r e v b r a s r e u m a t o l .2 0 1 7;5 7(2):100–106
w w w . r e u m a t o l o g i a . c o m . b r
REVISTA BRASILEIRA DE
REUMATOLOGIA
Original article
Effects of the use of growth hormone in children
and adolescents with juvenile idiopathic arthritis:
a systematic review
Renan
Bazuco
Frittoli
a,
Barbara
Sugui
Long
h
i
a,
Amanda
Meireles
Silva
a,
Antônio de Azevedo Barros Filho
b, Maria Ângela Reis de Góes Monteiro
b,
Simone Appenzeller
b,c,∗aUniversidade Estadual de Campinas (UNICAMP), Faculdade de Ciências Médicas, Laboratório de Reumatologia, Campinas, SP, Brazil bUniversidade Estadual de Campinas (UNICAMP), Faculdade de Ciências Médicas, Unidade de Reumatologia Pediátrica, Campinas, SP,
Brazil
cUniversidade Estadual de Campinas (UNICAMP), Faculdade de Ciências Médicas, Unidade de Reumatologia, Campinas, SP, Brazil
a r t i c l e
i n f o
Article history:
Received 23 November 2015 Accepted 7 April 2016
Available online 24 October 2016
Keywords:
Juvenile idiopathic arthritis Growth hormone
Children Adolescents
a b s t r a c t
Introduction:Children with juvenile idiopathic arthritis (JIA) often have impaired growth and short stature. There is evidence that the therapeutic use of growth hormone (GH) is useful and safe in these patients.
Objective:To analyze the effects of GH use in patients with JIA.
Method:A systematic review of the literature over the last 18 years in Medline and Embase databases. The criteria were analyzed independently by the researchers. We used the fol-lowing keywords: “growth hormone”, “arthritis, juvenile”, “arthritis, rheumatoid”, “child” and “adolescent”.
Results:Among the 192 identified articles, 20 corresponded to the inclusion criteria. Seven-teen longitudinal studies and 3 case reports were found. Most studies analyzed observed increased growth, muscle mass and bone mass using GH. Adverse effects observed were glucose intolerance, diabetes, bone deformities, osteonecrosis, reactivation of the disease and low final height.
Conclusion:The majority of studies reported positive effects after the therapeutic use of GH, but some variability in response to treatment was observed. The combination of growth hormone with other drugs seems to be a good option.
© 2016 Published by Elsevier Editora Ltda. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Corresponding author.
E-mail:appenzellersimone@yahoo.com(S. Appenzeller). http://dx.doi.org/10.1016/j.rbre.2016.07.009
Efeitos
do
uso
do
hormônio
de
crescimento
em
crianc¸as
e
adolescentes
com
artrite
idiopática
juvenil:
revisão
sistemática
Palavras-chave:
Artriteidiopáticajuvenil Hormôniodecrescimento Crianc¸as
Adolescentes
r
e
s
u
m
o
Introduc¸ão:Crianc¸ascomartriteidiopáticajuvenil(AIJ)frequentementeapresentamprejuízo nocrescimentoebaixaestatura.Existemevidênciasdequeousoterapêuticodohormônio decrescimento(GH)éútileseguronessespacientes.
Objetivo: AnalisarosefeitosdousodeGHempacientescomAIJ.
Método: Fez-serevisãosistemáticadaliteraturanosúltimos18anos,nasbasesdedados Medline e Embase. Os critérios foram analisadospelos pesquisadores de forma inde-pendente.Usaram-seosseguintesdescritores:growthhormone,arthritis,juvenile,arthritis, rheumatoid,childeadolescent.
Resultados: Entreos192artigosidentificados,20corresponderamaoscritériosdeinclusão. Foramencontrados17estudoslongitudinaisetrêsrelatosdecasos.Amaioriadosestudos analisadosobservouumaumentodecrescimento,massamuscularemassaósseacomouso doGH.Osefeitosadversosobservadosforamintolerânciaàglicose,diabetes,deformidades ósseas,osteonecrose,reativac¸ãodadoenc¸aealturafinalbaixa.
Conclusão:AmaioriadosestudosrelatouefeitospositivosapósusoterapêuticodoGH,porém certavariabilidadenarespostaaotratamentofoiobservada.Acombinac¸ãodohormôniode crescimentocomoutrosmedicamentospareceserumaboaopc¸ão.
©2016PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Juvenileidiopathicarthritis(JIA)isanautoimmune disease andtheleadingcauseofchronicarthritisinpediatricpatients. Itsannualincidencevariesfrom2to20cases/100,000 inhabi-tants,withaprevalenceof15–150cases/100,000inhabitants.1 Itsmainfeaturesarechronicarthritis,andinsomecases beingassociatedwithmultisysteminvolvementand progres-siontojointlimitationsandpermanentfunctionaldisability.2 Thediagnosis ofJIAisbased oncriteria ofthe Interna-tionalLeague ofAssociations forRheumatology (ILAR)and requiresthepresenceofarthritisinchildrenandadolescents agedunder16years,thatpersistsforatleastsixweeks,when excludingthepresenceofothercausesofchronicarthritis.3 Accordingtothe literature,themostfrequentJIAsubtypes areoligoarticularJIA(50–60%),polyarticularJIA(30–35%)and systemicJIA(10–20%).4
Children with JIA often exhibit an inadequate growth andshort stature,andthese effectsrelatetothe extentof organsinvolved,withtheactivityandextentofthedisease, malnutrition, malabsorption, increased catabolism, associ-ated complications, a constant inflammatory process, and effectsoftheuseofcertainmedications,beingthemost rel-evant ofthem the glucocorticoids.5–8 Several studies have observedimprovedgrowthandheightratesinpatientswith JIAtreated withgrowth hormone(GH).5,9–25 There are sev-eralindicationsoftheuseofGHapprovedfortreatmentof JIA;however,theuse ofthishormonecanresultinadverse events,26 includingnegativevariabilityinresponseto treat-mentwhichhasbeenobservedinsomestudiesofpatients withJIA.9,10,14,19,20,24,25,27,28
Duetothehighcostandpotentialsideeffects,9,10,20,27there are controversiesabout the idealindication, the dose, and
durationoftheGHtherapyinJIA.26Anunderstandingthe indi-cationsfortherapywithgrowthhormoneanditscontroversies canfacilitatetheevaluationofthepatientandhis/herreferral tothebesttreatment.26,29,30Theaimofthisstudyistoanalyze thevariouseffectsofGHuseinpatientswithJIA,basedonthe literaturereview.
Methods
This study consists of asystematic literaturereview, after a search conductedduring the monthsofJuly and August 2015, ofstudies publishedin thelast 18 years (1998–2015). Thesearchofthereferenceswasconductedthroughan elec-tronicdatabase(MedlineandEmbase)explorationandofthe referencelistofidentifiedarticlesbythreeresearchers inde-pendently. References that met the inclusion criteria were evaluated, regardlessof their journal.The selectionof the descriptorsusedinthereviewprocesswasconductedin con-sultationwithDeCS(HealthSciencesDescriptorsbyBIREME). In the search, the following descriptorsin Portuguese and Englishwereconsidered:“growthhormone”,“arthritis, juve-nile”,“arthritis,rheumatoid,”“child”and“adolescent”.31
102
rev bras reumatol.2017;57(2):100–106Selected studies (n=128)
Replicated studies, removed (n=25) Pubmed and embase
-electronic search (n=192)
Identified studies (summary and title)
(n=153)
Identification
Screening
Eligibility
Included
Excluded studies (n=109):
GH not used as therapy (n=61)
Not evaluated JIA (n=9)
Studies published over than 13 years ago (n=14)
Reviews (n=15)
Animal studies (n=1)
Other Language (n=8)
Unavailable journals (n=1)
Articles included in this study
(n=20)
Articles in reference lists of identified articles
(n=1) Full-text articles to
assess eligibility (n=19)
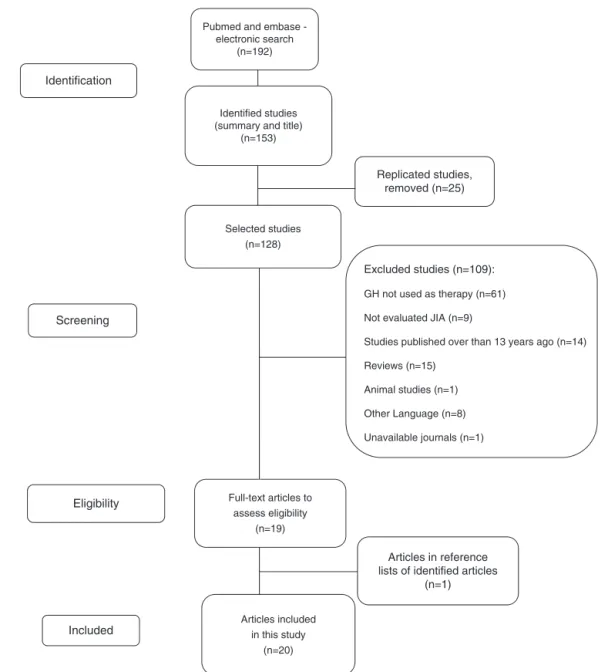
Fig.1–Explanatorydiagramontheprocessofselectionofarticles.
evaluatedpatientswithJIA;(5)Articlesthatincludedchildren andadolescentsintheirsample(Fig.1).
Results
Twentystudieswereincluded,ofwhich17hadlongitudinal design5,11–25,28 and threewere case reports.9,10,27 Atotal of 359patientswithJIA(systemic,polyarticular,and oligoartic-ular)weretreatedwithGH,withdosesrangingfrom0.028to 0.067mg/kg/day,andagedfrom4to17years;thetreatment timerangedfrom9monthsto6years(Tables1and2).
After use of GH, an increased growth of JIA patients was demonstrated in 80% of the studies,5,11–25 and 35% reported significant improvement in pubertal development.11–13,16,17,24,25
Among the positive effects of the treatment, 30% of the studies reported improvement in bone mineral
density and bone metabolism.12,17,19,21,23 In comparison withpre-treatment values,it wasreported thatthe forma-tion and resorption markers increasedsignificantly during treatment,17,21,23andthattheplasmalevelofosteocalcinwas thebestvariablepredictiveofgrowthinJIApatientswhohad beentreatedwithGH.21
Onestudy foundthat the fatlevelhas remainedstable, withnosignificantchangesversuspatientswhodidnotuse GH;12butin4(20%)studies,leanmassincreaseandfatmass decreasewereobserved.15,19,23,25
Regardingbloodglucoseandglucoseintolerancein5(25%) studies,14,19,24,25,28asignificantincreaseinfastingblood glu-cose wasnoted inpatients treatedwithGH versuscontrol group,whichled,insomecases,tothepossibledevelopment ofinsulinresistance,19,24,25,28andtodiabetes.25,28Increasesin glycosylatedhemoglobinwerealsodescribed.19,24,25
r e v b r a s r e u m a t o l . 2 0 1 7; 5 7(2) :100–106
103
group (years) duration (years)(mg/kg/day) duration(years)
Bechtold,201211
(Deutschland)
39patients(systemic/polyarticularJIA) 24controls
(8–13) ∼=5 0.047 6.32±1.96 GHhadpositiveeffectsonheightandpubertaldevelopment
BismuthE,201028
(France)
43patients(systemic/polyarticularJIA) 15controls
(4–14) (4–8)
3.9±2.7 4.3±2.9
0.065 3 GHcombinedwithglucocorticoidswasassociatedwithasignificant increaseinfastinginsulinlevel
Bechtold,201012
(Deutschland)
12patients(systemic/polyarticularJIA) (9–14) 8.49±2.9 – 5.35±0.7 Benefitsinanthropometriclevels,andfatlevelsstabilized
Bechtold,200713
(Deutschland)
31patients(systemic/polyarticularJIA) 18controls
(10–14) 3,7±1,2 0.047 8.4 Patientsreachedtheheightwithinthestandardtargetset
Simon,200714
(France)
30patients(systemic/polyarticularJIA) (7–12) – 0.065 3 Benefitsinanthropometriclevels
Bonemineralizationwithnosignificantdifference Increasedbloodglucose
Bechtold,200515
(Deutschland)
17patients(systemic/polyarticularJIA) (12–17) 8.2±4.4 (0.036–0.047) 4 GHhadpositiveandsignificanteffectsonheightandmusclemass
Saha,200416
(Finland)
25patients(12polyarticularJIA,10 oligoarticularJIAand3systemicJIA)
(8–11) – 0.033 0.5(6months) PatientswithGHtreatmentgrewfasterversuscontrolgroup
Bechtold,200417
(Deutschland)
11patients(systemic/polyarticularJIA) (9–11) 3.7±1.4 0.047 4 Significantincreaseinheight,andimprovementinboneremodeling
Bechtold,200318
(Deutschland)
38patients(systemic/polyarticularJIA) (5.5–13.8) ∼=3.8 (0.028–0.047) 4 Significantincreaseinfinalheight
Simon,200319
(France)
30patients(systemic/polyarticularJIA) (6.8–13.0) 8.2 (4.5–15.6)
0.065 3 Improvementingrowthrate
Increasesinleanbodymassandbonemineraldensity Glucoseintoleranceobservedin6patients
Increaseofglycosylatedhemoglobin Simon,20015
(France)
14patients(systemic/polyarticularJIA) ∼=9.7 ∼=3 0.065 1 Improvedgrowthrateandpreventionofmetaboliccomplications
Al-Mutair,200020
(SaudiArabia)
10patients(systemic/polyarticularJIA) (6–15) – 0.027 1and3 Improvedgrowthinthefinalheight
Onepatientdevelopeddeformitiesinbothknees Touati,200021
(France)
14patients(systemic/polyarticularJIA) (6–14) ∼=3 0.066 1 Significantincreasesinboneresorptionmarkers
Plasmalevelofosteocalcinwasthebestvariablepredictiveofgrowth Rooney,200022
(England)
20patients(systemic/polyarticularJIA) 7.6 – – 1 Improvementofbonemetabolismandheight
Simon,200023
(France)
14patients(systemic/polyarticularJIA) ∼=9 – 0.06 2 Increasesingrowthrateandleanmass
Increaseinbonemineraldensity Simon,199924
(France)
14patients(systemic/polyarticularJIA) (9–12) 3 (9months to∼=8years)
0.067 1 Improvementingrowthrate
Decreaseinglucosetoleranceandincreaseinglycosylated hemoglobinlevels
Touati,199825
(France)
14patients(systemic/polyarticularJIA) ∼=9 – 0.066 1–2 Increaseingrowth
Increaseinleanmassanddecreaseinfatmass
104
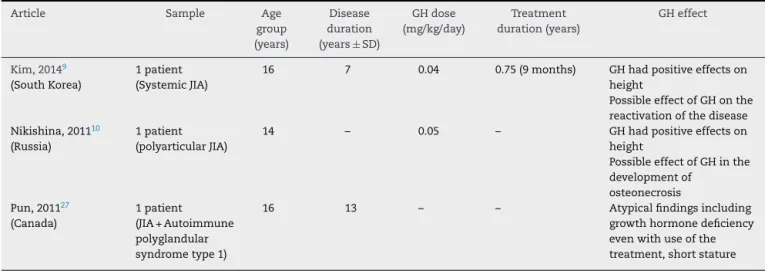
rev bras reumatol.2017;57(2):100–106Table2–DescriptionofarticlesonGrowthHormone(GH)effectsinpatientswithJuvenileIdiopathicArthritis(JIA)(case reports):1998–2015.
Article Sample Age
group (years)
Disease duration
(years±SD)
GHdose
(mg/kg/day)
Treatment
duration(years)
GHeffect
Kim,20149
(SouthKorea)
1patient (SystemicJIA)
16 7 0.04 0.75(9months) GHhadpositiveeffectson
height
PossibleeffectofGHonthe reactivationofthedisease Nikishina,201110
(Russia)
1patient (polyarticularJIA)
14 – 0.05 – GHhadpositiveeffectson
height
PossibleeffectofGHinthe developmentof
osteonecrosis Pun,201127
(Canada)
1patient (JIA+Autoimmune polyglandular syndrometype1)
16 13 – – Atypicalfindingsincluding
growthhormonedeficiency evenwithuseofthe treatment,shortstature
deformityinbothknees.20Incasereports,reactivationofthe diseaseintwostudies9,10 andlowfinalheightinonestudy wereobserved.27
Discussion
Recombinant GH hasrevolutionized the treatment of chil-drenand adolescentswithgrowth hormonedeficiencyand other growthdisorders,but theclinical and ethical contro-versies remain, about the diagnostic approach in patients withJIA,aswellastheoptimaldose,duration,andexpected results.11,12,19,26
Theresultsobtained bymany longitudinalstudies con-firmedpreviouslypublisheddata,thatthetreatmentwithGH ofpatientswithJIAallowspositivelyaffectthepubertal devel-opmentandfinalheight.5,11–25
Itisknownthatthetreatmentinstituted,particularlythe use ofglucocorticoids, alsocompromise thegrowth,which dependsonotherfactorssuchasageofonsetofthedisease,its severity,thepatient’sresponsetotreatment,timeof adminis-tration,anddoseoftheglucocorticoidadministered.8
Boneformationandresorptionmarkersincreased signif-icantlyduringthetreatmentwithGH14,17,19,21–23;butastudy observedlossofgrowthfollowingthediscontinuationofthe treatment.23 Itisbelieved thata prolongedtreatment with GHisneeded,inordertoallowanassessmentofapositive effectofthishormoneonthebonedensityandmetabolismin patientswithJIA.10,14,17,19,23,25 Themonitoringofpatientsto theirfinalheight,besidesbonemassevaluation,areneeded to better understand the potential beneficial effect of GH treatment.5,11–25,27
As toglucose intolerance,studies didnot find patients withabnormalglucosemetabolismsymptomsbeforeuseof GH.14,19,24,25,28Themetabolismofcarbohydratesmustbe care-fullymonitoredinJIApatientstreatedwithGH,particularly duringtheacutephaseoftheirdisease.Itisknownthat glu-cocorticoidsinterferewiththeGH/IGF-1axis,decreasingthe GHpulsatilesecretionbyincreasingofsomatostatinlevelsand thatthesepharmaceuticalsalsoreducetheexpressionofGH receptorsonhepatocytes,causingsomedegreeofresistance toGH,withaconsequentreductionofIGF-1levels.32,33
Theoretically,GHnotonlyregulatesgrowthbutalso con-trolstheimmunefunction,consideringthatitisnotcommon anoccurrenceofrelapseofdiseaseactivityafterseveralyears ofremissionwithoutadefinitecause.18,25 Ithasbeen ques-tionedifGHwasresponsibleforthereactivationofthedisease inthosecasereportspresentedinthispaper.9,10
One study reported an atypical case of JIA, with short stature afterthe use ofGH;however, this resultcannotbe generalizedtootherstudiesbecausethepatientsufferedfrom otherconcomitantdiseases.27
Itshould bestressedthat JIAisadiseasewithmultiple clinical manifestations among affected individuals (a sys-temic,polyarticular,oligoarticular,etc.,onset).Thus,thereare nodouble-blindrandomizedcontrolledstudiesortrialswith placeboversustheuseofGH.Abetterunderstandingofthe diseaseandmoreeffectivetreatmentsinrecentyearscan radi-callychangethegrowthstatusofthesepatients.Someauthors suggestthatthetreatmentwithGHisimplementedin combi-nationwithotherdrugs,andbiologicaldrugsareamongthose mostfavored.30
Conclusion
Thestudiespublishedinrecentyearsshowimprovedgrowth andheightratesinJIApatientstreatedwithGH.
Somevariationintheresponsetotreatmentwasobserved; insomecases,recoveryingrowth takesplace,andinother cases the treatment prevents the loss of height generally observedduringthenaturalcourseofthedisease.The selec-tion oftheresponsedependsontheinitiationoftreatment withGHandtheondiseaseseverityandactivity.Thestudies presentedinthisreviewaresingular,concerningthestudied factors(positiveandnegativeeffects)andcannotbe general-ized.Thecombinationofgrowthhormonewithotherdrugs maybeanoption.
Funding
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. ThierryS,FautrelB,LemelleI,GuilleminF.Prevalenceand incidenceofjuvenileidiopathicarthritis:asystematicreview. JointBoneSpine.2014;81:112–7.
2. Tugal-TutkunI,QuartierP,BodaghiB.Diseaseoftheyear: juvenileidiopathicarthritis-associateduveitis–classification anddiagnosticapproach.OculImmunolInflamm.
2014;22:56–63.
3. PettyRE,SouthwoodTR,MannersP,BaumJ,GlassDN, GoldenbergJ.InternationalLeagueofAssociationsfor Rheumatologyclassificationofjuvenileidiopathicarthritis: secondrevision,Edmonton,2001.JRheumatol.2004;31: 390–2.
4. PettyR,SouthwoodT,BaumJ,BhettayE,GlassDN,MannersP, etal.Revisionoftheproposedclassificationcriteriafor juvenileidiopathicarthritis:Durban1997.JRheumatol. 1998;25:1991–4.
5. SimonD,LucidarmeN,PrieurAM,RuizJC.Czernichowlinear growthinchildrensufferingfromjuvenileidiopathicarthritis requiringsteroidtherapy:naturalhistoryandeffectsof growthhormonetreatmentonlineargrowth.JPediatr EndocrinolMetab.2001;6:1483–6.
6. MacraeVE,WongSC,FarquharsonC,AhmedSF.Cytokine actionsingrowthdisordersassociatedwithpediatricchronic inflammatorydiseases(review).IntJMolMed.2006;18: 1011–8.
7. vonSchevenE,CorbinKJ,StagiS,CimazR. Glucocorticoid-associatedosteoporosisinchronic inflammatorydiseases:epidemiology,mechanisms, diagnosis,andtreatment.CurrOsteoporosRep. 2014;12:289–99.
8. StratakisCA.Cortisolandgrowthhormone:clinical implicationsofacomplex,dynamicrelationship.Pediatr EndocrinolRev.2006;3:333–8.
9. KimJY,KimHS,ParkSH.Diseaseflareafter7year-remission ofsystemictypejuvenileidiopathicarthritis:isgrowth hormonetherapyaculpritorinnocentbystander.IntJ Rheum.2015;18:377–8.
10.NikishinaIP,RodionovskayaSR,ShapovalenkoAN,Filippova LY,JostarevaOM.Anexperienceofsequentaluseofthree biologicsandrecombinantgrowthhormoneinapatientwith juvenileidiopathicarthritisassociatedwithunsuccessful outcomeofhipdamage.PediatrRheumatol.2011;9: 143.
11.BechtoldS,BeyerleinA,RippergerP,RoebJ,DallaPR,Hafner R,etal.Totalpubertalgrowthinpatientswithjuvenile idiopathicarthritistreatedwithgrowthhormone:analysisof asinglecenter.GrowthHormIGFRes.2012;22:180–5. 12.BechtoldS,RippergerP,DallaPR,RothJ,HafnerR,MichelsH,
etal.Dynamicsofbodycompositionandboneinpatients withjuvenileidiopathicarthritistreatedwithgrowth hormone.JClinEndocrinolMetab.2010;95:178–85.
13.BechtoldS,RippergerP,DallaPR,BonfigW,HafneerR,Michels H.Growthhormoneincreasesfinalheightinpatientswith juvenileidiopathicarthritis:datafromarandomized controlledstudy.JClinEndocrinolMetab.2007;92: 3013–8.
14.SimonD,PrieurAM,QuartierP,CharlesRuizJ,CzernichowP. Earlyrecombinanthumangrowthhormonetreatmentin glucocorticoid-treatedchildrenwithjuvenileidiopathic
arthritis:a3-yearrandomizedstudy.JClinEndocrinolMetab. 2007;92:2567–73.
15.BechtoldS,RippergerP,BonfigW,PozzaRD,HaefnerR, SchwarzHP.Growthhormonechangesbonegeometryand bodycompositioninpatientswithjuvenileidiopathic arthritisrequiringglucocorticoidtreatment:acontrolled studyusingperipheralquantitativecomputedtomography.J ClinEndocrinolMetab.2005;90:3168–73.
16.SahaMT,HaapasaariJ,HannulaS,SarnaS,LenkoHL.Growth hormoneiseffectiveinthetreatmentofseveregrowth retardationinchildrenwithjuvenilechronicarthritis.Double blindplacebo-controlledfollowupstudy.JRheumatol. 2004;31:1413–7.
17.BechtoldS,RippergerP,BonfigW,SchmidtH,BitterlingH, HafnerR,etal.Bonemassdevelopmentandbonemetabolism injuvenileidiopathicarthritis.JRheumatol.2004;31:
1407–12.
18.BechtoldS,RippergerP,HäfnerR,SaidE,SchwarzHP.Growth hormoneimprovesheightinpatientswithjuvenileidiopathic arthritis:4-yeardataofacontrolledstudy.JPediatr.
2003;143:512–9.
19.SimonD,LucidarmeN,PrieurAM,RuizJC,CzernichowP. Effectsongrowthandbodycompositionofgrowthhormone treatmentinchildrenwithjuvenileidiopathicarthritis requiringsteroidtherapy.JRheumatol.2003;30: 2492–9.
20.Al-MutairA,BahabriS,Al-MayoufS,Al-AshwalA.Efficacyof recombinanthumangrowthhormoneinchildrenwith juvenilerheumatoidarthritisandgrowthfailure.JPediatr EndocrinolMetab.2000;13:899–905.
21.TouatiG,RuizJC,PorquetD,KindermansC,PrieurAM, CzernichowP.Effectsonbonemetabolismofoneyear recombinanthumangrowthhormoneadministrationto childrenwithjuvenilechronicarthritisundergoingchronic steroidtherapy.JRheumatol.2000;27:1287–93.
22.RooneyM,DaviesUM,ReeveJ,PreeceM,AnsellBM,WooPM. Bonemineralcontentandbonemineralmetabolism:changes aftergrowthhormonetreatmentinjuvenilechronicarthritis. JRheumatol.2000;27:1073–81.
23.SimonD,PrieurA,CzernichowP.Treatmentofjuvenile rheumatoidarthritiswithgrowthhormone.HormRes. 2000;53:82–6.
24.SimonD,TouatiG,PrieurAM,RuizJC,CzernichowP.Growth hormonetreatmentofshortstatureandmetabolic
dysfunctioninjuvenilechronicarthritis.ActaPaediatrSuppl. 1999;88:100–5.
25.TouatiG,PrieurAM,RuizJC,NoelM,CzernichowP.Beneficial effectsofone-yeargrowthhormoneadministrationto childrenwithjuvenilechronicarthritisonchronicsteroid therapy.I.Effectsongrowthvelocityandbodycomposition.J ClinEndocrinolMetab.1998;83:403–9.
26.DensonLA.Growthhormonetherapyinchildrenand adolescents:pharmacokinetic/pharmacodynamic
considerationsandemergingindications.ExpertOpinDrug MetabToxicol.2008;4:1569–80.
27.PunT,ChandurkarV.Growthhormonedeficiency,short stature,andjuvenilerheumatoidarthritisinapatientwith autoimmunepolyglandularsyndrometype1:casereportand briefreviewoftheliterature.ISRNEndocrinol.
2011;2011:62759.
28.BismuthEA,ChevenneDB,CzernichowPA,SimonDA. Moderatedeteriorationinglucosetoleranceduringhigh-dose growthhormonetherapyinglucocorticoid-treatedpatients withjuvenileidiopathicarthritis.HormResPaediatr. 2010;73:465–72.
106
rev bras reumatol.2017;57(2):100–10630.DeBenedettiF,BrunnerH,RupertoN,SchneiderR,XavierR, AllenR,etal.PaediatricRheumatologyInternationalTrials OrganisationandthePediatricRheumatologyCollaborative StudyGroup.ArthritisRheumatol.2015;67:840–8.
31.HarrisJD,QuatmanCE,ManringMM,SistonRA,FlaniganDC. Howtowriteasystematicreview.AmJSportsMed.
2014;42:2761.
32.ZakM,MullerJ,KarupPF.Finalheight,armspan,subischial leglength,andbodyproportionsinjuvenilechronicarthritis. Along-termfollow-upstudy.HormRes.1999;52:80–5. 33.StratakisCA.Cortisolandgrowthhormone:clinical