Identifying Carinogeni Ativity of Methylated
and non-Methylated Polyyli Aromati
Hydroarbons (PAHs) Through
Eletroni and Topologial Indies
R.S. Braga 1
, P.M.V.B. Barone 2
, and D.S. Galv~ao 1
1
Institutode Fsia, UniversidadeEstadualde Campinas-UNICAMP,
Campinas-SP,Brasil,CP6165,CEP13081-970
2
Departamentode Fsia, UniversidadeFederalde Juizde Fora-UFJF
Juiz deFora, MG,Brasil,CEP36036-330
Reeived14Marh,2000.
Polyyliaromati hydroarbons(PAHs)area lassofplanar moleules, abundantinurban
en-vironment, whihanindue hemialarinogenesis. Their arinogeni powervariesina large
range,fromverystrongarinogenstoinativeones. Inapreviousstudy,weproposeda
methodol-ogytoidentifythePAHsarinogeniativityexploringeletroni andtopologial indies. Inthe
presentwork,weshowthatitispossibletosimplifythatmethodologyandexpanditsappliability
toinludemethylatedPAHsompounds. Usingverysimplerules,weanpredittheirarinogeni
ativitywithhighauray(89%).
I Introdution
Canerisadiseaseofmultiellularorganismsinvolving
multistep proesses in whih ells aumulate geneti
alterations astheyprogress to amoremalignant
phe-notype [1℄. In spite of many years of theoretial and
experimental work,thedetails ofthebiohemial
phe-nomena involved in the appearane of malignant
tu-morsarenotwell-understood. Itisbelievedtodaythat
although many fators an be assoiated with aner
indution, suh as virus, radiation, hemial agents,
et., the hemial omponent is the most important.
Amongthehemialsthatareknowntoindueaner,
the Polyyli Aromati Hydroarbons (PAHs) are of
speial relevane. PAHs are alass of planar organi
moleules (see Figs. 1 and 2) presentingarinogeni
power whih varies from someof the strongest known
arinogenstoinativeones[2℄.
The reasons why some of these very similar
moleules presentarinogeni ativity, and others do
not,havebeentheobjetof intenseresearh sinethe
thirties with thepioneerwork of Cook and
ollabora-tors [3℄. These rst works tried to orrelate the
ar-inogeniativitywithsomegeometrialfeaturesofthe
moleules. Theseideaswerefurtherdevelopedby
Pull-manandPullman[4℄usingquantummehanial
alu-lations (simple Hukel theory [5℄) and were expressed
intermsofritialindexvaluesoverspeimoleular
ilartheoriesevolvedtoinludeone,whihisalled the
bayregion'[6-9℄(seeinsetofFig. 1). Asemi-empirial
study hasbeenalso reported [10℄showingalose
or-relation betweentheeletrophilireativity atspei
arbon atoms of hrysene and methyl- hrysenes and
theirarinogeniativity.
These theories (based on eletroni indies) and
morereentones usingstatistial analysis,neural
net-works, and artiial intelligenemethods [11-13℄have
ahievedonlypartialsuess. Some ofthemworkwell
for aspei subsetof ompounds and fail forothers,
and vie-versa. Due to the inreasing levels of PAHs
presentinurbanair(partlyduetoautoexhaust)andin
manyommonproessedfoods,thesearhforatheory
thatouldpredit,atleastatqualitativelevel,whether
aspeiPAHwillbearinogeniornotisavery
im-portanthealthhallenge.
Reently [14℄ we proposed a new theoretial
ap-proahtoidentify PAHarinogeniativity. This
ap-proahisbasedontheoneptsofloaldensityof
ele-troni statesand ritialvalues fortheenergy
separa-tion between HOMO (highest oupied moleular
or-bital) and its next lower level HOMO-1. That study
wasarriedoutfortherst26moleulesshowninFig.
1. With afewsimplerules,wewereableto groupand
identify theirarinogeniativity.
One interesting experimental fat assoiated with
stitution(methylation,forinstane)in PAHmoleules
andrastiallyaettheirarinogeniativity[15℄,
de-pending onthesiteofsubstitutionandonthenumber
of substituted groups. Ative moleules an beome
inative or vie-versa, or the arinogeni power an
be largelyvaried (i.e., inreased ordereased). These
fats havenotbeenonsistentlyexplained in terms of
K-L theories. Although themethylation proess does
nothangethetotalnumberof-eletrons,itprodues
perturbationsinthe eletronidensityofstates,suh
as hanging the relative ontribution of HOMO and
HOMO-1to theloal densityofstates. Iftheruleswe
havepreviouslyproposed[14℄areorret,weould
ex-pet methylation to indue a disontinuous transition
in the arinogeni ativity, i.e., it ould makeative
moleules inative and vie-versa. Thus, thestudy of
methylatedompoundspresentsitselfasavery
interest-ingtestto ourpreviouslyproposedmethodology[14℄.
Figure. 1Struturalshemeofthe32non-methylated
poly-yli aromati hydroarbon(PAHs)moleules studied in
the present work. SeeTable 1fortheir desriptivenames.
The darkerbonds indiate the bondswith the alulated
highest bond orders. In the inset are shown the pyrene
strutureandalsotypialL,Kandbay(B)regionsforPAH
Figure.2Struturalshemeofthe49methylatedpolyyli
aromati hydroarbon(MPAHs) moleules studied in the
presentwork. SeeTable2for theirdesriptivenames. The
darkerbondsindiatethebondswiththealulatedhighest
bondorders.
II Methodology
Inthepresentworkwehavestudied81PAHmoleules
(49and 32methylated andnon-methylated PAHs,
re-spetively). Their shemati strutures are shown in
Figs. 1and 2. Most of these moleules were seleted
havinginmindtheriteriaofavailabilityof
experimen-tal data for hemial arinogenesis. For the rst 26
moleulesshowninFig. 1,theIballindexisavailable[9,
16,17℄. TheIballindexisdened astheperentageof
skinanerinmie(skinpaintingexperiments)divided
by the average latent period in days for the aeted
animals multiplied by 100 [16, 17℄. The remaining 6
moleules shown in Fig. 1 were hosen for
ompari-sonpurposes;theIballindexisnotavailable forthem,
sowe have hosen thearinogeni sale proposed by
Cavalieriet al. [18℄. For the methylated ompounds,
wehaveusedthesamesale[18℄,sinetheIballindies
arenotavailableforallofthem. Themethylated
stru-tures shown in Fig. 2 are struturally related to the
non-methylatedmoleulesshowninFig. 1,inorder to
provideadiret omparison.
PAHsareplanarmoleuleswithawell-dened
sep-aration the Hukel method is the simplest hoie due
toitssimpliityandgoodqualitativepowerpredition.
Also,itwillallowustoompareourresultswithalarge
amountof theoretialstudies arriedoutsinethe
for-tiesusing Hukel models [3,4, 12℄. Wehave usedthe
samemethodandparametersadoptedbyPullmanand
Pullman[4℄fortheirK-Ltheorytoallowadiret
om-parisonto theirresultsandtoourpreviousresults[14,
19℄. SeeRefs. [5℄and[19℄fordetailsabouttheHukel
method.
Inspiteofitssimpliity,theHukelmodeland
sim-ilar theories are still very useful in providing the
rel-evant physial information forthe qualitativeanalysis
of the eletroni and strutural properties of organi
ompounds. For instane, Hukel models have been
suessfullyusedtoinvestigatetheeletronistruture
ofondutingpolymersandmoleularrystals[20-24℄.
In the Hukel model, there is morethan one way
to treat methylated ompounds. In this work, we
haveusedtheso-alledindutivemethod, treatingthe
methylationthroughanappropriateresalingof the
parameter(=-0.5)[5℄. Wehavehosenthismodel
(in-stead of the heteroatom or hyperonjugation models)
beause, in the present ase, it is the best and
sim-plestwaytodiretlyomparetheeletronidensityof
states(DOS)andtheloaldensityofstates(LDOS)of
methylated andnon-methylated PAHs. Sine the
ma-tries will have thesame dimensions,the summations
are arried out overthe same number of sites for the
methylatedandtheirstruturalparents.
The DOS is dened as the number of eletroni
statesperenergyunit. Therelatedonept ofLDOS,
i.e., the DOS alulated over a spei moleular
re-gion, is introdued in order to also desribe the
spa-tial distribution of the states over the system under
onsideration. Due to the fat that we are arrying
outmoleularalulations,the eigenvaluesform a
dis-rete set and, in order to simulate a ontinuous set,
this Æ-funtionlike spetrum has to be smoothed out
usingGaussianorLorentzianfuntionsenteredonthe
eigenvalues [19, 25℄. For the LDOS alulations, the
ontributionofeaharbonatomtoaneletronilevel
isweightedbythesquareofthe(real)moleularorbital
oeÆient, i.e., bythe probabilitydensity
orrespond-ingtothelevelin thatsite. Inourpreviousworks[14,
19℄ wehave used a Lorentzian enveloping aordingly
tothefollowingexpression:
LDOS(E)= n X l=k 2 (E E l ) 2 + 2 n f X m=n1 j ml j 2 : (1)
Here, is thehalf-width ofthe Lorentzianpeak ( =
0:01), and the spetra aregenerated varying the
en-ergy for a desired energy range (E). For the results
shown here, we onsidered this range to be from -3.0
to 3.0 (histograms with 500 points). E refers to
the moleular energies and
ml
to the oeÆients of
theexpansionof moleularorbitals expressedasa
lin-ear ombination of atomi orbitals. The summation
isarriedoutoverthedesiredmoleular region(initial
(n
i
)tonal(n
f
)arbonsites)inludingalltheseleted
moleularenergies(l=k ton
).
However,thisproedurehassomedisadvantages.It
hassomeintrinsidependeneonthehosenvaluesfor
theLorentzianenvelopingandalsoontheinitialenergy
values used to generate the simulated spetra. This
preludesthediret omparisonwithLDOSgenerated
with othermethods. Forexample,ifweuseamethod
inluding all valene eletrons, the half-width of the
lorentzian-peak ouldprodueartiial hanges in the
LDOSvaluesthroughspuriousoverlapofthemoleular
levelsthat are verylose. This doesnot happen with
the Hukel method where only -eletrons are taken
into aount.
Tosolvetheseproblems,wehaverewritteneq. 1to
thefollowingform:
LDOS(E
i )=2
n f X m=ni j mi j 2 : (2)
Usingthedisretemodulationgivenbyeq. 2(insteadof
a ontinuousLorentzian envelope) weavoid the
prob-lems involving eq. 1 and we are also able to diretly
ompare DOS and LDOS alulated from any LCAO
(LinearCombinationofAtomiOrbitals)method.
Theuseofdensityofstates(DOS)andloaldensity
ofstates(LDOS)oneptsangiveusdetailed
informa-tionontheontributionsofspeigeometrialregions
of themoleules tothe hemial reativity, optial
re-sponse,et.,and,onsequently,totheirbiohemial
be-havior.
For the non-methylated PAHs moleules, it was
shown[14℄that theLDOSanalysisovertheK,L,and
Bay regions (onsidered by some authors [4-10℄ to be
therelevantmoleularregions)didnotprovidepatterns
that ould be orrelated with the arinogeni power.
The same was observed for the LDOS involving
ter-minal rings. However, when this analysis wasarried
out over the ring ontaining the highest bond-order
(RHBO) in assoiation with the dierene in energy
betweentheHOMOand HOMO-1(energy),alear
pattern appeared [14, 19℄. Throughverysimplerules,
itwaspossibletoassoiatetheseeletroniindieswith
the arinogeni ativity. For the present study of
methylated ompounds, we haveanalyzed these same
moleularregions.
III Results and disussions
The81PAHmoleuleswehavestudiedhere(49
methy-lated and 32 non- methylated ones) are indiated in
Figs. 1and2. Asanbeseenfrom Fig. 2,the
thehighestbond-ordersinrelationtotheparentPAHs
in Fig. 1.
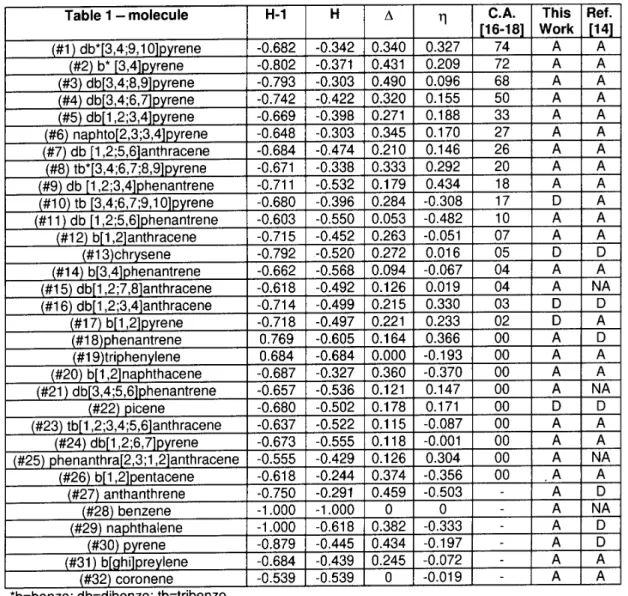
InTables1and2,weshowasummaryoftheHukel
results for the moleules shown in Figs. 1 and 2,
re-spetively. The values for the HOMO (highest
ou-pied moleular orbital), HOMO-1, their energy
dier-ene () and their LDOSrelative ontribution
dier-ene ()arepresented. Theexperimental arinogeni
ativity is also indiated when available. From these
tablesweannotiethatitisnotpossibletouseanyof
thesedataseparatelyasindiatorsforthearinogeni
ativity. Ourtheoretial preditionsare ontrasted to
theexperimentaldata(whenavailable)andwiththe
re-sultsobtainedwith themethodology ofpreviouswork
[14℄.
Table1-SummaryoftheHukelresultsforthemoleulesnumberedaordingtotheshemeshowninFig. 1. Thehighest
oupied moleular orbital (H), the next lower oupied level (H-1), their energy dierene value () and their relative
ontributiondierenetotheLDOS()areindiated. Thetheoretialresultsofthepresentwork(thiswork)andaprevious
work(Ref. 14) are ontrasted to theexperimentaldata for arinogeni ativity (C.A.). A andD indiateagreement or
disagreement,respetively. NAindiatestheasesnotanalyzedinref. 14. Alltheenergyresultsareexpressedintheusual
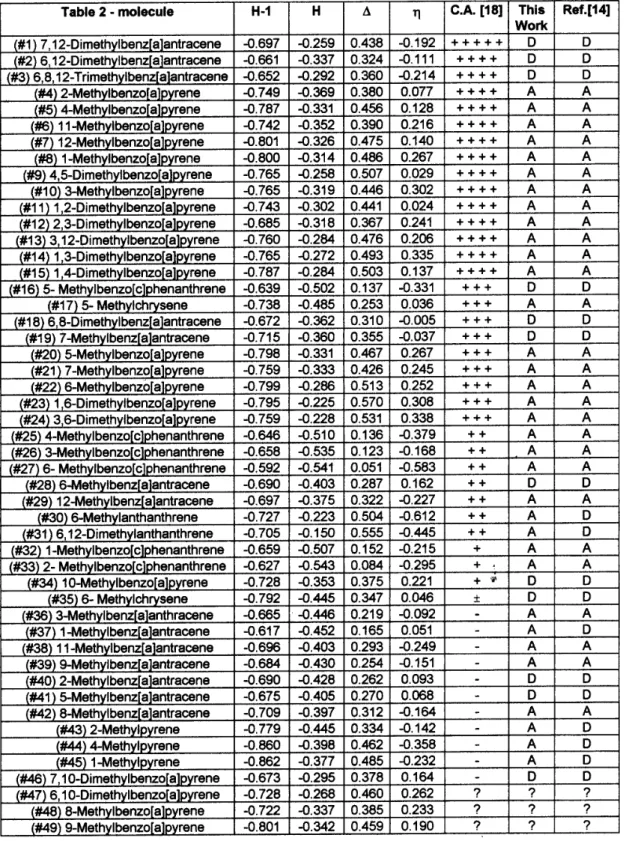
Table 2-Summaryofthe Hukelresults forthe methylatedompounds,numberedaording totheshemeshowninFig.
2. Thehighest oupiedmoleularorbital(H),thenextloweroupied level(H-1),theirenergydierenevalue(),their
relative ontribution dierene to the LDOS (), and the experimental arinogeni ativity (C.A.) are indiated. The
symbols(+++++),(++++),(+++),(++),(+),()and(-)meanextremelyative,veryative,ative,moderatelyative,
weaklyative,veryweaklyativeandinative,respetively. Thetheoretialresultsofthepresentwork(thiswork)andthe
onesobtainedusingthemethodologyofapreviouswork(Ref. 14)are ontrastedtotheexperimentaldatafor arinogeni
ativity(C.A.). AandDindiateagreementordisagreement,respetively. Alltheenergyresultsareexpressedintheusual
Hukelresonaneenergy(approximately2.4eV).Seetextfordisussions. Althoughforthelastthreemoleulesindiated
Baroneetal. [14℄havestudiedtherst26moleules
in Fig. 1andproposedthefollowingthreesimplerules
to identify arinogeni ativity (based on the
en-ergy valuesassoiatedwith the HOMO and HOMO-1
relativeontributiontotheLDOSovertheRHBO):
Pyrenelike moleules.
(a) If the moleuleontainsa pyrenelike struture
(see inset of Fig. 1) and is greater than 0.25
( 2.4eV), it will be strongly arinogeni.
Other-wise,themoleulewill beinative.
Nonpyrene moleules
(b)IftheHOMOisthehighest(peak)ontribution
to the LDOS over RHBO, the moleule will be
om-pletelyinative.
() If the HOMO ontribution to the LDOS over
RHBO is greater than that of HOMO-1 (but not
the highest peak) and > 0:15, the moleule will
presenta strong or moderate arinogeni ativity. If
the HOMO-1 ontribution is greater than that of the
HOMO, themoleulewill presentweak ornoativity
atall. TypialexamplesoftheserulesareshowninFig.
3.
Figure. 3Loaldensityofstates(LDOS)inarbitraryunits
(a.u.) over the ring that ontains the highest bond
or-der(RHBO)fortypialativeandinativenon-methylated
moleules. For simpliity, only the valenestates are
dis-played. H indiatesthehighestoupiedmoleular orbital
(HOMO)andNisthenextlowermoleularorbital
(HOMO-This set of rule presents some limitations. If a
ompound havethe HOMO ontribution greater than
HOMO-1(positive)and<0:15(asenotpresent
inthe Lorentziananalysis [14, 19℄),these rulesannot
be used todetermine whether theompound is ative
ornot.
However, this situation appears when we use the
disreterepresentationofthespetra(seetables1and
2)anditneedstobeonsidered. Thepresentstudy
in-ludingalargernumberofompounds(methylatedand
non-methylated)andusingdisretemodulationtoDOS
andLDOSspetraallowsustotreatthisase. Besides
that,theabovesetofthreerulesanbereduedtojust
onesimplerandmoreenompassingrule:
If the > 0 and > 0:17, the moleule
will present a strong or moderate arinogeni
ativity. Otherwise, the moleulewill presenta
weak ornull ativity.
Thisruleexploresthesameoneptsoftheoriginal
set of rules, ritial valuesand relative LDOS
on-tributionstoHOMOandHOMO-1. InFig. 3weshow
typial results for ativeand inative non-methylated
ompoundswhosearinogeniativityisorretly
pre-ditedbytheaboverule.
In what follows, we will disuss the results for
methylated ompounds. We would like to stress that
thedesriptionof the methylation proessin terms of
simpleperturbationoftheparameterfor thearbon
atwhihthehemialgroupisattahedisastrong
ap-proximation. Evenso,in thisapproah-astheresults
belowwillshow-atleastthequalitativebehaviorofthe
arinogeniativityisappropriatelydesribed. Thisis
alearindiationthatthemethodologyweare
propos-ingisphysiallysound.
We have examined 49 methylated moleules (with
experimental data available for 46 of them) and the
abovenewruleanorretly predittheabsolute
ar-inogeniativityof74%ofthem. Thisisanexellent
result,onsideringtheapproximationswehaveusedto
treatthemethylationproess. Ifweusetheaboverule
toanalyzethetendeny ofhangesinthevalues
un-dermethylation(i.e.,whetheritinreasesordereases)
theagreementwiththeexperimentaldatagoesto89%.
TypialLDOSresultsforthesemoleulesareshownin
Fig. 4.
Themostinterestingasesarethoseforwhih,
un-dermethylation, theativemoleules beomeinative
orvie-versa. Thishappensfor9outof46ompounds.
InTable3 we showthe variation of for these
om-pounds. We ansee from this table and from Fig. 5
that fortheativeompounds whih beomeinative,
thevaluedereases(#P2,#M34and#M46)andfor
inativeompounds whih beome ative,the value
inreases(#P12,#M01and#M03),followingthe
experimental data for 8 outof the 9ompounds. For
theremainingmethylated struture,itsativityis
or-retlypreditedbyourrule,whih,however,failsinthe
preditionofitsparentPAH struture.
Figure. 4Loaldensityofstates(LDOS)inarbitraryunits
(a.u.) overthe ring that ontains the highestbond order
(RHBO)formethylatedmoleulesrepresentativeoftherule
statedinthetext.Forsimpliity,onlythevalenestatesare
displayed. H indiates the highest oupied moleular
or-bital (HOMO) and N is the next lower moleular orbital
(HOMO-1). Seetextfordisussion.
For the 78 moleules with available experimental
data, our new single rule orretly desribes the
bio-logialativityof61 ofthem (78%). Ifweinludethe
tendenies, we orretly desribe 69 out of 78 (89%).
Three of the methylated moleules shown in Fig. 2
(#M47,#M48and#M49)arepreditedbyourruleto
bearinogenibutwedonothaveexperimentaldata
availableforthem.
Thebiohemialproessesleadingto hemial
ar-inogenesisareveryomplexphenomena, notwell
un-derstood in all the details. It is very intriguing that,
withoutassuminganybiohemial mehanismand
us-ingaverysimplerulebasedontheHukelmethod,we
areabletopreditwithhighauraythearinogeni
Figure. 5Loaldensityofstates(LDOS)inarbitraryunits
(a.u.) over the ring that ontains the highest bond
or-der(RHBO)fortypialmoleuleswhihundermethylation
presentvariationintheirarinogeniativity. For
simpli-ity, onlythe valenestatesare displayed. H indiatesthe
highest oupied moleular orbital (HOMO) and N is the
nextlowermoleular orbital (HOMO-1). See text for
dis-ussion.
The K-L theories, as well the Bay theories, both
assumetheexisteneofametaboliativationproess
induing arinogeni ativity. These theories use
en-ergetiindies, whihin fatrepresentativation
ener-gies [26℄. Onepossible explanationofwhythepresent
methodology works is that the loal density of states
(overthe ring that is the most suseptible to spei
hemial reations) measures these ativation energies
(believed to bediretly orrelated to the arinogeni
power[7℄)moreeÆiently. Butagain,the existeneof
aminimumvalueplayingadeisiverolein
determin-ing arinogeniativityis aruialfeature. This was
originallysuggestedbyBaroneet al. [14℄andit might
explain someof the K-L model failures. The physial
meaningoftheminimum anbeexpressedin terms
of frontierorbitals. It seemsthata`lean'frontier
or-bital, i. e., a HOMO well separated in energy from
the HOMO-1, is a neessary but not suÆient
ondi-tion for arinogeni ativity. It is the `balane'
be-tweenrelativeHOMO and HOMO-1ontributions (
values)andtheirenergyseparation(values)that
tiatedmethods[27℄(beyondHartree-Foklevel)
indi-ate that ative and inative moleules have dierent
patternsintermsofthemixingofongurationstates.
This suggeststhe existene of exited states with
dif-ferent lifetimes for ative and inative moleules and
onsequentlydierentspeireativitiesorativation
energies. Theseaspetsremaintobebettereluidated.
Table3-Relativevariationsofthevaluesforthe9
methy-lated ompounds (M) that hange the arinogeni
ativ-ityofitsrelatednon-methylatedparentstruture(P).The
numbering for the parent (P) and the related methylated
ompounds (M) is aording to Figs. 1 (P) and 2 (M).
Wewouldliketostressthatthepresentwork,based
on the eletroni features of isolated moleules, an
onlybeusedtolassifymoleules asativeornot,but
it annot be used to predit poteny. Environmental
aspets,suhas hydrophobiity(not onsideredhere),
playamajorroleindeterminingthepotenyofthe
a-tiveompounds, whilemainly eletroni fators
dier-entiatetheativefromtheinativeones. QSAR
stud-ies showno orrelation betweenthe eletroni indies
and the arinogeni poteny [28℄. Also, the
arino-geni powerofsomePAHsompoundsvaries,
depend-ing on the way of appliation (subutaneous injetion
or painted skin). Thus, the lassiation riteria for
isolated moleules is better dened in terms of ative
orinative[12℄.
In summary, we havepresentednew developments
intheeletroniindiesmethodology(EIM)toidentify
arinogeniativityofmethylatedandnon-methylated
PAH moleules,whih enlarge andgeneralize a
previ-ous methodology [14, 19℄. This improvement allowed
theonstrutionofasinglerulethatexploresthe
on-ept ofrelativeHOMO andHOMO-1ontributionsto
theloaldensityofstatesovertheringthatontainsthe
highest bond-order(RHBO) and on the separationin
Hukel method, but the methodology anbe adapted
tomoresophistiatedmethods,semi-empirialoreven
goodqualityabinitiomethods. Infat,itisan
interest-ingquestiontoknowwhethertheruleisartiially
pro-duedbytheHukelparameterization. Preliminary
al-ulations[27℄usingsophistiatedsemi-empirial
meth-odsfor non-methylated moleules haveprodued very
similarresultsandwebelievethatthisanbeextended
also forthe methylated ompounds. That study is in
progress.
Aknowledgments
The authors wish to thank Prof. L.V. Szentpaly,
Prof. M. A. Cotta and Prof. A. CamiloJr. for
help-ful disussions, and the Brazilian Agenies FAPESP,
CNPq,CAPES,FAPEMIG andPREVI/UFJFforthe
nanialsupport.
Referenes
[1℄ T.Sugimura,Siene258,603(1992).
[2℄ R.G.Harvey,N.E. Geaintov, A. Chem. Res.21,66
(1988).
[3℄ C.A. Coulson, Adv. Can. Res.1, 1(1953) and
refer-enestherein.
[4℄ A.Pullman,B.Pullman,Adv.Can.Res.3,117(1955)
andreferenestherein.
[5℄ A. Streitwieser, Moleular Orbital Theory, Wiley, N.
York(1961).
[6℄ J.P. Lowe, B.D. Silverman, A. Chem. Res.17, 332,
(1984).
[7℄ D.M. Jerina, et al., in Carinogenesis: Fundamental
MehanismsandEnvironmentalEets,B.Pullman,P.
O. Ts'o and H. Gelboin (Eds.), D. Reidel Publishing
Co.,Holland,1980,p.1.
[8℄ L.V.Szentpaly,J.Am.Chem.So.106,6021 (1984).
[9℄ J. Gayoso, S. Kimri, Int. J. Quant. Chem. 38, 461
(1990);38,487(1990).
[10℄ S. Kimri, J. Gayoso, J. Mol. Stru. (THEOCHEM)
362,141(1996).
[11℄ U.E. Nordun, W. Svante, Ata Chem. Sand. B 32,
602(1978).
[12℄ D.Villemin, D.Cherqaoui, A.Mesbah, J. Chem.Inf.
Comput.Si.34,1288(1994).
[13℄ X.-H.Song,M.Xiao,R.-Q.Yu,ComputersChem.18,
391(1994).
[14℄ P.M.V.B. Barone, A. Camilo Jr., D.S. Galv~ao, Phys.
Rev.Lett.77,1186(1996).
[15℄ D.W. Jones, R.S. Matthews, in Progress in
Medi-al Chemistry, G. Ellis and G.B. West (Eds.),
North-Holland,101,59(1974).
[16℄ P. Daudel, R. Daudel, Chemial Carinogenesis and
MoleularBiology,Wiley-Intersiene,NewYork,1966,
pp.1-5.
[18℄ E.L.Cavalieri, E.G.Rogan,R.W.Roth,R.K.Saugier,
A.Hakan,Chem.Biol.Interat.47,87(1983).
[19℄ R.S. Braga, P.M.V.B. Barone, D.S. Galv~ao, J. Mol.
Strut.(THEOCHEM)464,257(1999).
[20℄ D.S. Galv~ao, D.A. Santos, B. Laks, C.P. Melo, M.J.
Caldas,Phys.Rev.Lett.63,786(1989);65,527(1990).
[21℄ Z.G. Soos, S. Ramasesha, D.S. Galv~ao, Phys. Rev.
Lett.71,1609(1993).
[22℄ F.Lavarda,M.C.Santos,D.S.Galv~ao, B.Laks,Phys.
Rev.Lett.73,1264(1994).
[23℄ R.H.Baughman,D.S.Galv~ao,Nature365,735(1993).
[24℄ B.Laks,D.S.Galv~ao, Phys.Rev.B56,967(1997).
[25℄ L.E.Sansores, R.M. Valladares, J.A. Cogordan, A.A.
Valladares,J.Non-Cryst.Solids.143,232(1992).
[26℄ R. Benigni, C. Andreoli, A. Giuliani, Environ. Mol.
Mutagen.24,208(1994).
[27℄ P.M.V.B. Barone, R.S. Braga, A. Camilo Jr., D.S.
Galv~ao,J.Mol.Strut.(THEOCHEM),505,55(2000).
[28℄ R. Vendrame, R.S.Braga, Y.Takahata, D.S.Galv~ao,