Large Diamonds Grown at High Pressure Conditions
H. Kanda
NationalInstitute forResearhinInorganiMaterials(NIRIM),1-lNamiki,Tsukuba,
Ibaraki305-0044,Japan
Reeived4January,2000
A tehnique has been established to grow large diamonds up to 2 m. The rystals are bulky
polyhedronwithyellow,blue,greenorbrownoloraswellasolorless,whihdependonimpurities.
Theimpuritiesinorporatedintothediamondarelimited,i.e. nitrogen,boron,nikel,obalt,silion
andphosphorus.
I Introdution
Sineannounementofsuessindiamondsynthesisin
1955[1℄,thetehniquehasbeenimproveddramatially,
and alarge amount of syntheti diamondis produed
forommerialuseinthreeforms,i.e. largesingle
rys-tal, powderand polyrystalline ompat. This artile
is, however, onerned with only large single rystals
made by a growth tehnique named the temperature
gradient method [2-4℄. The largest diamond made so
far is 34.8arats (a. 7g)[5℄, and 10 mm diamondis
ommeriallyavailable [6℄. Synthetidiamondrystals
have appeared in gem market [7℄, although they have
notbeomepopular.
Thisartilesummarizesthegrowthtehnique,and
harateristisofthegrownrystalsarereviewed.
Sev-eralreviewartilespublishedalreadymayalsobe
help-fultolearnmoredetails ofthediamond[8-16℄.
II Growth of diamond:
the temperature gradient
method
The temperature gradientmethod for growth of large
synthetidiamonds was developedbytheGEresearh
group. A number of the diamonds are being made
in many ompanies and researh laboratories in these
days, although details of the method are not opened
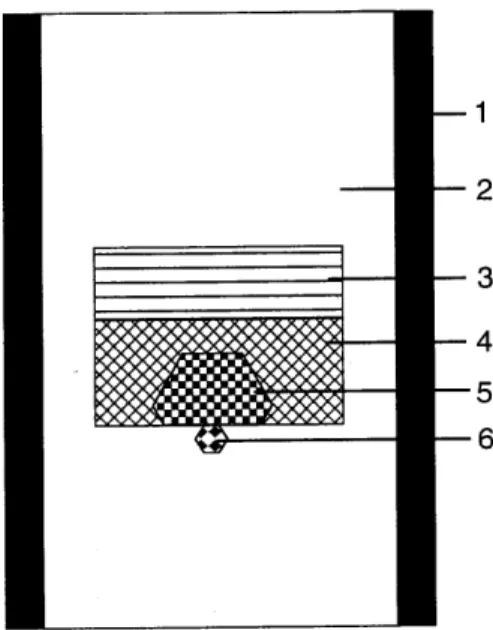
from ompanies. Fig. 1showsaellassembly used in
our laboratory. Carbon soure, a seed rystal and a
growthmediamadeofmetalsolvent-atalystare
essen-tialpartsfor thegrowth. Theyareplaedin aarbon
heatersothatthearbonsouresitsatahigher
temper-atureregionthantheseed. Inasimpleylindrialtube
heatershowninFig. 1,temperaturebeomeshighestat
theenterwheneletriurrentpassestheheater,and,
NaCl powder ompat prepared to t with the other
parts is lled in the graphite heater, surrounding the
three omponents, i.e. arbonsoure, solvent-atalyst
andseedrystal. Othermaterialsmaybeavailable
in-steadofNaClifitishemiallyinerttoarbonandthe
metal. Theseedrystalisembeddedintheompatso
that itontatswiththebottomofthemetal.
Figure1. Sampleassemblyfordiamondgrowthbythe
tem-perature gradient method. 1: Graphite heater, 2: NaCl
pressure medium, 3: Carbon soure, 4: Metalli
atalyst-solvent,5: Growndiamond,6: Seedrystal.
The ell assembly is ompressed up to 5 GPa in
a high pressure vessel and then heated to 1500 o
C.
The pressure and temperature must be so high that
themetalmeltsandthat diamondismorestablethan
graphite. Preise ontrol of pressure is not required,
temperature ontrol may be neessary, beause it
in-uenes growth rates, inorporation of inlusion and
impuritiesasdesribedinfollowingsetions.
At the P, T ondition, more than 10 atomi
per-ent of arbon is dissolved into the molten metal up
to saturation. In the presene of temperature
gradi-entin themetal,solubilityofarbonat thetopofthe
moltenmetalwhihontatswiththearbonsoureis
higher than that at the bottom of themetal, beause
thesolubilityinreaseswithaninreaseoftemperature.
The solubility dierene between the top and bottom
ofthemetalausesdissolutionofthearbonsoureand
transportationofarbonfromthearbonsouretothe
seedrystalwhihontatstothebottomofthemetal,
resulting in growth of new rystal on the seed. The
temperaturegradientisdrivingforetogrowarystal,
and the rystalontinues growingat a onstant
pres-sureandtemperature.
Graphite disk or powder is used as the arbon
soure. The graphite onverts to diamond in a few
minutesafterreahingthegiventemperatureunderthe
high pressure,and, therefore, diamondpowder is also
usefulasthearbonsoure. Qualityofgraphite isnot
sensitiveforthegrowthofdiamond. However,low
pu-rityarbonis notavailable. It hasbeen reportedthat
hydrogenpresentinarbonhasapoisonouseet[17℄.
Aording to the author's experiene, a arbon
mate-rialforapenildidnotonvertto diamond. Additives
suh asresin orlayadded to the arbonmay
deteri-orate the ability of the metal solvent-atalyst for the
onversion.
Carbon ontains1.1% of 13
C isotope with natural
abundane. Isotopially pure or 13
C enrihed arbon
has been used as the arbon soure to grow isotope
modied diamonds. Thermal ondutivity of the
iso-topially pure 12
Cdiamondhasbeenfoundto be50%
higherthanthatofnormaldiamondwithnatural
abun-dane isotope[18℄. 13
C enrihed diamond has helped
toidentifydefetstruturesbyoptialspetrosopyand
ESR[19,20℄. Whenmixed isotopesareused, some
ab-sorptionorluminesenepeakssplitorshift,suggesting
thestruturemodels.
Asmallsinglerystalofdiamondisusedastheseed.
Sizeandqualityinuenegrowthrateandqualityofthe
grownrystalasdesribedin followingsetions[6,21℄.
Anymaterialmaybeusefulforthegrowthmedium
in whih diamondgrows. However,metals ontaining
nikel,obaltorironas amain omponentareusually
used [22℄. Theyarealled atalyst-solventoratalyst.
High reativitywitharbonand lowmelting
tempera-ture are main fatorsto beonsidered for seletion of
the growth medium. Copper has lower melting
tem-perature than nikel, but the former has verylow
re-ativity, and diamond is not grown with high growth
rate [23,24℄. Alloying is a way to derease the
melt-ing temperature[3℄. The melting temperature means
metal-arbonsystem. If thesystemisin euteti
rela-tion, theliquid phase appears at a temperature lower
than that of pure metal. On the other hand, if solid
arbideisformed inthesystem,highertemperatureis
requiredtoformliquidphase. Magnesiumhasalsobeen
usedastheatalyst-solvent,butveryhightemperature,
1800 o
C,is required,beausemagnesiumarbidemelts
atsuh ahightemperature[25℄.
III Crystal size
Crystal size is roughly proportional to growing time,
and, therefore, long time is required to grow a large
rystal. Growthrateis inuenedbytemperatureand
temperaturegradient. The growthrate inreaseswith
inreasing temperature and/or temperature gradient.
Highergrowthratesmaybedesirablefromaviewpoint
of prodution, but inlusions are readilyinorporated
at higher rates. Vagarali et al.[11℄ have summarized
optimumgrowthratesatwhihgoodqualitydiamonds
grow. Agurequotedbythemshowsgoodquality
rys-tals upto 0.5 mg are madewith growthrates of 1 to
3 mg/hr. De Beers and Sumitomo have made larger
diamonds at higher growth rates; 14.2 arats for 500
hrs[11℄and10aratsfor150hrs[6℄,respetively. This
highgrowthrate wasahieved withemployingalarge
seed rystal of 5 mm in diameter. The world largest
rystalis34.80aratsmadebyDeBeersin1992.
Transportationofarboninthegrowthmediumhas
been simulated to alulate growth rates [26℄. The
study revealed that the growth rate has a maximum
at aninitial stage followedby agradual dereaseto a
onstant. Metalliinlusion is readilyinorporated at
theinitial stage ofgrowthbeauseof the high growth
rate. Li et al. [27℄ have proposed amodied ell
as-sembly to suppress theinorporation of theinlusion.
Intheassembly,themetalsolvent-atalysthasalower
partwherediameterissmall,resultinginlowering
ar-bonsupply.
IV Morphology
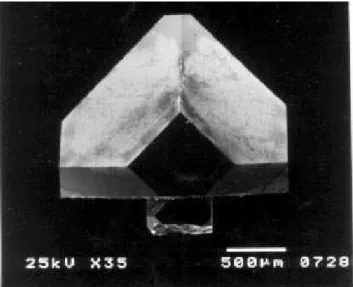
Fig.2showsanexampleofarystalgrownonaseed. It
hassmoothsurfaesexeptabottomsurfae,whih is
roughbeausethissurfaehadfaedwithinnerwallof
theNaClgrowthell. Thesmoothsurfaesareindexed
asf111gandf100g. Inaddition,f113gandf110g
sur-faes appear ommonly as minor surfaes [3℄. f115g
and f117g have also been observed [3,28℄. The
mor-phologydepends ongrowthtemperatureand
omposi-tionof themetalli solvent-atalyst. f111gandf100g
areknowntoappearmostdominantlyonsyntheti
dia-mond. Whenthesurfaesareompared,f100gdevelop
moredominantlythan f111gat lowergrowth
appear when boron is doped [28℄. Aording to
au-thor's experiments, they were seen on rystals grown
fromCo-Ti alloyat lowtemperatures.
Figure2.Diamondgrownonaseedrystal. Asmallrystal
atthebottomistheseed rystalwith0.5 mmindiameter.
(Sanningeletronmirograph).
Crosssetionofagrownrystalrevealsgrowth
his-toryof therystalbeauseofinhomogeneous
distribu-tionofimpurities[30℄. AnexampleisshowninFig. 3.
Change of area of eah growth surfaeduring growth
anbedeterminedfromsuhapiture. Aregion,whih
isformedwith pilingupofagrowthlayer,seenonthe
ross setion is named a growth setor. It has been
demonstrated that utuationof growthondition
in-uenesmorphologyverysensitively[31℄.
Figure 3. Cross setion of a grown rystal after polished
along (110) plane,takenundertransmitted light. A (001)
growth setor is paler yellow than adjaent f111g growth
setorswhihappeardarkerinthisblakandwhitepiture.
Thispitureshowsthatrelativedimensionofthe(001)
sur-faetof111gsurfaeshangedabruptlyinamidwayofthe
growth.
Twin planesparallel to f111gare sometimes seen.
Iftheseedrystalontainsatwinplane,thisextendsto
planeshavegrownat lowertemperaturesaordingto
ourexperiments,whihlooks tobeanaggregates[32℄.
V Growth surfae
The growth surfaes appear smooth marosopially,
butnepatternsarerevealedwithaidofoptial
miro-sopes. Dendriti patterns are ommonlyseen on the
syntheti diamonds ommerially available [33℄. The
patternsdependonompositionofthemetalli
solvent-atalysts,beausetheyarereetedbytexturesformed
whenmoltenmetalssolidify[34℄.Arystalgrownfrom
Fe-30%Ni alloy exhibits dendriti patterns with high
symmetry. Onthe other hand,striations independent
of orientation of diamondare seenonarystal grown
from iron. A growth hillokwhih suggeststhespiral
growthmehanismhasbeenobservedon arystal,
al-thoughitisnotlearlyseenbeauseofthepreseneof
the dendriti pattern [35℄. However,
athodolumines-ene topography showed a lear zoning whih
orre-sponds to viinal surfaes of the growth hillok. The
pattern suggeststhat thehillok onsistsof twotypes
of viinal surfaes, whih have dierent luminesene
nature aused by orientation dependent inorporation
ofimpurities[36℄.
VI Defets
Reently thegrowth tehnique hasbeen sophistiated
to produe defet free rystals [21℄, but a variety of
defets havebeenobserved depending on growth
on-ditions. Thedefets are lassied into four types;
in-lusion, stakingfault, disloationand atomi
impuri-ties, whih orrespond to three-, two-, one- and
zero-dimensional defets, respetively. The defets exept
forstakingfaultaredesribedinthefollowingsetions.
VI.1 Inlusion
Sizeof inlusions varies from 1 m to 1mm. The
largeinlusionisthemetallisolvent-atalyst
enapsu-lated by growingdiamond, andhaveplaty orrod like
shapewithroundorner[37℄. Inorderto suppress
for-mationoftheinlusion,lowergrowthratesarerequired.
Ingeneral,inlusionismorereadilyaptured at
ondi-tionswhere olorlessrystalsgrow[2℄,andprodution
ost of olorless rystal is higher than that of yellow
rystals.
Miron size inlusions are also ommonly present
eveninrystalswhihdonotexhibitanymetalli
inlu-sions[37℄. Darkeldilluminationisasensitivemethod
todetettheneinlusionsdispersingwithlowdensity.
They are sometimes presentas an aggregate.
Chemi-al omposition of the inlusions hasnot been
aggre-from obaltmetalemployingamiro-beamX-ray
uo-resene tehnique[38℄. However, theaggregatehasa
ompletelydierentformfromthelargeinlusion,
sug-gesting that the aggregateis ofsolid partiles suh as
oxide or halides. It may be probable that they were
trapped as solid partiles whih had dispersed in the
moltenmetal. Whentitanium isaddedtothemetalli
solvent-atalyst as anitrogen getter, TiCne rystals
are aptured into grownrystal. Inorporation of the
TiCrystalhassuessfullybeensuppressedbyadding
oppertothesolvent-atalyst[39℄.
VI.2 Disloation
Anumberofdisloationsareobservedevenin
inlu-sion freerystals[30℄. Mostofthem extendtogrowth
diretionsoriginatingfromtheseedrystal. X-rayand
athodoluminesenetopographytehniquesdetetthe
disloationslearly[40℄. Loalstrainausedby
disloa-tions is readilyobserved using apolarized mirosope
[41℄. Reently rystals with low density disloations
havebeenmadeemployingadisloationfreerystalas
theseedrystal[21℄.
VI.3 Impurities in relation to olor
Diamond aptures limited kinds of elements as
atomi impurities that beome optial ative enters.
They are hydrogen, boron, nitrogen, silion,
phospho-rus, nikel and obalt. The impurities exept
hydro-genprodueabsorptionandluminesenebandsaused
byeletronitransitions. Optialpropertiesassoiated
with theimpurities aredesribedin the following
se-tions. Hydrogen is the mostmajorimpurity forCVD
diamond,butnotforhighpressuresynthetidiamond,
andthis isomittedhere.
VI.3.1 Nitrogen
Most of syntheti diamond ommerially available
exhibits yellow olor. The yellow olor is attributed
to 50 to 500 ppm of nitrogen impurity [3,42℄, whih
areinorporatedwithoutintention,probablyfromhigh
pressure media surrounding the growth ell. The
ni-trogenisinorporatedasanisolatedatomondiamond
lattiesite,produingstrongabsorptionwithapeakin
aUV region,atailof whih extendtoavisible region
givingrise toyellowolor. If arystalisgrownabove
1500 o
C,nitrogen impuritiespartlyaggregateto pairs
during growth [43℄. More details on the aggregation
aredesribedbelowin thissetion. Itmaybepossible
todopehigheronentrationsofnitrogenbyadding
ni-trogenontainingompounds. 15
N-enrihedanilinehas
been addedto agrowth ellin order to study isotope
shift ofnitrogen relatedoptial enters [44℄. However,
itisnoteasytodopehigheronentrationsofnitrogen.
800 ppm of the nitrogen onentration is the highest
in rystals grown from onventional metalli
solvent-diamondthat morethan1000 ppmofnitrogen is
on-tained. Reentlymorethan1000ppmofnitrogenhave
beeninorporatedusingnonmetalliatalyst,Na
2 SO
4
[46℄. Non-metalliatalysts are essentialto dope high
onentrationsofnitrogen,beauseitissurethat
natu-raldiamondalsogrowsfrommoltenroks,i.e. akindof
non-metallimaterials. Ontheotherhand,itiseasyto
reduetheonentrationsofnitrogento growolorless
rystals,usingthemetalli solventatalysts.
Inordertoreduethenitrogenonentrations,
om-positionofthealloyusedassolvent-atalystismodied
[3℄. Ni,Co,Feandtheiralloyproduediamond
ontain-ing50 to 300ppmof nitrogen. With adding elements
suh as Ti, Zr and Al to the metals, onentrations
of nitrogen are redued. The elements are, therefore,
alled\nitrogengetter". Afewperentsof Tiadditive
issuÆientto growaolorlessrystal,in whih
nitro-genonentrationsarelessthan1ppm. V,CrandMn,
whih sit at rightpositions of Ti in the priodi table,
havealsoeet to redue the nitrogenonentrations,
buttheir powerislowerthanTi[47℄. Higherontents
oftheelementsmustbepresentinthemetalli
solvent-atalystsinordertogrowolorlessrystals.
Thenitrogenonentrationsindiamondmaybe
re-latedto hemialaÆnity ofnitrogen withthe
solvent-atalystmetal. Ifthesolvent-atalysthavestrong
aÆn-itywithnitrogen,morenitrogenstayinthesolvent
at-alystandlessamountsofnitrogenareinorporatedinto
diamond[47℄. Anotherinterpretationaboutthe
nitro-gengettereethasbeenproposed[39℄;ifthenitrogen
getterelementsarepresentin thesolventatalysts,
ni-tridessuh asTiN and ZrN are formed, and nitrogen
onentrationsinthemoltenmetalbeomeslow,
result-inginlowonentrationsin thegrowingdiamond.
Growth temperature also inuenes nitrogen
on-entrations [29,48℄. The nitrogen onentrations
de-rease with inreasing growth temperature, when Ni
or Co is used as the solvent-atalyst. On the other
hand,thenitrogenonentrationsinreasewith
inreas-ing growth temperature, when solvent-atalysts
on-taining the nitrogen getters are used. This may be
beausethegetter eet beomes weakat higher
tem-peratures.
The inuene of the atalyst-solvent and
temper-ature in inorporation of nitrogen into diamond has
beeninterpretedintermsofsolubilityofnitrogeninthe
atalyst-solvent metals [49℄. If the solubility is large,
morenitrogen stays in the metal and less nitrogen is
inorporatedinto diamond. The solubilityof nitrogen
may beanother wordof aÆnitydesribedabove. The
solubilityinreaseswithinreasingtemperature,
result-inginaderease ofnitrogenonentrations.
Mirosopi observation of the grown rystalgives
usandingofinhomogeneousdistributionofthe
nitro-genrelatedyellowolor. Fig.3isarosssetionofa
hibitthedeepestyellowolor[3℄. Orderofthedepthof
yellowoloris; f111g>f100g>f113g=f110g.
Pre-iseomparisonshowsthatf113gsetorsontainsmore
nitrogenthanf110g[50℄.Thereisareportthata
rys-tal grown at low growth temperatures ontains more
nitrogeninf100gthaninf111g[29℄,indiatingthatthe
nitrogenonentrationsare inuenedbytemperature
moresensitivelyinf100gsetorsthaninf111g. Itmay
beinterestingthat nitrogen isotoperatio, 15
N/ 14
N, in
f111gsetors isdierentfromthatin f100g[51℄.
The nitrogen related yellow olor fades with heat
treatmentabove1500 o
C,beausesinglesubstitutional
nitrogen atomsmigrate tobepairsin the rystal[52℄.
Withheatingabove2500 o
C,thenitrogenpairsonvert
toanotheraggregates,whihareproposedtoonsistof
fournitrogensandavaany[53℄. Theheattreatment
mustbearriedoutunderhighpressureonditions,5to
8GPa,inordertopreventfromgraphitization.Mostof
olorlessnaturaldiamondsontainsigniantamounts
of nitrogen in the aggregatedforms, whereasolorless
syntheti diamonds arenitrogen free. Itis well
estab-lishedthatnaturaldiamondshavebeenheat-treatedin
theEarth solongthatthe nitrogenshavebeen
aggre-gated. Measurementsofthe aggregationrateof
nitro-genhelpustoestimateageofnaturaldiamond[13,54℄.
Theaggregation ratedetermined for the rsttime
wasbasedontheseondorderkinetis[52℄. Thereport
onthekinetisgaveppm 1
valueofkinetioeÆient
of 1:7410 4
(min 1
/ppm 1
) at 1900 o
C, whih
orre-spondsto50%ofonversionofsingleformsto pairsof
100ppm nitrogen with heattreatment at1900 o
Cfor
lhr.
Theaggregationrate is afuntion of temperature,
butithasbeomeapparentthetemperatureisnotonly
a fator whih inuenes the aggregation rate. The
aggregation rate strongly depends on growth setors.
Thereisareportshowingthatitishigherinf111g
se-torsthaninf100g[29℄. Ithasalsobeenpresentedthat
the aggregation rate is not uniform even in a growth
setor[55℄. Thenonuniformityaresuggestedtobe
at-tributedtoinhomogeneousdistributionofotherdefets
[56℄. Ithasbeenpointedoutthat theseondorder
ki-netis an not be applied for the aggregation proess
[57,58℄. Further study is required to understand the
aggregationproess.
Nitrogen impurities produe absorption and
lumi-nesenepeaksotherthanthat givingtheyellowolor
[9,16℄, ommonones ofwhihare theN3enter
(zero-phononpeakat 415nm)onsisting ofthreenitrogens
andavaany,theH3enter(zero-phononpeakat503
nm)onsistingoftwonitrogensandavaany,575nm
zero-phonon peak aused by a nitrogen and vaany,
andtheNVenterwithazero-phononpeakat637nm.
Theseareproduedbyirradiationofhighenergybeam
suh as eletron and neutron followed by heat
treat-ment. However, it is not unusual that these are
ob-produe these bands [59℄. It is well known that the
H3luminesenebandispresentinf100gsetorsof
as-grownsyntheti diamondommeriallyavailable [30℄.
VI.3.2 Boron
Inorporationofboronimpurityproduesblueolor
in diamond [50, 60-62℄ and the diamond has p-type
semiondutingproperty. Theolorisgivenbya
grad-ual inreaseof absorptionfrom highenergy tolow
en-ergy in a visible region. No sharp peak is present in
thevisibleregion,but sharpabsorptionpeaksareseen
around3.5mofaninfraredregion[63℄.
Theblue olordiamondisreadilymade, ifasmall
amountofboronisaddedinagrowthell[50℄.Depthof
blueolorinreaseswithinreasingtheboronadditives.
However,moreamountsofboronadditivearerequired
to produe blue olor, when diamond is grown from
metalsolventatalystswithoutnitrogengetter,beause
boron aeptoris ompensated by nitrogendonorand
onlyunompensatedaeptorontributestoblueolor.
Aordingtoourexperiene,whenpurenikelwhih
produesdiamondontaining200to300ppmof
nitro-genwasused, morethan 10mgofboron wasrequired
to beadded intothegrowthellin orderto growblue
oloreddiamond. Yellowolorpersistedwhenlessthan
10mg boron was added. On the other hand,addition
of 0.1 mgof boron wasenoughfor growthofthe blue
oloredrystalwhenthesolventatalystwasNi-3%Ti,
fromwhihnitrogenfreerystalwasgrown.
The blue olor is not uniform even in a rystal.
Growth setor dependene is observed. f110gsetors
mostsensitivelyhange to blue,whenboron isadded,
whereas f111g setors are the most insensitive. The
f111g setors persist yellow olor, ifboron additiveis
notsuÆient. However,f111gsetorsbeomethe
deep-est in setors,whenalargeamountofboronisadded.
Namely, the order of depth of blue hanges with an
inrease of boron additive. The setor dependene is
understandable,ifweassumetwofators;(1)f111g
se-tors apture both boron and nitrogenmosteasily and
(2) depth of blue olor is proportional to
unompen-satedboron,i.e. [boron℄-[nitrogen℄,wherethebrakets
indiate the onentrationsof boronand nitrogen. As
for f111g setors, if boron additive is little, the
on-entration is lowerthan that of nitrogen, resulting in
persisteneof yellow olor. If boron additive is large,
theonentrationsofunompensatedboronbeomethe
highest.
Theborondopeddiamondsgivebluishgreen
lumi-nesene with eletronbeamexitation
(athodolumi-nesene),inwhihfourbroadbandsarepresentinUV
and visible regions[64℄. Boundexiton peaks arealso
observedin aUV regionof230to240nm. UV
illumi-nationproduesbroadphosphoresenebandspeaking
at2.1and2.5eVfromtheborondopedrystals. They
VI.3.3 Nikel
More than ten of optial bands have been
dou-mentedfromdiamondgrownfromnikelontaining
al-loys. The nikel related bands are also dependent on
onentrationsandstrutureof nitrogenimpurities. A
diamondgrownfrompure Niexhibits brownishyellow
olor,andzero-phononpeaksat2.51eV,1.883eVand
1.4eVareseenin absorptionspetrareordedata
liq-uidnitrogentemperature[66,67℄.Inontrast,diamond
grown from iron does not produe any sharp peak in
spite of having similar yellow olor. A monotoni
in-reaseinabsorptiontoaUVregionisobserved. When
nitrogen getters suh as Ti are added to nikel, green
orbrownrystalsgrow,inwhihharateristi
absorp-tionpeaks,whiharedierentfromthoseobservedfrom
pureNi, areobserved[68,69℄.
The rystalsontainingnikelalso giveunique
lu-minesene bands[67℄. Zero-phononlines ofthebands
areat2.56eVand1.4eV.Thelatterisobservedin
ab-sorptionspetraaswell. Thispeakhasbeenonrmed
to berelatedtoNi fromisotopesplittingmeasuredby
high resolutionspetrosopy[70℄.
ESR studies have determined onentrations and
struture of nikelimpuritiesin pre-annealed rystals
[20, 71℄. Ni 1
whihsubstitutesaarbonatomon
dia-mondlattiehasbeendeteted. Thehighest
onentra-tionmeasuredsofaris70ppm. InterstitialNi +
hasalso
been deteted. Correlation between the Ni impurities
and theoptial bands havenotbeen well established,
althoughithasreentlybeenreportedthatthe
onen-trationsofNi 1
isproportionaltointensityofthe2.51
eVpeak[72℄.
The nikel ontaining rystals hange their olor
withheattreatmentabove1500 o
C.Thebrownish
yel-lowolorhangestobrown,inwhihanumberofsharp
absorptionpeaksareobserved[73℄. Thegreenoloralso
beomes brown. This brown olored rystal does not
haveharateristipeaks,but stronggreenolor
lumi-nesene is produed by UV illumination, and several
luminesene peaks have been reorded [74-76℄. The
olorhangeproduedbytheheattreatmentisrelated
to migration of nitrogenimpurities to form omplexes
withnikelimpurity[74℄.Struturemodelsofthe
om-plexes onsisting of nikel, nitrogen and vaanyhave
beenproposed,basedonESRmeasurements[74-77℄.
Thenikelrelatedoptialentersareloatedonlyin
f111ggrowth setors[68,78℄. X-ray uoresene
teh-nique has also deteted the nitrogen impurity in
syn-theti diamonds, and hasonrmed that the nikelis
onnedtof111ggrowthsetors[79℄. Detailed
observa-tionhasrevealedthatitisnotuniformevenina(111)
setor. Theolorsbeomedeeperinregionswhihgrew
at highergrowthrates[48℄.
VI.3.4 Cobalt
TheX-rayuoresenetehniquehasalsodeteted
loatedonlyinf111ggrowthsetorsasnikelimpurity
is. However,uniqueabsorptionband isveryweak,but
strongyellowlumineseneisobservedfromtherystals
afterheattreated[81℄. Severalluminesenepeakshave
beenseeninphoto-andathodo-luminesenespetra.
Thepeaks depend on degreesof aggregation of
nitro-gen.Cobaltimpuritieshavebeenlessstudiedompared
withnikel.
VI.3.5 Silion
Silionimpurityproduesonlyonepeakat1.681eV
inbothabsorptionand luminesene. It isobservedin
CVDdiamondsommonly,beausesiliaglassand
sil-ionisusually used in areationelland asubstrate,
respetively[82℄. IthasalsobeenobservedinSiion
im-planted diamond [83℄. As for high pressurediamond,
the peak is produed, when silion ontaining alloys
are used as the metalli atalyst-solvents. The peak
wassharperthanthoseobservedintheCVDdiamond,
whih madea preise investigation possible [84℄. The
peak split to 12 lines orresponding to natural
abun-dane silion isotope by high resolution spetrosopy.
This is a diret evidene that silion impurity is
in-volved in the1.681 eVenter. Struturemodelof the
silionrelateddefethasnotbeenestablished,although
ithasbeenproposedthat avaanyisalso involved.
VI.3.6 Phosphorus
n-Type semionduting diamond has been made
withdopingphosphorusbytheplasmaassisted
hemi-alvapordeposition(CVD) tehnique[85℄. The
phos-phorus impurity inorporated in the rystal has been
detetedbytheSIMStehnique,andaunique
athodo-luminesenepeakat5.175eVinaUVregionhasbeen
deteted, whih is expeted to be of a bound exiton
of phosphorus donor [86℄. However, phosphorus
ele-ments or phosphorus related optial enters have not
beenfound in thelargerystalsgrownbythe
temper-aturegradientmethod. A omplexofphosphorus and
nitrogenhasbeendetetedbyESRin arystalgrown
frompurephosphorusunderahighpressureondition,
although thegrown rystalinvestigatedis a verythin
layerformedonaseedrystal[87℄.
VII Conlusion
Sine announement of suess in large diamonds by
thetemperaturegradientmethodin1970,thetehnique
hasbeenimprovedto produe10mmrystalsand
de-fetfreerystalsin aommerialsale. Somekindsof
defettuninghavebeomepossible. Then,therystals
areavailableforbothindustrialappliationsand
sien-tistudy. However,aproblemwehaveis prodution
growth tehnique and an inrease of demands to the
rystals will allow us to produe the diamonds with
heaperost.
Referenes
[1℄ F.P. Bundy, H.T. Hall, H.M.Strong and R.H.
Wen-torf,Jr.,Nature176,51(1955)
[2℄ R.H.Wentorf,Jr.,J.Phys.Chem.75,1833(1971).
[3℄ H.M. Strongand R.M. Chrenko, J.Phys.Chem. 75,
1838(1971).
[4℄ H.M.StrongandR.H.Wentorf,Jr.DieNaturwiss.59,
1(1972).
[5℄ DeBeers,Personalommuniation,(1992).
[6℄ S.SatohandH.Surniya,Rev.HighPressureSi.
Teh-nol.2,315(1993)inJapanese.
[7℄ J.E. Shigley, E. Fritsh, I. Reinitz and T.M. Moses,
GemsGemol.31,256(1995).
[8℄ F.P. Bundy, H.M. Strong and R.H. Wentorf, Jr. in
Chemistry and Physis of Carbon, Vol.10, edited by
P.L. Walker and P.A. Thrower(Marel Dekker, New
York,1973)pp.213-263.
[9℄ G. Davies, in Chemistry and Physis of Carbon,Vol.
13, edited by.P.L.Walkerand P.A. Thrower(Marel
Dekker,NewYork,1977)pp.1-143.
[10℄ G. Munke, inThe Properties of Diamond, edited by
J.E.Field(AademiPress,London,1979)pp.473-99.
[11℄ S.Vagarali,M.LeeandR.C.DeVries,J.HardMater.
1,233(1990).
[12℄ H.M.StrongandR.H.Wentorf,Jr.Amer.J.Phys.59,
1005(1991).
[13℄ T.Evans, inThe Properties ofNaturaland Syntheti
Diamond,editedbyJ.E. Field(Aademi Press,
Lon-don,1992)pp.259-290.
[14℄ R.C.BurnsandG.J.Davies,inThePropertiesof
Nat-uralandSynthetiDiamond,editedbyJ.E.Field
(Aa-demiPress,London,1992)pp395-422.
[15℄ H. Kanda and T. Sekine, in Properties and Growth
of Diamond, edited by G.Davies (INSPEC, London,
1994)pp.403-426.
[16℄ A.M.Zaitsev,inHandbookofIndustrialDiamondsand
Diamond Films, edited by M.A. Prelas, G. Popovii,
L.K.Bigelow(MarelDekker,NewYork1997)
pp.227-376.
[17℄ A.Tsuzuki,S.HiranoandS.Naka,J.Mater.Si. 20,
2260(1985).
[18℄ T.R.Anthony,W.F.Banholzer,J.F.Fleisher,L.Wei,
P.K. Kuo,R.L.Thomas,andR.W.Peyoe,Phys.Rev.
B42,11104(1990).
[19℄ A.T. Collins, P.J.Word, G.S. Woods and H. Kanda,
DiamondRelat.Mater.2,136(1993).
[20℄ J. Isoya, H. Kanda, J.R. Norris, J. Tang and M.K.
Bowman,Phys.Rev.B41,3905(1990).
[21℄ H.Sumiya,N.Toda, Y.NishibayashiandS.Satoh,J.
[22℄ H.P.Bovenkerk, F.P.Bundy, H.T.Hall, H.M.Strong
andR.H.Wentorf,Jr.,Nature184,1094(1959).
[23℄ F.P.Bundy,Nature241,116(1973).
[24℄ H. Kanda, M. Akaishiand S.Yamaoka, Appl. Phys.
Lett.65,784(1994).
[25℄ N.V. Novikov and A.A. Shulzhenko, in Siene and
Tehnology of New Diamond, edited by S. Saito, O.
Fukunagaand M.Yoshikawa,(KTKSi. Pub,Tokyo,
1990)pp.217-219.
[26℄ M. Wakatsuki,W.Li,Y.GohdaandL. Y.Ding,
Dia-mondRelat.Mater.5,56(1996).
[27℄ W. Li, H. Kagi and M. Wakatsuki, J. Cryst. Growth
160,78(1996).
[28℄ M.-L.T.Rooney,J.Cryst.Growth116,15(1992).
[29℄ S.Satoh,H.Sumiya,K.TsujiandS.Yazu,inSiene
andTehnologyofNewDiamond,editedbyS.Saito,O.
Fukunagaand M.Yoshikawa,(KTKSi. Pub,Tokyo,
1990)pp.351-355.
[30℄ G.S.WoodsandA.R.Lang,J.Cryst.Growth28,215
(1975).
[31℄ H.Kanda,T.Ohsawa,O.FukunagaandI.Sunagawa,
J.Cryst.Growth94,115(1989).
[32℄ H. Kanda, T. Ohsawa and S. Yamaoka, J. Cryst.
Growth99,1183(1990).
[33℄ S.TolanskyandI.Sunagawa,Nature184,1526(1959).
[34℄ H.Kanda,M.Akaishi,N.Setaka,S.YamaokaandO.
Fukunaga,J.Mater.Si.15,2743(1980).
[35℄ H.KandaandT.Ohsawa,DiamondRelat.Mater.5,8
(1996).
[36℄ F.C.Frank,A.R.Lang,D.J.F.Evans,M.-L.T.Rooney,
P.M.SpearandC.M.Welbourn,J.Cryst.Growth100,
354(1990).
[37℄ J. Shigley, E. Fritsh, C.M. Stokton, J.I. Koivula,
C.W.Fryer,R. Kane,D.R. Hargett andC.W.Welh,
GemsGemol.23,187(1987).
[38℄ S.Shimomura,H.KandaandH.Nakazawa,Diamond
Relat.Mater.6,1680 (1997).
[39℄ H. Sumiya and S. Satoh, Diamond Relat. Mater. 5,
1359(1996).
[40℄ I.KiawiandA.R.Lang,Philos.Mag.30,219(1974).
[41℄ A.R.Lang,J.Appl.Cryst.27,988(1994).
[42℄ R.M. Chrenko, H.M. Strong and R.E. Tuft, Philos.
Mag.23,313(1971).
[43℄ H.Kanda,T.OhsawaandS.Yamaoka,inSiene and
Tehnology of New Diamond, edited by S. Saito, O.
Fukunagaand M.Yoshikawa,(KTKSi. Pub,Tokyo,
1990)pp.339-344.
[44℄ A.T.Collins,M.StanleyandG.S.Woods,J.Phys.D:
Appl.Phys.20,969(1987).
[45℄ A.T.Collins and S.C.Lawson,Philos. Mag.Lett. 60,
117(1989).
Re-[47℄ H. Kanda, Y. Sato, N. Setaka, T. Ohsawa and O.
Fukunaga, J. Chem. So. Japan, No. 9, 1349 (1981)
inJapanese.
[48℄ H. KandaandS.C. Lawson,IndustrialDiamondRev.
55,56(1995).
[49℄ R.C. Burns, J.O. Hansen, R.A. Spits, M. Sibanda,
C.M. Welbourn and D.L. Welh, Diamond Relat.
Mater.8,1433(1999).
[50℄ R.C.Bums, V.Cvetkovi, C.N.Dodge,D.J.F. Evans,
M.-L.T. Rooney, P.M. Spear and C.M. Welbourn, J.
Cryst.Growth104,257(1990).
[51℄ S.R. Boyd C,T. Pillinger, H.J. Milledge, M.J.
MendelssohnandM.Seal,Nature331,604(1988).
[52℄ R.M. Chrenko, R.E. Tuft and H.M. Strong, Nature
270,141(1977).
[53℄ T. Evansand Z. Qi, Pro.R. So.Lond. A381, 159
(1982).
[54℄ W.R. Taylor,D.Canil andH.J.Milledge,Geohimet
Cosmohim.Ata60,4725(1996).
[55℄ H.KandaandS.Yamaoka,DiamondRelat.Mater.2,
1420 (1993).
[56℄ I.Kiawi,H.Kanda,D.FisherandS.C.Lawson,
Dia-mondRelat.Mater.6,1643(1997).
[57℄ D.Fisher,S.C.Lawson,DiamondRelat.Mater.7,299
(1998).
[58℄ I.Kiawi, H.KandaandA.Mainwood,Diamond
Re-lat.Mater.7,327(1998).
[59℄ E.J. Brookes, A.T. Collins and G.S. Woods, J. Hard
Mater.4,97(1993).
[60℄ R.H.Wentorf,Jr.andH.P.Bovenkerk,J.Chem.Phys.
36,1987(1962).
[61℄ R.M. Chrenko,Phys.Rev.B7,4560(1973).
[62℄ M.-L.T. Rooney, C.M. Welbourn, J.E. Shirley, E.
FritshandI.Reinitz,GemsGemol.29,38(1993).
[63℄ S.D.SmithandW.Taylor,Pro.Roy.So.London79,
1142 (1962).
[64℄ S.C. Lawson, H. Kanda, H. Kiyota, T.Tsutsuni and
H.Kawarada,J.Appl.Phys.77,1729(1995).
[65℄ K.Watanabe,S.C.Lawson,J.Isoya,H.KandaandY.
Sato,DiamondRelat.Mater.6,99(1997).
[66℄ A.T. Collins and P.M. Spear, J. Phys. D 15, L183
(1982).
[67℄ A.T.CollinsandP.M.Spear,J.Phys.C16,963(1983).
[68℄ A.T.Collins. H.KandaandR.C.Burns,Philos.Mag.
B61, 797(1990).
[69℄ S.C. Lawson, H. Kanda and M. Sekita, Philos. Mag.
B68,39(1993).
[70℄ G.Davies, A.J. Nevesand M.H. Nazare, Phys.Rev.
B43,14196 (1991).
[71℄ J.Isoya,H. Kanda andY. Uhida, Phys.Rev.B42,
9843(1990).
[72℄ A.T.Collins, H. Kanda, J. Isoya, C.A.J. Ammerlaan
and J.A. van Wyk, Diamond Relat. Mater. 7, 333
(1998)
[73℄ S.C. Lawson and H. Kanda,J. Appl. Phys.73, 3967
(1993).
[74℄ V.A. Nadolinny and A.P. Yelisseyev, Diamond Relat.
Mater.3,17(1993).
[75℄ I.N. Kupriyanov, V.A. Gusev, Yu.M. Borzdov, A.A.
Kakininand Yu.N.Pal'yanov, DiamondRelat. Mater.
8,1301(1999).
[76℄ H. Kandaand K. Watanabe, DiamondRelat. Mater.
8,1463(1999).
[77℄ R.I. Mashkovtsev and Yu.N. Pal'yanov, Solid State
Com.111,397(1999).
[78℄ A.R.Lang andG.M. Meaden, J.Cryst. Growth108,
53(1991).
[79℄ M.Wakatsuki,S.Hayakawa,A.Aoki,Y.GohshiandA.
Iida,inNewDiamond SieneandTehnology,edited
by R. Messier, J.T. Glass, J.E. Butler and R. Roy
(Mater.Res.So.,Pittsburgh,1991)pp.143-148.
[80℄ X.Jia,S.Hayakawa,W.Li,Y.GohshiandM.
Wakat-suki,DiamondRelat.Mater.8,1895 (1999).
[81℄ S.C. Lawson, H. Kanda,K. Watanabe, I.Kiawi, Y.
SatoandA.T.Collins,J.Appl.Phys.79,4348(1996).
[82℄ A.T.Collins,M.KamoandY.Sato,J.Mater.Res.5,
2507(1990).
[83℄ A.M. Zaitsev, V.S. Vavilov and A.A. Gippius, Sov.
Phys.Lev.Inst.Rep.10,15(1981).
[84℄ C.D.Clark,H.Kanda,I.KiawiandG.Sittas, Phys.
Rev.B51,16681 (1995).
[85℄ S.Koizumi,M. Kamo, Y. Sato,H. Ozaki and T.
In-uzuka,Appl.Phys.Lett.71,1065(1997).
[86℄ H.Stemshulte,K.Thonke andR. Sauer,inPro. of
The5thNIRIMInternationalSymposiumonAdvaned
Materials,editedbyM.Kamoetal.,NIRIM,Tsukuba,
1998)pp.113-116.
[87℄ J. Isoya, H. Kanda, M.A. Kaishi, Y. Morita and T.