RevistaBrasileiradeFarmacognosia25(2015)353–355
w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
New
lycosinine
derivative
from
Hippeastrum
breviflorum
Camila
Sebben
a,
Raquel
Brandt
Giordani
a,b,
Jean
Paulo
de
Andrade
a,c,
Strahil
Berkov
d,
Edison
Javier
Osorio
c,
Marcos
Sobral
a,
Mauro
Vieira
de
Almeida
e,
Amélia
Teresinha
Henriques
a,
Jaume
Bastida
c,
José
Ângelo
Silveira
Zuanazzi
a,∗aProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,FaculdadedeFarmácia,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil bProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,DepartamentodeFarmácia,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil cDepartmentofNaturalProducts,PlantBiologyandSoilScience,FacultyofPharmacy,UniversityofBarcelona,Barcelona,Spain
dAgroBioInstitute,Sofia,Bulgaria
eDepartamentodeQuímica,UniversidadeFederaldeJuizdeFora,CampusMartelos,JuizdeFora,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received29May2015 Accepted29June2015 Availableonline26July2015
Keywords:
Hippeastrumbreviflorum Amaryllidaceae Alkaloids Lycorine
9-O-demethyllycosinineB LycosinineB
a
b
s
t
r
a
c
t
Anewlycosininederivative,9-O-demethyllycosinineB,wasisolatedfromthenativeBrazilian Hippeas-trumbreviflorumHerb.,Amaryllidaceae,alongwiththewell-knownalkaloidslycosinineBandlycorine. Thestructureofthenew compoundwas establishedbyphysical andspectroscopicmethods.9-O -demethyllycosinineBisthethirdlycosininevariantidentifiedintheAmaryllidaceaefamily.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The Amaryllidaceaefamily is one of the20 mostimportant alkaloid-containingplantfamilies(Cordell,2001).Amaryllidaceae speciesareabletosynthesizespecificisoquinolinealkaloidsofeight typicalskeletontypes,whichhavedemonstratedawiderangeof biologicalactivitiesincludingantitumoral,antiviral,antiparasitic, andacetylcholinesteraseinhibitoryactivity,amongothers(Bastida etal.,2006;Berkovetal.,2008;Giordanietal.,2011a;McNultyetal.,
2007).HippeastrumisanendemicAmaryllidaceaeAmericangenus
distributedfromMexicotoSouthernBrazilandArgentina(Dutilh etal.,2013).Althoughmanychemicalandbiologicalevaluationsof alkaloidsisolatedfromBrazilianAmaryllidaceaeplantshavebeen carriedoutsince1997,mostoftheBrazilianHippeastrumspecies arestillunexplored.
Among the alkaloids foundin Hippeastrum species, lycorine (1) and montanine are the most studied. Lycorine is a well-known antitumoral agent (Bastida et al., 2006; Ghosal et al., 1985) and has been successively isolated from H. glaucescens,
∗ Correspondingauthor.
E-mail:zuanazzi@ufrgs.br(J.Â.S.Zuanazzi).
H. striatum, H. vittatum and H. santacatarina (da Silva, 2005; da Silva et al., 2006; Giordaniet al., 2011a; Hoffman Jr.et al., 2003). Recently, lycorine has shown to be active against the amitochondriate Trichomonas vaginalis Donné through a new paraptotic mechanism, which prompted the semi-synthesis of novelesterifiedderivatives(Giordanietal.,2011a, 2012). Mon-tanine wasisolated inan appreciableamountfromH. vittatum
and showed remarkable cytostatic and psychopharmacological effects (da Silva et al., 2006, 2008), along with the ability to inhibittheacetylcholinesteraseenzymeandincreasetheprotein phosphorylationoftheMAPKsignalingpathway,whosecascade of reactions is strongly related with the memory process (da Silva, 2005; Pagliosa et al., 2010). Very recently, six Amaryll-idaceae alkaloids were characterized from Hippeastrum species forthefirsttime(deAndradeetal.,2011,2014;Giordanietal., 2011b).
Hereinisreportedthecompletespectroscopicdataofa new lycosininederivative,9-O-demethyllycosinineB(2),from
Hippeas-trum breviflorum Herbert. Lycosinine derivatives mayrepresent
anewkindofskeleton-typeofAmaryllidaceaealkaloids(Ünver, 2007).The completephytochemicalprocedureinH.breviflorum
also afforded the purification of the alkaloids lycorine (1) and lycosinineB(3).
http://dx.doi.org/10.1016/j.bjp.2015.06.005
354 C.Sebbenetal./RevistaBrasileiradeFarmacognosia25(2015)353–355
Materialsandmethods
Generalexperimentalprocedures
Ultraviolet(UV)spectraweredeterminedinMeOHonan 8452-A Hewlett Packard UV-Vis spectrophotometer. 1H NMR, DEPT, HMQC,HMBCandNOESYspectrawererecordedinCD3OD,ona Varian500spectrometer.Chemicalshiftsarereportedinunitsofı (ppm)andcouplingconstants(J)inHz.EI-MSat70eV.HR-ESI-MS spectrawereobtainedonanLC/MSD-TOF(2006)Mass spectrom-eter(Agilent technologies). Analytical and preparative TLCwas performedonsilicagel platesandthespots onchromatograms werevisualizedbyexposureunderUVlight(254nm)andby Dra-gendorff’sreagent.SilicagelMerck60(70–230mesh)wasusedfor CCandVLC.
Plantmaterial
HippeastrumbreviflorumHerb.,Amaryllidaceae,wascollected
inOctober2012(floweringstage)inSãoFranciscodePaula,inthe BrazilianstateofRioGrandedoSul.Sampleswereidentifiedby JulieDutilh(Unicamp).Avoucherspecimenhasbeendepositedat ICNHerbarium/UFRGSunderthereferencenumber123123.
Extraction,purificationandidentificationofthecompounds
Thefreshbulbs(3.8kg)werecrushedandmaceratedseparately withEtOHfor48h,threetimes.Thecombinedextractswere con-centratedunderreducedpressure.Theresiduewasacidifiedwith dilutedhydrochloricacid(10%)andwashedwithpetroleumether (4×250ml) and then CH2Cl2 (3×250ml). Both fractions were
negativeforalkaloids.ThesolutionwasbasifiedtopH8–9with aqueousNH4OH (25%)and extracted usingCH2Cl2 (8×250ml).
TheCH2Cl2 fractionfrombulbs(2.15g)wassubjectedtoVLCon silicagel(Kieselgel–mesh0.15/0.30,Val-de-Reuil,France)by gra-dientelutionwithCH2Cl2:MeOH(100:0–A,90:10–B,80:20– Cand50:50–D).FractionC(0.39g)wasrechromatographedby centrifugalthinlayerchromatographyusingCH2Cl2asthe start-ingsolvent,graduallyenrichedwithMeOH(0–50%)yielding 50 fractions(200mleach).ThefractionsweremonitoredbyTLCand 9-O-demethyllycosinineB(2,47.1mg)precipitatedspontaneously fromfraction7.Lycorine(1,4.0mg)alsoprecipitatedfromfraction 20.FractionD(0.2g)waschromatographedbyCCstartingwith CH2Cl2 andgraduallyenrichedwithMeOH (0–50%).After semi-preparativeTLC,lycosinineB(3,16.2mg)wasisolated.Theknown compoundswereidentifiedbycomparingoftheirphysicaldata(IR, 1HNMR,13CNMRandMS)withreferencesamples.
Resultsanddiscussion
ThealkaloidcontentofH.breviflorumhasbeenpreviously eval-uatedthroughaGC–MSapproachandtenalkaloidswereidentified (deAndradeet al.,2015).TheGC–MSanalysisalsodetectedan undefinedalkaloid,whichafterpurificationin thepresentwork
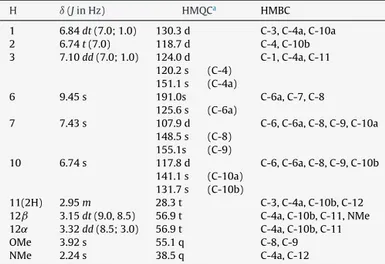
Table1
1HNMR,HMQCandHMBCofcompound1.
H ı(JinHz) HMQCa HMBC
1 6.84dt(7.0;1.0) 130.3d C-3,C-4a,C-10a
2 6.74t(7.0) 118.7d C-4,C-10b
3 7.10dd(7.0;1.0) 124.0d C-1,C-4a,C-11 120.2s (C-4)
151.1s (C-4a)
6 9.45s 191.0s C-6a,C-7,C-8
125.6s (C-6a)
7 7.43s 107.9d C-6,C-6a,C-8,C-9,C-10a 148.5s (C-8)
155.1s (C-9)
10 6.74s 117.8d C-6,C-6a,C-8,C-9,C-10b 141.1s (C-10a)
131.7s (C-10b)
11(2H) 2.95m 28.3t C-3,C-4a,C-10b,C-12 12ˇ 3.15dt(9.0,8.5) 56.9t C-4a,C-10b,C-11,NMe 12˛ 3.32dd(8.5;3.0) 56.9t C-4a,C-10b,C-11
OMe 3.92s 55.1q C-8,C-9
NMe 2.24s 38.5q C-4a,C-12
aMultiplicitiesdeterminedbyDEPTexperiment.
wasisolatedasthenewcompound9-O-demethyllycosinineB(2). ItsHRESIMS gave a massof 284.1295, which is correct forthe molecularformulaC17H18NO3andinagreementwiththe theoret-icalmass(284.1281)oftheparent[M+H]+ion.The1HNMRdata for 9-O-demethyllycosinine Bwerevery similartothose previ-ouslyreportedforlycosinineB(3),thepresenceofonearomatic methoxylgroupbeingtheonlyascribabledifferenceobserved.An evidentNOESYcorrelationbetweenthemethoxylgroupandH-7 (ı7.43)confirmedthe8-OMegroup.HMBCcorrelationbetween thealdehydiccarbonylgroupatC-6(ı191.0)and H-7wasalso observed,confirminganonfusedindolderivative.Theremaining signalswereinagreementwithlycosinineB(3)andtheNMRdata for9-O-demethyllycosinineB(2)areshowninTable1.
LycosinineAand B, previouslypurified fromChineseLycoris
aurea (L’Héritier) Herbert, are the only representatives of the
lycosinineseries(Yangetal.,2005)andhavebeenconsidereda newskeleton-typeamongtheAmaryllidaceaealkaloids,aswellas galanthindolederivatives(Ünver,2007).Abiosyntheticroutehas beenproposedforlycosininecompounds(Yangetal.,2005).The
9-O-demethyllycosinineB(2)isthethirdalkaloidfromthelycosinine seriestobeisolatedandourchemicalstudiesonAmaryllidaceae speciesshouldproveusefulinobtainingabetterunderstandingof thebiosynthetic,chemicalandbiologicalaspectsofthesebioactive compounds.
9-O-demethyllycosinineB(2):Amorphoussolid;mp138–140◦;
UV(MeOH)maxnm(logε):243(3.56)and295(3.70);1Hand13C NMR:Table1;EI-MSm/z(rel.int.):283[M]+(100),267(13);254 (54),240(45),222(33),194(22).HR-ESI-MS[M+H]+m/z284.1295 (C17H18NO3,calcd:284.1281).
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Author’scontribution
C.Sebbenetal./RevistaBrasileiradeFarmacognosia25(2015)353–355 355
Acknowledgments
ThisinvestigationwassupportedbygrantsofCAPES,FAPERGS CNPq (Brazil). JPA the Agencia Espa˜nola de Cooperación Inter-nacional para el Desarollo (BECASMAEC-AECID) for a doctoral fellowship.JASZandATHaregratefultoCNPqforresearcher fel-lowships.
References
Bastida,J.,Lavilla,R.,Viladomat,F.,2006.ChemicalandbiologicalaspectsofNarcissus
alkaloids.In:Cordell,G.A.(Ed.),TheAlkaloids,vol.63.ElsevierInc.,Amsterdam, pp.87–179.
Berkov,S.,Codina,C.,Viladomat,F.,Bastida,J.,2008.N-alkylatedgalathamine deriva-tives:potentacetylcholinesteraseinhibitorsfromLeucojumaestivum.Bioorg. Med.Chem.Lett.18,2263–2266.
Cordell,G.A.,2001.Thepotentialofalkaloidsindrugdiscovery.Phytother.Res.15, 183–205.
daSilva,A.F.S.,(Ph.D.thesis)2005.EstudoQuímicoeBiológicodeHippeastrum vit-tatum(LHér.)HerberteHippeastrumstriatum(Lam.)Moore(Amaryllidaceae). ProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederal doRioGrandedoSul,PortoAlegre,pp.119.
da Silva,A.F.S., Andrade, J.P.,Bevilaqua, L.R.M.,De Souza, M.M.,Izquierdo,I., Henriques, A.T., Zuanazzi, J., 2006. Anxiolitic-, antidepressant- and anti-convulsant-likeeffectsofthealkaloidmontanineisolatedfromHippeastrum vittatum.Pharmacol.Biochem.Behav.85,148–154.
daSilva,A.F.S.,Andrade,J.P.,Machado,K.R.B.,Rocha,A.B.,Apel,M.A.,Sobral,M., Henriques,A.T.,Zuanazzi,J.A.S.,2008.Screeningforcytotoxicactivityofextracts andisolatedalkaloidsfrombulbsofHippeastrumvittatum.Phytomedicine15, 882–885.
deAndrade,J.P.,Berkov,S.,Viladomat,F.,Codina,C.,Zuanazzi,J.A.S.,Bastida,J.,2011. AlkaloidsfromHippeastrumpapilio.Molecules16,7097–7104.
deAndrade,J.P.,Guo,Y.,Font-Bardia,M.,Calvet,T.,Dutilh,J.,Viladomat,F., Cod-ina,C.,Nair,J.J.,Zuanazzi,J.A.S.,Bastida,J.,2014.Crinine-typealkaloidsfrom
HippeastrumaulicumandH.calyptratum.Phytochemistry103,188–195. deAndrade,J.P.,Giordani,R.B.,Torras-Claveria,L.,Pigni,N.B.,Berkov,S.,Font-Bardia,
M.,Calvet,T.,Konrath,E.,Bueno,K.,Sachett,L.G.,Dutilh,J.H.,Borges,W.S., Vilado-mat,F.,Henriques,A.T.,Nair,J.J.,Zuanazzi,J.A.S.,Bastida,J.,2015.TheBrazilian Amaryllidaceaeasasourceofacetylcholinesteraseinhibitoryalkaloids. Phy-tochem.Rev.,http://dx.doi.org/10.1007/s11101-015-9411-7.
Dutilh,J.H.,Fernandez,E.P.,Penedo,T.S.A.,deMoraes,M.M.V.,Messina,T.,2013. Amaryllidaceae.In:Martinelli,G.,Moraes,M.A.(Eds.),LivroVermelhodaFlora doBrasil.CNCFLORA,RiodeJaneiro,pp.126–139.
Ghosal,S.,Saini,K.S.,Razdan,S.,1985.Crinumalkaloids:theirchemistryandbiology. Phytochemistry24,2141–2156.
Giordani,R.B.,Vieira,P.B.,Weizenmann,M.,Rosember,D.B.,Souza,A.P.,Bonorino,C., deCarli,G.A.,Bogo,M.R.,Zuanazzi,J.A.S.,Tasca,T.,2011a.Lycorineinducescell deathintheamitochondriateparasite,Trichomonasvaginalis,viaanalternative non-apoptoticdeathpathway.Phytochemistry72,645–650.
Giordani,R.B.,deAndrade,J.P.,Verli,H.,Dutilh,J.H.,Henriques,A.T.,Berkov,S., Bastida,J.,Zuanazzi,J.A.S.,2011b.AlkaloidsfromHippeastrummorelianumLem. (Amaryllidaceae).Magn.Reson.Chem.49,668–672.
Giordani,R.B.,RezendeJunior,C.O.,Andrade,J.P.,Bastida,J.,Zuanazzi,J.A.S.,Tasca, T.,DeAlmeida,M.V.,2012.LycorinederivativesagainstTrichomonasvaginalis. Chem.Biol.DrugDes.80,129–133.
HofmannJr.,A.E.,Sebben,C.,Sobral,M.,Dutilh,J.,Henriques,A.T.,Zuanazzi,J.A.S., 2003.AlkaloidsofHippeatrumglaucescens.Biochem.Syst.Ecol.31,1455–1456. McNulty,J.,Nair,J.J.,Codina,C.,Bastida,J.,Pandey,S.,Gerasimoff,J.,Griffin,C.,2007. Selectiveapoptosis-inducingactivityofcrinum-typeAmaryllidaceaealkaloids. Phytochemistry68,1068–1074.
Pagliosa,L.B.,Monteiro,S.,Andrade,J.P.,Dutilh,J.,Bastida,J.,Cammarota,M., Zua-nazzi,J.A.S.,2010.EffectofisoquinolinealkaloidsfromtwoHippeastrumspecies oninvitroacetylcholinesteraseactivity.Phytomedicine17,698–701. Ünver,N.,2007.NewskeletonsandnewconceptsinAmaryllidaceaealkaloids.
Phy-tochem.Rev.6,125–135.