease.AmJKidneyDis.1997;29(5):651–7.
8. SessoRC,LopesAA,ThomeFS,LugonJR,WatanabeY,Santos
DR.RelatóriodoCensoBrasileirodeDiálise2012.JBras
Nefrol.2014;36(1):48–53.
9. LocatelliF,AljamaP,BaranyP,CanaudB,CarreraF,Eckardt
KU,etal.RevisedEuropeanbestpracticeguidelinesforthe
managementofanaemiainpatientswithchronicrenal
failure.NephrolDialTransplant.2004;19Suppl.2:ii1–47.
10.KDOQI.KDOQIClinicalPracticeGuidelineandClinical
PracticeRecommendationsforanemiainchronickidney
disease:2007updateofhemoglobintarget.AmJKidneyDis.
2007;50(3):471–530.
11.LocatelliF,CovicA,EckardtKU,WiecekA,VanholderR,
ERA-EDTAERBPAdvisoryBoard.Anaemiamanagementin
patientswithchronickidneydisease:apositionstatementby
theAnaemiaWorkingGroupofEuropeanRenalBestPractice
(ERBP).NephrolDialTransplant.2009;24(2):348–54.
12.KDIGO.KDIGOclinicalpracticeguidelineforanemiain
chronickidneydisease.KidneyIntSuppl.2012;2:279–335.
13.LocatelliF,PisoniRL,CombeC,BommerJ,AndreucciVE,Piera
L,etal.AnaemiainhemodialysispatientsoffiveEuropean
countries:associationwithmorbidityandmortalityinthe
DialysisOutcomesandPracticePatternsStudy(DOPPS).
NephrolDialTransplant.2004;19(1):121–32.
14.Brasil.MinistériodaSaúdeProtocoloClínicoeDiretrizes
Terapêuticas.Anemiaempacientescominsuficiênciarenal
crônica–alfaepoetina.Brasília:MinistériodaSaúde;2010.
15.Ribeiro-AlvesMA,GordanPA.DiagnósticodeAnemiaem
PacientesPortadoresdeDoenc¸aRenalCrônica.JBrasNefrol.
2014;361Suppl.1:9–12.
16.AbensurH.Anemiadadoenc¸arenalcrônica.JBrasNefrol.
2004;26(3):26–8.
17.BrugnaraC.Irondeficiencyanderythropoiesis:new
diagnosticapproaches.ClinChem.2003;49(10):1573–8.
18.MittmanN,SreedharaR,MushnickR,ChattopadhyayJ,
ZelmanovicD,VaseghiM,etal.Reticulocytehemoglobin
contentpredictsfunctionalirondeficiencyinhemodialysis
patientsreceivingrHuEPO.AmJKidneyDis.1997;30(6):912–22.
19.MastAE,BlinderMA,DietzenDJ.Reticulocytehemoglobin
content.AmJHematol.2008;83(4):307–10.
20.FishbaneS,ShapiroW,DutkaP,ValenzuelaOF,FaubertJ.A
randomizedtrialofirondeficiencytestingstrategiesin
hemodialysispatients.KidneyInt.2001;60(6):2406–11.
21.BrugnaraC,LauferMR,FriedmanAJ,BridgesK,PlatO.
Reticulocytehemoglobincontent(CHr):earlyindicatorofiron
deficiencyandresponsetotherapy.Blood.1994;83(10):3100–1.
22.ThomasC,ThomasL.Biochemicalmarkersandhematologic
indicesinthediagnosisoffunctionalirondeficiency.Clin
Chem.2002;48(7):1066–76.
23.LeaJP,NorrisK,AgodoaL.Theroleofanemiamanagementin
improvingoutcomesforAfricanAmericanswithchronic
kidneydisease.AmJNephrol.2008;28(5):732–43.
24.Kalantar-zadehK,IkizlerTA,BlockG,AvramMM,KoppleJD.
Malnutrition–inflammationcomplexsyndromeindialysis
patients:causesandconsequences.AmJKidneyDis.
2003;42(5):864–81.
25.BamgbolaO.Resistancetoerythropoietin-stimulatingagents:
etiology,evaluation,andtherapeuticconsiderations.Pediatr
Nephrol.2010;27(2):195–205.
26.WishJB.Assessingironstatus:beyondserumferritinand
transferrinsaturation.ClinJAmSocNephrol.2006;Suppl.
1:S4–8.
27.MeansRT,KrantzSB.Progressinunderstandingthe
pathogenesisoftheanemiaofchronicdisease.Blood.
1992;80(7):1639–47.
28.PigeonC,IlyinG,CourselaudB,LeroyerP,TurlinB,BrissotP,
etal.Anewmouseliver-specificgene,encodingaprotein
homologoustohumanantimicrobialpeptidehepcidin,is
overexpressedduringironoverload.JBiolChem.
2001;276(11):7811–9.
29.RiveraS,NemethE,GabayanV,LopezMA,FarshidiD,GanzT.
Synthetichepcidincausesrapiddose-dependent
hipoferremiaandisconcentratedinferroportin-containing
organs.Blood.2005;106(6):2196–9.
30.NemethE,TuttleMS,PowelsonJ,VaughnMB,DonovanA,
WardDM,etal.Hepcidinregulatescellularironeffluxby
bindingtoferroportinandinducingitsinternalization.
Science.2004;306(5704):2090–3.
31.DeDomenicoI,WardDM,KaplanJ.Hepcidinregulation:
ironingoutthedetails.JClinInvest.2007;117(7):
1755–8.
32.NemethE,GanzT.Theroleofhepcidininironmetabolism.
ActaHaematol.2009;122(2–3):78–86.
33.NemethE,RiveraS,GabayanV,KellerC,TaudorfS,Pedersen
BK,etal.IL-6mediateshypoferremiaofinflammationby
inducingthesynthesisoftheironregulatoryhormone
hepcidin.JClinInvest.2004;113(9):1271–6.
34.GanzT,NemethE.Hepcidinandironhomeostasis.Biochim
BiophysActa.2012;1823(9):1434–43.
35.Canc¸adoRD,ChiattoneCS.AnemiadeDoenc¸aCrônica.Rev
BrasHematolHemoter.2002;24(2):127–36.
36.MacdougallIC,CooperAC.Erythropoietinresistance:therole
ofinflammationandpro-inflammatorycytokines.Nephrol
DialTransplant.2002;17(S11):39–43.
37.JuradoRL.Iron,infections,andanemiaofinflammation.Clin
InfectDis.1997;25(4):888–95.
38.FuchsD,HausenA,ReibneggerG,WernerER,
Werner-felmayerG,DierichMP,etal.Immuneactivationand
theanaemiaassociatedwithchronicinflammatorydisorders.
EurJHaematol.1991;46(2):65–70.
39.BetjesMG,HuismanM,WeimarW,LitjensNHR.Expansionof
cytolyticCD4+CD28−Tcellsinend-stagerenaldisease.
KidneyInt.2008;74(6):760–7.
40.CooperAC,BreenCP,VyasB,OcholaJ,KemenyDM,
MacdougallIC.Poorresponsetorecombinanterythropoietin
isassociatedwithlossofT-lymphocyteCD28expressionand
alteredinterleukin-10production.NephrolDialTransplant.
2003;18(1):133–40.
41.BetjesMG,WeimarW,LitjensNHR.CMVseropositivity
determinesepoetindoseandhemoglobinlevelsinpatients
withCKD.JAmSocNephrol.2009;20(12):2661–6.
42.BrownKE,AndersonSM,YoungNS.ErythrocytePantigen:
cellularreceptorforB19parvovirus.Science.
1993;262(5130):114–7.
43.ChisakaH,MoritaE,YaegashiN,SugamuraK.ParvovirusB19
andthepathogenesisofanaemia.RevMedVirol.
2003;13(6):347–59.
44.HsuC,BatesDW,KupermanGJ,CurhanGC.Relationship
betweenhematocritandrenalfunctioninmenandwomen.
KidneyInt.2001;59(2):725–31.
45.LuiSL,LukWK,CheungCY,ChanTM,LaiKN,PeirisJS.
Nosocomialoutbreakofparvovirusb19infectioninarenal
transplantunit.Transplantation.2001;71(1):59–64.
46.OzekiM,FukushimaT,OhzekiM,SasakiT,KashiharaN.A
nosocomialparvovirusB19infection-inducedtransient
aplasticcrisisinapatientwithchronicrenalfailure.Clin
47.YoungNS,BrownKE.ParvovirusB19.NEnglJMed. 2004;350(6):586–97.
48.BeckerMR,SchneiderB,ReberU,PogeU,KleinB,KlehrHU,
etal.Renalanemiaaggravatedbylong-termparvovirusB19
andcytomegalovirusinfectioninarenaltransplantpatient:
casereportandevaluationofB19seroprevalenceindialysis
patients.TransplantProc.2005;37(10):4306–8.
49.GuiserixJ,RamdaneM,HoarauJM,FinielzP,MichaultA.
ParvovirusB19andhemodialysis.Nephron.1996;72(4):
719.
50.MalyszkoJ,HryszkoT,MalyszkoJS,WolczynskiS,Mysliwiec
M.ParvovirusB19infectionandIGFsystemcomponentsin
relationtoerythropoiesisindialyzedpatientsandkidney
transplantrecipients.TransplantProc.2002;34(8):3211–4.
51.DuranayM,BaliM,SahinM,YakinciG,VurgunN,DilmenU.
ParvovirusB19infectionandunresponsivenessto
erythropoietintherapyinhaemodialysispatients.Nephrol
DialTransplant.1998;13(3):779–80.
52.AlvesMT,Vilac¸aSS,CarvalhoMG,FernandesAP,DusseLM,
GomesKB.HumanparvovirusB19infectioninarenal
transplantrecipient:acasereport.BMCResNotes.2013;
6(28).
53.EgbunaO,ZandMS,ArbiniA,MenegusM,TaylorJ.Acluster
ofparvovirusB19infectionsinrenaltransplantrecipients:a
prospectivecaseseriesandreviewoftheliterature.AmJ
Transplant.2006;6(1):225–31.
54.SmithSR,ButterlyDW,AlexanderBD,GreenbergA.Viral
infectionsafterrenaltransplantation.AmJKidneyDis.
2001;137(4):659–76.
55.KeungY,ChuahirunT,WessonD.Concomitantparvovirus
B19andcytomegalovirusinfectionsafterliving-relatedrenal
transplantation.NephrolDialTransplant.1999;14(2):
469–71.
56.FishP,HandgretingerR,ShaeferH.Pureredcellaplasia.BrJ
Haematol.2000;111(4):1010–22.
57.AvesaniCM,CarreroJJ,AxelssonJ,QureshiAR,LindholmB,
StenvinkelP.Inflammationandwastinginchronickidney
disease:partnersincrime.KidneyInt.2006;70:8–13.
58.QureshiAR,AlvestrandA,DanielssonA,Divino-FilhoJC,
GutierrezA,LindholmB,etal.Factorspredictingmalnutrition
inhemodialysispatients:across-sectionalstudy.KidneyInt.
1998;53(3):773–82.
59.LocatelliF,AndrulliS,MemoliB,MaffeiC,VecchioLD,Aterini
S,etal.Nutritional-inflammationstatusandresistanceto
erythropoietintherapyinhaemodialysispatients.Nephrol
DialTransplant.2006;21:991–8.
60.Kalantar-ZadehK,LeeGH,MillerJE,StrejaE,JingJ,Robertson
JA,etal.Predictorsofhyporesponsivenessto
erythropoiesis-stimulatingagentsinhemodialysispatients.
AmJKidneyDis.2009;53(5):823–34.
61.GawedaAE,GoldsmithLJ,BrierME,AronoffGR.Iron,
inflammation,dialysisadequacy,nutritionalstatus,and
hyperparathyroidismmodifyerythropoieticresponse.ClinJ
AmSocNephrol.2010;5(4):576–81.
62.ShaeferR,TeschnerM,KoschM.Folatemetabolisminrenal
failure.NephrolDialTransplant.2002;17(S5):24–7.
63.PernaAF,IngrossoD,SantoNG,GallettiP,BrunoneM,Zappia
V.Metabolicconsequencesoffolate-inducedreductionof
hyperhomocysteinemiainuremia.AmSocNephrol.
1997;8(12):1899–905.
64.VecchiAF,Bamonti-CatenaF,FinazziS,CampoloJ,TaioliE,
NovembrinoC,etal.Homocysteine,vitaminb12,andserum
anderythrocytefolateinperitonealdialysisandhemodialysis
patients.PeritDialInt.2000;20(2):169–73.
65.CostaE,BeloL,Santos-SilvaA.Specialproblemsin
hemodialysispatientsInternet.In:Resistancetorecombinant humanerythropoietintherapyinhaemodialysispatients. Portugal:InTech;2011[Chapter3].Availablefrom:
http://www.intechopen.com/books/special-problems-in-
hemodialysis-patients/resistance-to-recombinant-human-erythropoietin-therapy-in-haemodialysis-patients[cited
14.10.13].
66.ShahabI,KhannaR,NolphKD.Peritonealdialysisor
hemodialysis?Adilemmaforthenephrologist.AdvPeritDial.
2006;22:180–5.
67.NationalKidneyFoundation.K/DOQIclinicalpractice
guidelinesfornutritioninchronicrenalfailure.AmJKidney
Dis.2000;356Suppl.2:S1–40.
68.MovilliE,CancariniGC,ZaniR,CameriniC,SandriniM,
MaiorcaR.Adequacyofdialysisreducesthedosesof
recombinanterythropoietinindependentlyfromtheuseof
biocompatiblemembranesinhaemodialysispatients.
NephrolDialTransplant.2001;16(1):111–4.
69.DruekTB,EckardK.Roleofsecondaryhyperparathyroidism
inerythropoietinresistanceofchronicrenalfailurepatients.
NephrolDialTransplant.2002;17(S5):28–31.
70.BrancaccioD,CozzolinoM,GallieniM.Hyperparathyroidism
andanemiainuremicsubjects:acombinedtherapeutic
approach.JAmSocNephrol.2004;15Suppl.1:S21–4.
71.NationalKidneyFoundation.Kidneydisease-dialysis
outcomequalityinitiative:K/DOQIClinicalPractice
Guidelinesforbonemetabolismanddiseaseinchronic
kidneydisease.AmJKidneyDis.2003;424Suppl.3:S1–202.
72.RaoDS,ShihM,MohiniR.Effectofserumparathyroid
hormoneandbonemarrowfibrosisontheresponseto
erythropoietininuremia.NEnglJMed.1993;328(3):171–5.
73.VlahakosDV,MarathiasKP,MadiasNE.Theroleofthe
renin–angiotensinsystemintheregulationoferythropoiesis.
AmJKidneyDis.2010;56(3):558–65.
74.RodgersKE,diZeregaJS.ContributionofthelocalRASto
hematopoieticfunction:anoveltherapeutictarget.Front
Endocrinol(Lausanne).2013;23(4):157.
75.ErdösEG.AngiotensinIconvertingenzymeandthechanges
inourconceptsthroughtheyears.Hypertension.
1990;16(4):363–70.
76.LenfantM,Wdzieczak-bakalaJ,GuittetE,PromeJC,SottyD,
FrindelE.Inhibitorofhematopoieticpluripotentstemcell
proliferation:purificationanddeterminationofitsstructure.
ProcNatlAcadSciUSA.1989;86(3):779–82.
77.RiegerK,Saez-serventN,PapetMP,Wdzieczak-BakalaJ,
MorgatJ,ThierryJ,etal.Involvementofhumanplasma
angiotensinI-convertingenzymeinthedegradationofthe
haemoregulatorypeptideN-acetylserylaspartyllysylproline.
BioremJ.1993;296(Pt.2):373–8.
78.AziziM,RousseauA,EzanE,GuyeneT,MicheletS,GrognetJ,
etal.Acuteangiotensin-convertingenzymeinhibition
increasestheplasmalevelofthenaturalstemcellregulator
N-acetyl-seryl-aspartyl-lysyl-proline.JClinInvest.
1991;97(3):839–44.
79.MeurYL,LorgeotV,ComteL,SzelaqJC,Leroux-RobertC,
PraloranV.PlasmalevelsandmetabolismofAcSDKPin
patientswithchronicrenalfailure:relationshipwith
erythropoietinrequirements.AmJKidneyDis.
2001;38(3):510–7.
80.ColeJ,ErtoyD,LinH,SutliffRL,EzanE,GuyeneTT,etal.Lack
ofangiotensinII–facilitatederythropoiesiscausesanemiain
angiotensin-convertingenzyme-deficientmice.JClinInvest.
2000;106(11):1391–8.
81.IodiceC,BallettaMM,MinutoloR,GiannattasioP,TuccilloS,
BellizziV,etal.Maximalsuppressionofrenin–angiotensin
systeminnonproliferativeglomerulonephritis.KidneyInt.
2003;63(6):2214–21.
82.JacobsenP,AndersenS,JensenBR,ParvingHH.Additiveeffect
ofACEinhibitionandangiotensinIIreceptorblockadeintype
Idiabeticpatientswithdiabeticnephropathy.JAmSoc
83.MarathiasKP,AgroyannisB,MavromoustakosT,MatsoukasJ,
VlahakosDV.Hematocrit-loweringeffectfollowing
inactivationofrenin–angiotensinsystemwithangiotensin
convertingenzymeinhibitorsandangiotensinreceptor
blockers.CurrTopMedChem.2004;4(4):483–6.
84.CasadevallN.AntibodiesagainstrHuEPO:nativeand
recombinant.NephrolDialTransplant.2002;17(S5):
42–7.
85.MacdougallIC,RogerSD,FranciscoA,GoldsmithDJA,
SchellekensH,EbbersH,etal.Antibody-mediatedpurered
cellaplasiainchronickidneydiseasepatientsreceiving
erythropoiesis-stimulatingagents:newinsights.KidneyInt.
2012;81(8):727–32.
86.CasadevallN.Pureredcellaplasiaandanti-erythropoietin
antibodiesinpatientstreatedwithepoetin.NephrolDial
Transplant.2003;18Suppl.8:37–41.
87.ThorpeR,SwansonSJ.Assaysfordetectinganddiagnosing
antibody-mediatedpureredcellaplasia(PCRA):an
assessmentofavailableprocedures.NephrolDialTransplant.
2005;20(S4):iv16–22.
88.BehlerC,TerraultN,EtzellJ,DamonL.Rituximabtherapyfor
pureredcellaplasiaduetoanti-epoetinantibodiesina
womantreatedwithepoetin-alfa:acasereport.JMedCase
Rep.2009;3:7335.
89.ShinS,MoonSJ,HaSK,JoY,LeeT,LeeYS,etal.
Immunogenicityofrecombinanthumanerythropoietinin
Korea:atwo-yearcross-sectionalstudy.Biologicals.
2012;40(4):254–61.
90.KharagjitsinghAV,KorevaarJC,VandenbrouckeJP,Boeschoten
EW,KredietRT,DahaMR,etal.Incidenceofrecombinant
erythropoietin(EPO)hyporesponse,EPO-associated
antibodies,andpureredcellaplasiaindialysispatients.
KidneyInt.2005;68(3):1215–22.
91.SchonholzerC,KeuschG,NiggL,RobertD,WautersJ.High
prevalenceinSwitzerlandofpurered-cellaplasiadueto
anti-erythropoietinantibodiesinchronicdialysispatients:
reportoffivecases.NephrolDialTransplant.
2004;19(8):2121–5.
92.Brasil,MinistériodaSaúdeProtocoloClínicoeDiretrizes
Terapêuticas.Aplasiapuraadquiridacrônicadasérie
vermelha.Brasília:MinistériodaSaúde;2010.
93.CasadevallN.Whatisantibody-mediatedpureredcell
aplasia(APASV).NephrolDialTransplant.2005;20(S4):iv3–8.
94.JeongK,LeeT,IhmC,LeeS,MoonJ.Polymorphismsintwo
genes,IL-1BandACE,areassociatedwitherythropoietin
resistanceinKoreanpatientsonmaintenancehemodialysis.
ExpMolMed.2008;40(2):161–6.
95.MiddletonSA,BarboneFP,JohnsonDL.Sharedandunique
determinantsoftheerythropoietin(EPO)receptorare
importantforbindingEPOandEPOmimeticpeptide.JBiol
Chem.1999;74(20):14163–9.
96.NgT,MarxG,LittlewoodT,MacdougallI.Recombinant
erythropoietininclinicalpractice.PostgradMedJ.
2003;79(933):367–76.
97.InrigJ,BryskinS,PatelU,ArcasoyM,SzczechL.Association
betweenhigh-doseerythropoiesisstimulatingagents,
inflammatorybiomarkers,andsolubleerythropoietin
receptors.BMCNephrol.2011;12:67.
98.KhankinE,MutterW,TamezH,YuanH,KarumanchiS,
ThadhaniR.Solubleerythropoietinreceptorcontributesto
erythropoietinresistanceinend-stagerenaldisease.PLoS