Plasma-deposited a-C(N):H Films
D.F. Franeshini
InstitutodeFsia,Universidade FederalFluminense
AvenidaLitoraneas/n,Niteroi,R.J.,24210-340,Brazil
Reeived15February,2000;reeivedinnalformon22Marh,2000
Thegrowth behaviour, lmstruture and mehanial properties of plasma-deposited amorphous
hydrogenated arbon-nitrogen lms are shortly reviewed. The eet of nitrogen-ontaining gas
additiontothedepositiontothehydroarbonatmospheresusedisdisussed,onsideringthe
mod-iations observed inthe hemial omposition growth kinetis, arbon atom hybridisation and
hemialbondingarrangementsofa-C(N):Hlms. Theoverallstruturebehaviourisorrelatedto
thevariationofthemehanialproperties.
I Introdution
In the last ten years there has been a great interest
in the study of plasma-deposited amorphous
hydro-genated arbon-nitrogen (a-C(N):H) lms. The main
reason for the initial interest was the suggestion, by
LiuandCohen[1℄,thatthehypothetialompound
-C
3 N
4
wouldhavemehanialpropertiessimilartothat
of rystalline diamond. Sine that suggestion, muh
work has been done on the searh for arbon nitride
synthesis, eortthat wasreentlyreviewed[2,3℄. The
aimoftheworkonplasma-depositeda-C(N):Hwas
ob-viously not to produe rystalline arbon nitride, but
to study thesupposedbeneial eetsof nitrogen
in-orporation on the mehanial, eletrial and optial
propertiesofa-C:Hlms.
Theresearhona-C(N):H lmsstartedevenbefore
LiuandCohen'ssuggestion,withthestudyofnitrogen
eletroni dopingofa-C:HlmsreportedbyJonesand
Stewart[4℄. Afterthat,followedthepioneeringworksof
Han andFeldman [5℄andAmirandKalish[6℄,mainly
onsidering optial and eletrial properties, and the
work by Kaufman et al [7℄ on Infrared Spetrosopy
of a-C(N):H lms, whih stated important strutural
eets of nitrogen inorporation into amorphous
hy-drogenatedarbonlms. Furtherresearhontheeld
showedthatsomeaspetsofthenitrogeninorporation
proessin a-C:H lms plaeobstalesto the
ahieve-mentoftheexpetedbenets. Therstoneisthe
lim-ited nitrogen uptake observed in a-C(N):H lms. No
more than about 20 at.% N ould be ahieved up to
now in plasma deposited a-C(N):H lms. Theseond
oneisthatnitrogeninorporationresultsinastrong
de-reaseinthesp 3
arbonatomfration[8℄,whihisthe
mainresponsiblefora-C:Hlmrigidity. Andnallythe
preferentialbondingofhydrogenatomstothenitrogen
atoms, whihmakesdiÆultarbon-nitrogenextended
networkformation,andaddsterminatinggroupstothe
amorphousnetwork.
Despite suh limitations, plasma-deposited
a-C(N):H lms were found to be used in a number of
appliations. The stress redution indued by
nitro-geninorporation[9℄andonsequentadhesion
improve-ment,allowedthedevelopmentof a-C(N):H
antiree-tiveoatingsforGe-basedinfrareddetetors[10℄. Itwas
foundalsothatnitrogenaneletroniallydopea-C:H
lms,andstronglydereasethedefetdensity,enabling
itsuse asasemiondutor material [11℄. Nitrogen
in-orporation was found also to derease the threshold
eletrieldineletron-eldemissionproess[12℄,
mak-ingpossibletheuseofa-C(N):Hlmsasaoveroaton
emissiontipsinat-paneldisplaydevies[13℄.
Inasimpleway, harda-C:Hlmstruture maybe
pituredbyanover-onstrainedrandomnetwork
om-posedbysp 3
andsp 2
-hybridisedarbonatomsand
hy-drogenatoms. Thismeansthatthemeanoordination
number of the atoms forming the network is greater
than the ideal geometrial value [14℄. This high
de-gree of overonstraining in a-C:H lms is due to the
preseneofalargefrationof sp 3
atoms,highly
ross-linked,whihresultsintheobservedrigidity,aswellas
inthedevelopmentofahighinternalompressivestress
duetohemialbonddistortion. So,in thestudyof
a-C(N):Hlms struturespeial attention must be paid
to the struture hanges that aet the network
on-netivity, suh as hemial omposition, arbon atom
hybridisation,andhemialbondingsheme.
The proesses involved in plasma deposition of
amorphous hydrogenated arbon lms are quite
om-plex. Besides the hydroarbon plasma hemistry
de-tails, eah dierent speies from the plasma interat
withthegrowinglayerinadierentway[15℄. Atrst,
wehavethepositivelyhargedions,mainlythe
arbon-arryingones,that areextratedfrom theplasmaand
aeleratedtowardsthesubstratebythenegatively
bi-ased substrateeletrode. Theyare theresponsible, by
energy deposition, forthe ativation of C-Cbond
for-mation, among other onsequenes. Carbon-arrying
slow neutralradials ontributemanly to lm growth
itself, bystiking to dangling bonds at thelm
grow-ing surfae. Hydrogen fastions andslowneutralsan
be involved in dangling bond reation or saturation,
among other proesses. Nitrogen addition to the
de-position atmosphere may, besidesaltering the plasma
hemistry, also alter the surfaeproess ating in the
growinglayer,aswillbedisussedin thispaper.
The aim of the present work is to shortly review
aspets onerned to the nitrogen inorporation
pro-ess,lmgrowthkinetis,andmodiationofa-C(N):H
lmstrutureandmehanialproperties. Noreferene
will be made on eletrial or optial properties of
a-C(N):Hlms. Furtherrefereneonthissubjetmaybe
found in a number of papers [4,5,6,11,16,17℄. In
se-tionII aredisussed resultsonthe eets of
nitrogen-ontaininggas addition to thedeposition atmosphere,
on lm hemial omposition and growthkinetis. In
setionII,Themain struturalhangesobservedin
a-C(N):Hlms,asdeterminedbyseveralharaterisation
tehniques,aredisussedinsetionIII.Inaddition,the
struturehangesare relatedto theobserved
mehan-ialproperties. SetionIII summarisestheoverall
dis-ussion.
II Chemial Composition and
Growth Kinetis
Several plasma deposition methods were used in the
studyofa-C(N):Hlms. Mostoftheworkwasdoneon
lmsdepositedbyonventionalradio-frequenyPlasma
Enhaned Chemial Vapour Deposition (rf-PECVD)
[18℄, in a hydroarbon / nitrogen-ontaining gaseous
mixture,withthesubstrateplaedontherf-biased
ele-trode [19-22℄. Inthis method theapaitiveoupling
of therfpowerto theathode allowsthedevelopment
of an averaged-in-time DC negative bias at the
pow-eredeletrode,knownasself-biaspotential(V
B ). The
potentialV
B
extrats the ions in the plasma, and
a-eleratethem towardsthelmgrowingsurfae. There
were somereportsonlmsdepositedbytheDC
glow-disharge method [23℄, and also a variation of the rf
ow-disharge, in whih the substrate is plaed in a
negativelyDC-biasedeletrodeparallelandopposedto
therf-poweredone[7℄.
In order to ahieve higher plasma ionisation and
dissoiation, some authors used high density plasma
soures to study a-C(N):H lms. The Eletron
Cy-lotron Resonane- Mirowave (ECR-MW) plasma
soure[24,25℄,andthehelialresonator plasmasoure
[26℄ were used to generate a high density plasma,
to ether with rf-biasing of the substrate eletrode, in
ordertoextrat theionsfrom theplasma. Avariation
oftherf-glow-dishargemethod,inwhihasteady
mag-neti eld is perpendiularly imposed to the rf-biased
eletrode surfae, was used to deposit a-C(N):H lms
with a gaseous mixture largelydiluted in helium [11℄.
Prodution of a-C(N):H lms was also performed by
using highly ionised plasma soures [27℄ or ion beam
soures[28℄,inordertodepositorassistthedeposition
proess . Theeets ofnitrogen-ontaininggasonthe
hemialompositionoflmsdepositedbyseveral
teh-niques, and preursor atmospheres may be viewed on
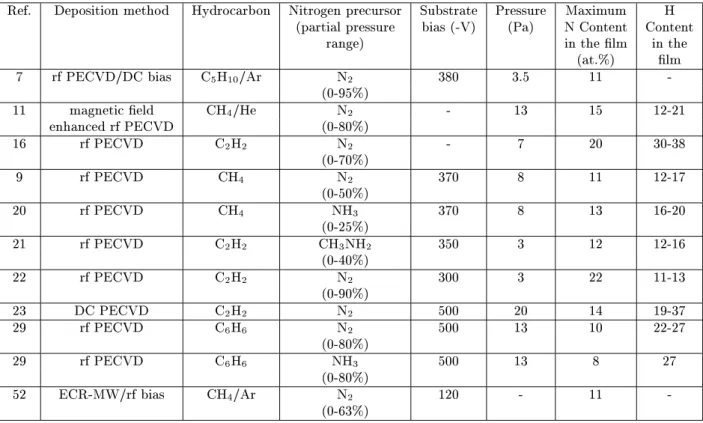
Table1. Inthistablearedisplayedthemaximum
nitro-gen ontent and thehydrogen ontentrange obtained
for the whole variation of the nitrogen-ontaining gas
partial pressure. Foreasieromparison of the results,
the table also displays the deposition tehnique used
andthemaindepositionparameters.
Asshownintable1,themaximumnitrogenuptakes
are about20 at %,and were obtainedusing aetylene
- nitrogen atmospheres. On the other hand, results
using other gasmixtures hardlyreah15 at. %
nitro-gen. Despite this dependene onthe preursorgas, it
seemsthatthemaximumnitrogenontentdoesnot
de-pendonthedepositiontehniqueused. Itisinteresting
to notealso that themaximumnitrogenuptakeis not
diretly orrelated to the nitrogen inorporationyield
of the partiular nitrogen-ontaininggases. It is
eas-ily seen by omparing results from referenes [9℄ and
[20℄inthetable,whihwereobtainedbyusingCH
4 -N
2
and CH
4 -NH
3
mixtures. Both works obtained about
thesamemaximumnitrogenuptakes,despitethegreat
dierenesinthenitrogenatomifrationin the
depo-sition atmosphere at the maximum nitrogen
inorpo-ration . Thus, proess other then hemial reativity
may drive the nitrogen inorporation. In whih
on-erns to the hydrogen ontent some authors report a
ontinuouslydereasingbehaviouruponnitrogen
inor-poration,whileothersreportarelativelyinsensitive
be-haviour,showing alsodierent levelsof hydrogen
on-tent.
Theobservedrelativelylowupperlimit for the
ni-trogenontentin a-C(N):H lmshasbeenasribedto
thestrongdereaseonthelmdepositionrateupon
ni-trogenpreursoradditiontothedepositionatmosphere,
asshownonFig.1. Inthisgureareshownplotsof
de-position rate againstthenitrogen ontent, normalised
to that of the nitrogen-free lm, for several preursor
gas mixtures [22℄. All theplots showa learderease
onthedepositionrateagainstnitrogenontent. Inthe
gure, the deposition rate plots for CH
4 -N
2 or NH
3 ,
and C
2 H
2 -CH
3 NH
2
gaseousmixturesseemto followa
very similar dependene with nitrogen ontent. This
ommon dependene showsan about 13 at. % N
ap-parentvanishingofdepositionrate. Ontheotherhand,
theplotorrespondingtotheC
2 H
2 -N
2
mixturefollows
alearly dierentdependene,showingthe deposition
ratefallathighernitrogenontents,allowingthe
Table1. Chemialompositionanddepositiondetailsfora-C(N):Hlmsdepositedbyseveraltehniques.
Ref. Depositionmethod Hydroarbon Nitrogenpreursor Substrate Pressure Maximum H
(partialpressure bias(-V) (Pa) NContent Content
range) inthelm inthe
(at.%) lm
7 rfPECVD/DCbias C5H10/Ar N2 380 3.5 11
-(0-95%)
11 magnetield CH
4
/He N
2
- 13 15 12-21
enhanedrfPECVD (0-80%)
16 rfPECVD C2H2 N2 - 7 20 30-38
(0-70%)
9 rfPECVD CH
4
N
2
370 8 11 12-17
(0-50%)
20 rfPECVD CH4 NH3 370 8 13 16-20
(0-25%)
21 rfPECVD C
2 H
2
CH
3 NH
2
350 3 12 12-16
(0-40%)
22 rfPECVD C2H2 N2 300 3 22 11-13
(0-90%)
23 DCPECVD C
2 H
2
N
2
500 20 14 19-37
29 rfPECVD C
6 H
6
N
2
500 13 10 22-27
(0-80%)
29 rfPECVD C6H6 NH3 500 13 8 27
(0-80%)
52 ECR-MW/rf bias CH
4
/Ar N
2
120 - 11
-(0-63%)
Figure1. Variationoftherelativedepositionrateasa
fun-tionofthenitrogenontentinthelm,forlmsdeposited
from CH
4 -N
2
[9℄, CH
4 -NH
3 [20℄, C
2 H
2 -CH
3 CH
2
[21℄, and
C
2 H
2 -N
2
mixtures[22℄.
Althoughdataonplasmahemistryof
hydroarbon-nolearonnetionis madewith thelmgrowth
pro-ess. Thehangesobservedintheoptialemission
spe-traofhydroarbon-N
2
mixturesaretheinreaseinthe
CNandN-derivedemissionlines,inparallelwitha
de-rease in the intensity of the CH emission lines [31℄.
Sinethesehangesannotbythemselvesbeorrelated
withthelmsurfaeproess, littleinformationanbe
extratedfromthiskindofanalysis.
Two fators were identied as being the ause for
the observed strong derease on the deposition rate
[22℄. The rst one is the erosion of arbon atoms by
energeti nitrogen ions like N
2
+ that omes from the
plasma. This eet wasstudied in detail by Hammer
andGissler[32℄. Theyfoundthatlow-energy(150eV)
N +
2
ion bombardment of a hydrogen-free amorphous
arbon lm resultedin arbon atom removal at rates
as high as 0.5 Catom perN +
2
ion, mainly as CN and
C
2 N
2
moleules. Thisproesswasidentiedasa
hem-ial sputtering proess, sine the observed sputtering
rateisfarhigherthanthatexpetedbyphysial
sput-teringproess. AsimilarproesswasobservedbyHong
andTurban [33℄ whenstudying the ething proess of
a-C:HlmsbyN
2
ECRplasmas. Theyfoundthat
rf-biased, previously grown a-C:H lms were eroded by
theN
2
plasma,beingevolvedin this proessthesame
kindoffragmentsastheobservedin N +
2
ion
bombard-mentofhydrogen-freearbonlms. Theseondfator
istheevaporationofN
2
andarbonatomsinorporationmayshowadisordered
nature in a-C(N):H lm growth, nitrogen evaporation
is verylikely to ourwhen the lm nitrogen ontent
inreases.
BothfatorsweretakenintoaountbyTodorovet
al[34℄inordertomodelion-beamdepositeda-CN
x lm
growth,for apartiular C/Nionarrivalratio. Inthis
work,besidesarbonatomhemialsputteringarbon
removal by N+ ions and N
2
evaporation, the authors
also inluded N atom sputtering by C +
ions, within
a Monte-Carlo simulation of the ollision proess
fol-lowing ion subsurfae penetration. With this model
theyouldtresultsofthehemialompositiondepth
prole,but no attemptwasmade tomodeldeposition
rates.
Asimplestatistialmodelfora-C(N):Hlmsgrowth
kinetis and nitrogen inorporation was reently
pro-posed [35℄. This model inorporates the main
ef-fets aused by the plasma nitrogenated speies on
the growth kinetis: The hemial sputtering of
ar-bonatomsbynitrogenions,andtheevaporationofN
2
moleules. This was ahievedby onsideringonly two
speiesarrivingatthelmsurfaewithomplimentary
probabilities: a fully aggregating \C" speies,
repre-senting arbon-arryingions and radials, and a \N"
speies, representing the N +
2
ions extrated from the
plasma. In themodel, when a\N" speies fall overa
\C" atomin thedeposit,the interation betweenand
inoming \N" speies and the deposit is desribed by
theinterationparameterq. Thisparameterrepresents
theprobabilityof aN atom to removeaCatom from
thelm,being(1 q)theprobabilityofaggregationof
the\N"speies. Whena\N"speiesfallsoveranother
a N atom, both leavethe deposit asan N
2
moleule.
N atom removalbya \C"speieswasnotonsidered,
sineformosta-C:Hlmdepositionproessesthemain
hannel for material aggregationat thelm surfaeis
thestikingofarbonarryingslowradials. Byusing
aninterationparameterqequalto0.25(veryloseto
thearbonremovalratereportedinreferene[19℄),this
modelwasfoundtotverywellthedepositionrate
re-sultsobtainedfromplasmadepositionusing
aetylene-nitrogen mixtures reported in referene [22℄. In
ad-dition, the obtained maximum nitrogen uptake as a
funtion of the interation parameter showed that no
morethanabout33at .%N ouldbe inorporatedin
a-C(N):H lms, even onsidering no hemial
sputter-ingbutonlyN
2
evaporation. Moreover,notouldbe
obtainedfordeposition rateexperimental resultsfrom
depositionatmospheresotherthanC
2 H
2 -N
2 .
Thelowernitrogenuptakeassoiatedwithasharper
dereaseonthedepositionrateuponnitrogenshownon
gure 1 (for the CH
4 -N
2 or NH
3 and C
2 H
2 -CH
3 NH
2
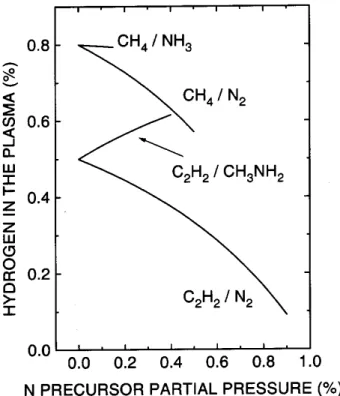
mixtures) was asribed to the role of hydrogen in the
deposition proess [22℄. Aording to Jaob [15℄, the
mainroleofhydrogenina-C:Hlmdepositionatroom
higher the hydrogen ontent in the deposition
atmo-sphere, lower would be the surfae sites available to
lm growth by slowradials. Fig. 2showsthe
hydro-gen atomifrationin thedepositionatmosphere, asa
funtion ofthenitrogenpreursorgaspartialpressure,
for the lm deposition experiments shown on Fig. 1.
The ranges shown orrespondto thenon-zero
deposi-tionraterange. Itis easytonote thatwhenusing the
C
2 H
2 -N
2
mixture,thedepositionatmospherehydrogen
frationisfarlowerthanthatshownbytheother
mix-tures. Furtherimprovementoftheabovedisussed
sta-tistial model for a-C(N):H lm growth inorporated
suh an eet, by bloking axed fration of surfae
sites to lm growth [36℄. With this modiation, the
modelwasableto t thetwokindsof deposition rate
urvesshownin Fig. 1.
Figure2. Hidrogenontentinthedepositionatmospheres,
forthegaseousmixturesindiatedintheplot,indenon-zero
depositionraterange.
III Film struture and
mehan-ial properties
As it was mentioned in the introdution, the main
struturalaspetonerningamorphousarbonlmsis
thearbonatomhybridisation. Thepreseneofalarge
frationofsp 3
arbonatomsina-C:Hlms,withahigh
ross-linkingof the amorphousnetwork,givesrise the
rigidityshownbythislms. So,therstonerninthe
state ofarbonatoms. Unfortunatelythere areonlya
fewreportsofsuhkindofanalysis.
Seth and Babu [37℄ reported a study on the
arbon sp 3
fration of a-C(N):H lms deposited
from butadiene-nitrogen and butadiene-ammonia
at-mospheres, as determined by 13
C-NMR spetrosopy.
They reported that nitrogen inorporation resulted
in an inreased C sp 3
fration for ammonia-derived
lms, whereasnot remarkablehanges were found for
nitrogen-derivedlms.
The above nding was not orroborated by later
studies. Table 2 summarises results on the
hybridi-sation state of arbon atoms in plasma deposited
a-C(N):Hlms. There areshownresultsobtainedbythe
omparison of theof the
and
peaksof the
CK-edgeEletronEnergyLoss(EELS)spetraoflms
deposited byrf-PECVD [8℄(alsoshownin Fig. 3)and
magnetially-enhanedrf-PECVD[11℄.Inaddition,are
also shown results obtained from the ombination of
the EELS spetra in the plasmon region and Auger
Eletron Spetra(AES) takenfrom lmsdeposited by
ECR-MWontorf-biasedsubstrates[24℄. Togetherwith
the sp 3
fration results,thedepositiondetails are also
shown.
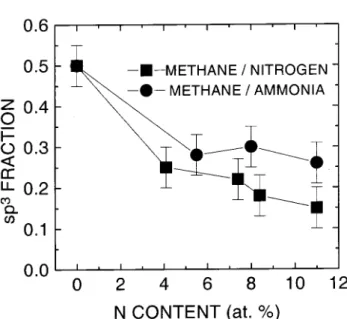
Figure3.VariationoftheCatomsp 3
frationasafuntion
of nitrogen ontent for lmsdeposited CH4-N2 and
CH4-NH3 mixtures(referene[8℄).
Table2. Thesp 3
arbonatomfrationvariationuponnitrogeninorporation.
Ref. Depositionmethod Maximumnitrogen sp 3
Catom
ontent(at%) frationrange(%)
8 rf-PECVD 11 50-10
CH4/NH2
8 rf-PECVD 11 50-25
CH
4 /NH
3
24 ECR-MW 4.6 41-15
CH
4 /N
2
11 Magnetially enhaned 15 35-20
PECVD
CH
4 /N
2 /He
Asdisplayedintable2nitrogeninorporationinall
ases results in aderease of the sp 3
C atom fration
upon nitrogeninorporation. Nosensitivityonthe
ni-trogen preursor gas was observed. Even the use of
a high density plasma soure ould not avoid thesp 3
fration derease. This strong derease in the arbon
sp 3
frationonthenitrogen ontentwasalsoobserved
uponnitrogenontentinhighlytetrahedralamorphous
arbon(t-a-C)lms[38℄.
Ramanspetrosopyangiveomplimentary
infor-mation aboutsp 2
arbonatom arrangementon
amor-phous arbonlms. As in a-C:H lms, the main
fea-tures of theRaman spetraof a-C(N):H lms are the
so-alled D and G bands present in graphiti arbon
materials. Information onsp 2
Catom strutureis get
from Ramanspetraby theanalysis of the integrated
band intensityratioI
D /I
G
,andthepeakpositionsand
lution by tting two gaussian lines to it. Mariotto
et al. [39℄ studied the Raman spetra of a-C(N):H
lmsdeposited byrf-PECVD in methane-nitrogen
at-mospheres. They found a ontinuous inrease of the
I
D /I
G
bandratiouponnitrogen inorporation,andan
inreaseof the G band peak position towardsthat of
rystallinegraphite. Thisbehaviourwasasribedtoan
inreasein thesizeornumberofthegraphiti lusters
presentin the lm,in aordanewith the
interpreta-tionstatedby Dillonand o-workers[40℄,in thestudy
of dierent kinds of amorphous arbons, heat-treated
at inreasing temperatures. Similar onlusions may
bedrawnbyomparisonto resultsreportedbyTamor
et al. [41℄, on the study of a-C(H) plasma-deposited
lmsat severalself-biasvoltages.
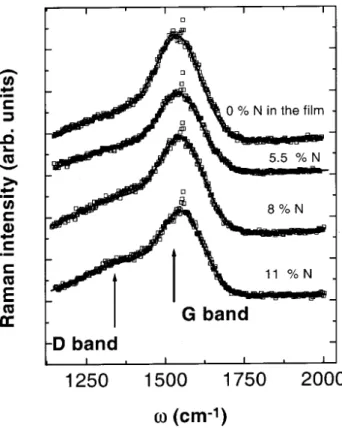
Fig. 4 shows a typial variation of Raman
show-methane-ammoniaatmospheres[20℄. Themain results
ofthetting proedure(I
D /I
G
ratio andthepeak
po-sitions !
G
of theG band) are displayed ontable 3as
funtions of the nitrogen atomi fration in the lm.
This table learly shows the inrease on I
D /I
G and
G
uponnitrogeninorporation. Ramanspetrataken
from a-C(N):H lms deposited by dierentdeposition
tehniquesandpreursorgasmixtures[42℄alwaysshow
thiskindofbehaviour.
Figure4. Ramanspetratakenfromlmsdeposited using
CH4-NH3 forseveralnitrogenontents(referene[20℄).
Table3. TheRamanbandintensityratioI
D /I
G andG
bandpeakpositionasfuntionsofthenitrogenontentin
thelm(Ref. 20).
Nitrogenontent(at%) ID/IG !G (m 1
)
8 0.73 1545
5.5 0.83 1549
7.9 0.98 1555
11 1.07 1557
IfweanalyseRamanSpetrosopyresultstogether
withtheabovearbonatomhybridisationstudies,one
an say that nitrogen inorporation into a-C:H lms
maygiverisetoaninreasingpreseneoflusteredSp 2
arbonatoms. Suh struturemodiationwouldgive
risetoadereaseintheamorphousnetwork
onnetiv-ity,assuggestedbyRobertson[43℄.
Infrared(IR)Spetrosopy has been used to probe
loal hemial bonds onguration in a-C(N):H lms.
lms are the C-H band, at about 2925 m 1
, the
N-H strething band, at about 3500 m 1
, the C N
strethingband,at aboutand abroadband plaedin
the 1400-1600m 1
wave-number range. By
ompar-ingspetratakenfrom 14
Nand 15
N-inorporateda-C:H
lms,Kaufmanetal. [7℄showedthethisbroadbandis
infatanIRobservationoftheIR-forbiddenRamanD
and Gbands. Thisis madepossiblebythe symmetry
breaking introduedby thepresene ofpolararbon
-nitrogenbondsinthelms. Thepreseneofthisband
learly shows that there are N atoms bonded to sp 2
arbonlustersina-C(N):H lms.
Fig. 5 shows a typial IR spetra evolution upon
nitrogen inorporation into a-C:H lms. The gure
shows, besides the ontinuous inreasein the Raman,
NH, andCNband intensities,aontinuousdereasein
the CH band intensity, whih almost vanishes for the
higher nitrogenontents, showingthat hydrogen
pref-erentiallybondstonitrogen. Asanumberofworkson
a-C(N):Hlmdepositionshowednosensitivevariation
of hydrogen ontent upon nitrogen inorporation, we
mayexpetthatmostoftheNatomswouldbepresent
asterminal bonds in the amorphousnetwork. This is
alsotheasefortheCN bonds. So,wemayexpet
that nitrogenatoms,atleastasagreatpart,mustnot
beontributingtothenetworkonnetivity.
Figure5. Infrared spetratakenfromlmsdeposited using
Furthersupportforthisonlusionisgivenbya
X-RayPhoto-EletronSpetrosopy(XPS) study on
hy-drogen inorporation into a-CN
x
sputtered lms [44℄.
The XPS results showed that hydrogen interrupts
C-N bond formation, and also evideniated preferential
hydrogenationofN atoms.
Thisis alsotheaseof the 13
C NMRspetrosopy
studyarriedoutbyLaMannaetal. [45℄ona-C(N):H
lms deposited by rf-PECVD on the grounded
ele-trode. Contrary to most NMR studies on amorphous
arbonlms,whihingeneralshowbroadbands
assoi-ated tosp 2
andsp 3
-hibridizedatoms, thisstudyfound
verythin linessuperimposedto thebroadones,whih
were assoiated to ordered strutures. Theyould t
the obtained spetrato strutures similar to nitrogen
ontaining fused aromati rings terminated by NH
2
groups. Suh a struture is very similar to sp 2
ar-bon lusters bonded to mostlyhydrogenated nitrogen
atoms.
The formation of network-terminating bonds as a
onsequene of nitrogen inorporationfound also
par-allel from other observations, not diretly related to
atomi oreletronistruture. Dopler-broadening
ob-servationofpositronannihilationina-C(N):Hlms[46℄
showedthat nitrogeninorporationinreasestheopen
volumefrationinthelm,whatwewouldexpetfrom
the breaking of network interonnetions. Hydrogen
thermaleusionstudiesshowed,forthesame
nitrogen-inorporatedlms, lowtemperature(about200 Æ
C)
ef-fusionpeaks. Astheompata-C:Hlmsusuallyshows
onlyhightemperature(about600 Æ
C)hydrogeneusion
peaks,thelowtemperaturepeaks presentina-C(N):H
lmswereassignedtothepreseneofopenvoidsinthe
lm[47℄.
Theinueneofnitrogeninorporationonthe
stru-ture modiation of amorphous arbon lms has also
beensubjetfortheoretialinvestigation. Although
al-most totallyfousedonhydrogen-freearbon-nitrogen
materials, those works may give some insight to the
disussionofhydrogen-ontaininglms.
Weih et al. [48℄ reported a moleular dynamis
simulation study on the formation of arbon-
nitro-gensolids. Theystudiedthenitrogeninorporationon
amorphous arbon within several density ranges, and
found that the presene of nitrogen indues the
on-tinuous inrease of the sp 2
C atom fration in all the
ases. Hu et al. [49℄ studied by semiempirial
meth-ods the nitrogen inorporation eets in arbon
lus-ters formed by diamond ells, and found that for N
ontents greater than about 12 at. % a transition to
the sp 2
stateis likelyto our. Both ndings at least
support the possibility that the sp 2
C atom inrease
observed in a-C(N):H lmsmay be due to a hemial
bondingeet.
Another approah is to onsider the eets of ion
bombardment. It iswell known that fastionispeies
bombardmentof lm growingsurfae plays akeyrole
in the formation of C atoms tetrahedralbonding. As
it wasdisussed in session II, theinidene of arbon
or nitrogen fastspeies over alm ontainingarbon
andnitrogen,maygiverisetohemialsputtering. As
pointedoutby Martonet al. [50℄,damageby ion
im-patmayatasasourefortrigonalbondformationin
arbon-nitrogen lms,and thus may be playing arole
ina-C(N):Hstruture.
Asdisussedabove,thestrutureofa-C(N):Hlms
maybeviewed asaover-onstrained,stressedrandom
network. Sothemeasurmentoftheintemalstress,
be-sidesbeingofpratialinterest,maygiveadiagnostis
of the degreeof overonstrainig, and thus of the
on-netivityoftheamorphousnetwork.
Nitrogen inorporation into a-C:H lms result, as
a general rule in the redution of the internal stress
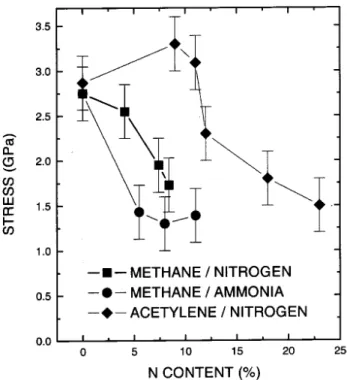
[22,42,51℄. Fig. 6showstheinternalompressivestress
variationforthreeseriesofa-C(N):H lms
rf-PECVD-deposited from dierent gaseous mixtures [9,20,22℄.
The three urves show a lear stress derease upon
nitrogen inorporation, at least aboveaertain
nitro-genontent. Thisbehaviourisin loseorrespondene
to the abovedisussed struture hanges in a-C(N):H
lms. The inreaseonthe sp 2
fration,the lustering
ofsp 2
arbonbonds, andtheintrodutionof terminal
sitesin thenetwork,allofthemmayontributetothe
dereaseofthenumberofonstrainsin theamorphous
network,andthus tostressrelaxation.
Figure 6. Variation of the internal ompressive stress for
lmsderivedfromCH4-N2,CH4-NH3andC2H2-N2gaseous
mixtures,asafuntionofnitrogenontent.
Butisalsolearinthegurethatthestressrelease
isaomplishedindierentwaysforeahofthe
series-haveshownalmostthesameCsp 2
fration
vari-ation [8℄. The diereneobservedin thetheirinternal
stress variation was asribed to dierent variations in
thehydrogenontentofthetwoseriesofsamples[9,22℄.
The hydrogen ontentmayalso explain the
dierenti-ated behaviour of the C
2 H
2 -N
2
series, whih shows a
relatively large nitrogen ontent range with no stress
variation,andthelowerhydrogenontentsofthethree
series(Seetable 1).
Although the internal stress derease has been
always observed upon nitrogen inorporation in
a-C(N):H lms, the mehanial hardness has shown a
spreadintheobservedbehaviour. Someworksreported
no remarkable hanges in the hardnessupon nitrogen
inorporation,atthesametimethat astrongderease
is observed in theinternal stress[9,10,29℄. Other ones
reported ontinuous hardness derease upon nitrogen
inorporation [16,19℄, as it would be expeted from a
dereasednetworkonnetivity. Dierenthardness
be-haviours-onstantordereasinguponNinorporation
- as the deposition parameters hanged were also
ob-served[16℄.
The reasonfor the observed disrepanies may be
duetothedierentmethodsemployedin thehardness
measurements. Mostoftherstworksonthe
mehan-ialproperties of a-C(N):H properties used miro-
in-dentation methods. In this methods, sine the depth
of indentation may be omparable to thin lm
thik-ness, thehardnessvalues measuredmay be inuened
bythesubstratehardness,reduingthevalidityofthe
omparisonbetweenexperiments.
More reent studies used nano-indentation
teh-niques for hardness measurement of a-C(N):H lms.
In this ase a hardness derease has been always
ob-served. This wastheaseofa-C(N):H lmsdeposited
byECR-MWplasmasinCH
4 -N
2
atmospheresreported
by Chanet al. [52℄. Hauert et al. observedthe same
behaviourforlmsdepositedfrom
pentadiene-nitrogen-argon mixtures by DC-biased rf-PECVD [53℄. In this
ase,earliermiro-indentationhardnessmeasurements
haveshown essentiallyonstanthardness, in lms
de-positedbythesametehniqueandgaseousmixture[10℄.
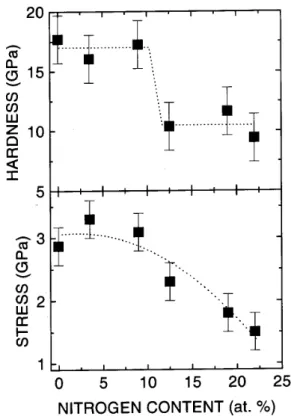
Fig. 7showsthehardnessvariationfora-C(N):Hlms
rf-PECVDdepositedinC
2 H
2 -N
2
atmospheres,plotted
together with the stress variation [22℄. The observed
variationisratherstep-likethanaontinuously
dereas-ingone. Butthehardnesslearlyfallswhenthestress
showdereasingbehaviour.
Atomi Fore Mirosopy was used to investigate
the frition oeÆient and surfae roughness of
a-C(N):Hlmsobtainedrf-plasmadeompositionofCH
4
-NH
3
mixtures [54℄. The frition oeÆient was found
tobeinsensitivetonitrogeninorporation,beinginthe
0.2range. Thesurfaeroughnesswasfoundtoinrease
byafatoroftwofora11at %nitrogenontent. The
roughnessinreaseasuponnitrogeninorporationwas
latedtothedetailsoflmgrowthkinetis[36℄.In
refer-ene[36℄arandomdepositionmodelfora-C(N):Hlm
growthwasused toalulatesurfaeroughnessvalues.
A inreasingbehaviourwasfound forinreasing
nitro-genontent,asaonsequenebetweengrowthandthe
erosiondrivenbyN +
2
ionbombardment.
Figure7. Variationofthenanohardnessandinternal
om-pressivestressasfuntionsofthenitrogenontent(referene
22).
IV Summary
The hemial omposition, growth kinetis, struture
and mehanial omposition hanges on the nitrogen
inorporationproessarereviewed. With minor
dier-enes, a-C(N):H lmsdepositedby severaltehniques,
using arange of deposition parametersand preursor
gasmixtures,showedthesameoverallbehaviour.
Nitrogen inorporation in the deposition
atmo-sphereauseseveredereaseonthelmdepositionrate,
whihlimitsthemaximumnitrogenuptaketoabout20
at%N.Thebehaviourofthelmgrowthkinetisseems
to bedrivenby theompetition betweenerosion
(ar-bonatom hemialsputteringby N
2
fastions andN
2
moleules evaporation)and aggregationproesses,
be-ingmodiedbythehangesinthehydrogenuxtothe
growinglayer.
The main struture hanges observedin a-C(N):H
deposition,thedereaseoftheCatomspfration,
lus-tering oftheCsp 2
atoms,andpreferential
the mehanial properties of the lms hange
drasti-ally,asisshownbythestrongdereaseobservedinthe
mehanial hardness and internal ompressive stress.
Besidesthestruturehanges,thehydrogenontent
it-self wasfoundto alterthemehanialpropertiesalso.
Referenes
[1℄ A.Y.LiuandM.L.Cohen-Siene245,841(1989).
[2℄ D.Marton,K.J.Boyd,J.W.Rabalais,I.J.Mod.Phys.
B9,3527(1995).
[3℄ S.Muhl,J.M.Mendez,DiamondRelat.Mater.8,1809
(1999).
[4℄ D.I.Jones,A.D.Stwart,Phil.Mag.B46,423(1982).
[5℄ H.X.Han,BernardJ.Feldman,SolidStateComm.65,
921(1988).
[6℄ O.Amir,R.Kalish,J.Appl.Phys.70,4958(1991).
[7℄ J.H. Kaufman,S.Metin, D.D. Saperstein, 39(1989),
13053,Phys.Rev.B39,13053(1989).
[8℄ D.F. Franeshini, F.L. Freire Jr, S.R.P Silva, Appl.
Phys.Lett.68,2645(1996).
[9℄ D.F. Franeshini,C.A.Ahete, F.L.FreireJr.,Appl.
Phys.Lett.60,3229(1992).
[10℄ S.Metin, J.H.Kaufman,D.D.Saperstein,J.C. Sott,
J.Heyman,E.Haller,J.Mater.Res.9,396(1994).
[11℄ S.R.P. Silva, J. Robertson, G.A.J. Amaratunga, B.
Raferty,L.M.Brown,J.Shwan,D.F.Franeshini,G.
Mariotto,J.Appl.Phys.81,2026(1997).
[12℄ G.A.J.Amaratunga,S.R.P.Silva,Appl.Phys.Lett.68,
2529 (1996).
[13℄ E.J. Chi, J.Y. Shim,H.K.Baik, H.Y. Lee, S.M. Lee,
S.J.Lee,J.Va.Si.Tehnol. B17,731(1999).
[14℄ J.C. Angus,F.Jansen,J.Va.Si.Tehnol.A6,1778
(1988).
[15℄ W.Jaob,ThinSolidFilms326,1(1998).
[16℄ J.Shwan,W. Dworshak,K.Jung, H.Ehrardt,
Dia-mondRelat.Mater.3,1034(1994).
[17℄ O.Stenzel,M.Vogel,S.Ponitz,R.Petrih,T.
Wallen-dorf, C.V. Borzyskowski, F.Rozploh, Z. Krasilnik,
N.Kalugin,Phys.StatusSolidA140,179(1993).
[18℄ J.W. Zou, K. Reihelt, K. Shmidt, et al., J. Appl.
Phys.65,3914(1989).
[19℄ P. Wood, T. Wyedeven, O. Tsuji, Thin Solid Films
258,151(1995).
[20℄ F.L. Freire Jr., D.F. Franeshini, Thin Solid Films
293,236(1997).
[21℄ M.M. Laerda, D.F. Franeshini, F.L. FreireJr., G.
Mariotto,DiamondRelat.Mater.6,631(1997).
[22℄ L.G. Jaobsohn, F.L. Freire Jr., M.M.Laerda, D.F.
Franeshini,J.Va.Si. Tehnol.A17,545(1999).
[23℄ A. Grill, V. Patel, Diamond Films Tehnol. 2, 61
(1992).
[24℄ S.Bhattaharyya,C.Valle,C.Cardinaud,O.Chauvet,
[25℄ H.Saitoh,T.Inque,S.Ohshio,Jpn.J.Appl.Phys.37,
4983(1998).
[26℄ J.H.Kirn,D.H.Ahn, Y.H. Kim,H.K. Baik,J.Appl.
Phys.82,658(1997).
[27℄ J. Shwan, V. Batori, S. Ulrih, H. Ehrardt, S.R.P.
Silva,J.Appl.Phys.84,2071(1998).
[28℄ H.W.Song,F.Z.Cui,X.M.He,W.Z.Li,J.Phys.
Con-dens.Matter6,6125(1994).
[29℄ K-RLee,K.Y.Eun,J.SRhee,Mat.Res.So.Symp.
Pro.356,233(1995).
[30℄ S.F.Durrant,N.Maral,S.G.Castro,R.C.G.Vinhas,
M.A.Bia de Moraes, J.H. Niola, Thin Solid Films
259,139(1995).
[31℄ K.J. Clay, S.P.Speakman, G. s
J. Amaratunga,S.R.P.
Silva,J.Appl.Phys.79,7227(1996).
[32℄ P.Hammer,W.Gissler,DiamondRelat.Mater.5,1152
(1996).
[33℄ J. Hong, G. Turban, Diamond Relat. Mater. 8, 572
(1999).
[34℄ Todorov-J.Va.Si.Tehnol.A12,3192(1994).
[35℄ F.D.A. Aar~ao Reis, D.F. Franeshini, Appl. Phys.
Lett.74,209(1999).
[36℄ F.D.AAar~aoReis,D.F.Franeshini,Phis.Rev.E61,
3417(2000).
[37℄ J.Seth,A.J.I. Ward,V. Babu, Appl.Phys.Lett. 60,
1957(1992).
[38℄ V.S. Veerasamy, J. Yuan, G.A.J. Amaratunga, W.I.
Milne,K.W.R.Gilkes, M. Weiler,L.M. Brown, Phys.
Rev.B48,17954(1993).
[39℄ G. Mariotto, F.L. Freire Jr., C.A. Ahete, hin Solid
Films241,255(1994).
[40℄ R.O.Dillon, J.A.Woolam, V. Katkanant, Phys.Rev.
B29,3482(1984).
[41℄ M.A. Tamor, W.C. Vassell, K.R. Carduner, Appl.
Phys.Lett.58,592(1991).
[42℄ F.L.FreireJr.,Jpn.J.Appl.Phys.136,4886(1997).
[43℄ J.Robertson,Phys.Rev.Lett.68,220(1992).
[44℄ S.Souto,F.Alvarez,Appl.Phys.Lett.70,1539(1997).
[45℄ J.LaManna,J.Bradok,Wilking, S.H.Lin,B.J.
Feld-man,SolidStateComm.109,573(1999).
[46℄ F.L.FreireJr.,D.F. Franeshini,C.A.Ahete, Phys.
StatusSolidB192,493(1995).
[47℄ D.F.Franeshini,F.L.FreireJr.,W.Beyer,G.
Mari-otto,DiamondRelat.Mater.3,(1993),(1993).
[48℄ F.Weih,J.Widany,Th.Frauenheim,Phys.Rev.Lett.
78,3226(1997).
[49℄ J.Hu, P. Yang, C.M. Lieber, Phys. Rev.B57, 3185
(1998).
[50℄ D.Marton,K.J. Boyd,J.W. Rabalais, Y.Lishitz, J.
Va.Si.Tehnol.A16,455(1998).
[52℄ Wai-ChungChan,Man-KeungFung,Kai-HoLai,Igor
Bello,Shuit-TongLee,Chun-SingLee,J.Non-Cristall.
Solids124,180(1999).
[53℄ R.Hauert,A.Glisenti,S.Metin,J.Goitia,J.H.
Kauf-man, P.H.M. vanLoosdreht, A.J. Kellok, P.
Ho-mann,R.L.White,B.D.Hermsmeier,ThinSolidFilms
268,22(1995).
[54℄ R.Prioli,S.I.Zanette,A.O.Caride,D.F.Franeshini,
F.L.Freire,J.Va.Si.Tehnol.A14,2351 (1996).
[55℄ S.R.P. Silva,G.A.J. Amaratunga,J.R. Barnes, Appl.