RevistaBrasileiradeFarmacognosia27(2017)698–701
w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Plectraterpene,
a
new
ursane-type
triterpene
ester
and
other
steroids
from
the
aerial
parts
of
Plectranthus
montanus
Musarat
Amina
a,∗,
Nawal
M.
Al
Musayeib
a,∗,
Gamal
A.
Mohamed
b,c,
Sabrin
R.M.
Ibrahim
d,eaDepartmentofPharmacognosy,CollegeofPharmacy,KingSaudUniversity,Riyadh,SaudiArabia
bDepartmentofNaturalProductsandAlternativeMedicine,FacultyofPharmacy,KingAbdulazizUniversity,Jeddah,SaudiArabia cDepartmentofPharmacognosy,FacultyofPharmacy,Al-AzharUniversity,AssiutBranch,Assiut,Egypt
dDepartmentofPharmacognosyandPharmaceuticalChemistry,CollegeofPharmacy,TaibahUniversity,Al-MadinahAl-Munawarah,SaudiArabia eDepartmentofPharmacognosy,FacultyofPharmacy,AssiutUniversity,Assiut,Egypt
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10October2016 Accepted30May2017
Availableonline15November2017
Keywords:
Plectranthuscylindraceus Lamiaceae
Plectraterpene Ursane-typetriterpene
a
b
s
t
r
a
c
t
Anewursane-typetriterpeneester,plectraterpene[3-(decanoyloxy)-19-hydroxy-urs-12-ene]andfour knownsteroidalcompoundshavebeenisolatedfromtheaerialpartsofPlectranthusmontanusBenth. (syn.PlectranthuscylindraceusHochst.exBenth.),Lamiaceae.Theknowncompoundswerestigmasterol, sitosterylferulate,cholest-5-en-3-O--d-glucopyranosideandstigmasterol-3-O--d-glucopyranoside.
Compoundsplectraterpene,sitosterylferulateandstigmasterol-3-O--d-glucopyranosidearereported
forthefirsttimefromthisplantwhereascompoundcholest-5-en-3-O--d-glucopyranosidefirsttime
fromthegenus.Thestructuresofthesecompoundsweredeterminedthroughspectralanalysis,including extensive2DNMRdataaswellaschemicalmethodsandcomparisonwithliterature.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Genus Plectranthus belonging to family Lamiaceae includes
morethan300species(Retief,2000).Thetherapeutic,nutritional, andhorticulturalvaluesofthisgenusareattributedtoitsaromatic natureandessentialoilproducingcapability(Lukhobaetal.,2006;
Grayeretal.,2010;AlasbahiandMelzig,2010).Plectranthus
mon-tanusBenth.(syn.PlectranthuscylindraceusHoechst.Ex.Benth.)is astronglyaromatic,succulentandhighlybranchedherborshrub, knownasAl-SharandZefubrekinSaudiArabiaandOman, respec-tively(Orabi etal.,2000;Asresaetal.,2013).It isusedtotreat sore throats,skin, digestive,respiratory, and inflammatory dis-eases(Orabietal.,2000;Rahmanetal.,2004).Itsoilpossessed antibacterialandantifungalactivities(Marwahetal.,2007;Asresa etal.,2013).Previously,sesquiterpenesandflavonoidshavebeen reportedfromthisplant(Orabietal.,2000).Asapartofour ongo-ingsearchfornewconstituentsfromSaudimedicinalplants,anew ursane-typetriterpeneester,plectraterpene(1),togetherwithfour knowncompoundswereisolatedfromtheaerialpartsofP. cylin-draceus.Theirstructureswereverifiedbyvariousspectroscopicand chemicalmethods.
∗ Correspondingauthors.
E-mails:mamina@ksu.edu.sa(M.Amina),nalmusayeib@ksu.edu.sa (N.M.Musayeib).
Materialsandmethods
Generalexperimentalprocedure
Optical rotations were measured on a Perkin-Elmer Model
341LCpolarimeter(Perkin-Elmer,Waltham,MA,USA).IRspectra
weremeasuredwithaShimadzuInfrared-400
spectrophotome-ter (Shimadzu, Kyoto, Japan). EIMS spectra were recorded on
JEOLJMS-SX/SX102Amassspectrometer(Joel,Peabody,MA,USA).
ESIMSspectrawererecordedonanAgilent6320Iontrapmass
spectrometer(Agilenttechnologies,USA).HRESIMSspectrawere
obtained using an LTQ Orbitrap mass spectrometer (Thermo
Fisher,Waltham, MA, USA). NMR spectrawere measuredon a
BrukerDRX 700spectrometer(Bruker,Rheinstetten, Germany).
Vacuumliquid chromatography (VLC)wasperformed using
sil-icagel60(0.04–0.063mm; 500g;Merck,Darmstadt,Germany).
Column chromatographic separations were performed on
sil-ica gel 60 (0.04–0.063mm, Merck, Darmstadt, Germany), RP18
(0.04–0.063mm,Merck,Darmstadt,Germany),andsixmlstandard LiChrolut extraction tube (RP18, 40–63m, Merck, Darmstadt, Germany).TLCanalyseswereconductedonpre-coatedsilicagel
F254aluminumsheets(Merck,Darmstadt,Germany).Compounds
weredetectedbysprayingthesheetswithp-anisaldehyde/H2SO4 reagentfollowedbyheatingat110◦Cfor1–2min.
https://doi.org/10.1016/j.bjp.2017.05.015
M.Aminaetal./RevistaBrasileiradeFarmacognosia27(2017)698–701 699
Table1
NMRspectraldataofcompounds1and2(CDCl3,700and176Hz).
Position 1 Position 2
ı(H)(mult.,J[Hz]) ı(C)(mult.) ı(H)(mult.,J[Hz]) ı(C)(mult.)
1 1.56–1.58(m)1.01–1.04(m) 38.8CH2 1 1.85–1.87(m)1.11–1.13(m) 37.2CH2
2 2.21–2.24(m)1.86–1.88(m) 28.8CH2 2 1.88–1.90(m)1.31–1.33(m) 28.0CH2
3 4.31(dd,J=11.0,5.0) 79.0CH 3 4.26–4.29(m) 78.4CH
4 – 39.6C 4 2.45–2.47(m)2.28–2.30(m) 38.3CH2
5 1.53–1.56(m) 55.2CH 5 – 141.0C
6 1.61–1.63(m)1.46–1.48(m) 18.4CH2 6 5.40(brs) 121.4CH
7 1.64–1.66(m)1.23–1.26(m) 32.9CH2 7 1.95–1.98(m)1.57–1.59(m) 31.7CH2
8 – 40.0C 8 0.94–0.96(m) 45.9CH
9 1.69(dd,J=13.1,3.9) 47.7CH 9 0.91–0.93(m) 50.3CH
10 – 36.9C 10 – 36.5C
11 1.94–1.96(m)1.83–1.85(m) 23.7CH2 11 1.59–1.61(m)1.50–1.53(m) 20.8CH2
12 5.14(t,J=3.5) 124.4CH 12 2.05–2.07(m)1.21–1.24(m) 39.7CH2
13 – 139.6C 13 – 42.1C
14 – 42.8C 14 1.05–1.07(m) 56.8CH
15 2.18–2.20(m)1.10–1.13(m) 27.3CH2 15 1.25–1.27(m) 25.7CH2
16 2.04–2.06(m)1.90–1.92(m) 25.8CH2 16 1.92–1.94(m)1.63–1.65(m) 29.3CH2
17 – 33.1C 17 1.17–1.19(m) 56.1CH
18 1.93(d,J=7.2) 59.1CH 18 1.06(s) 18.4CH3
19 – 72.9C 19 0.75(s) 11.9CH3
20 1.20–1.22(m) 42.1CH 20 1.54–1.56(m) 31.9CH
21 1.34–1.36(m)1.27–1.29(m) 31.3CH2 21 0.98(d,J=6.8) 18.0CH3
22 1.97–1.99(m)1.67–1.68(m) 39.7CH2 22 1.41–1.46(m)1.07–1.09(m) 33.7CH2
23 0.83(s) 28.2CH3 23 1.66–1.68(m)1.12–1.15(m) 23.9CH2
24 0.89(s) 15.9CH3 24 1.47–1.49(m) 36.1CH2
25 1.02(s) 16.9CH3 25 1.25–1.27(m) 29.0CH
26 1.03(s) 17.6CH3 26 0.87(d,J=6.3) 21.8CH3
27 1.14(s) 23.5CH3 27 0.86(d,J=6.3) 21.9CH3
28 0.81(s) 28.4CH3 1′ 4.40(d,J=7.0) 101.0CH
29 1.15(s) 26.6CH3 2′ 3.18–3.20(m) 73.7CH
30 0.98(d,J=6.3) 21.4CH3 3′ 3.27–3.29(m) 76.5CH
1′ – 170.3C 4′ 3.29–3.31(m) 70.3CH
2′ 2.34(t,J=6.8) 33.8CH2 5′ 3.35–3.38(m) 76.7CH
3′ 1.78–1.80(m) 25.2CH2 6′ 3.85–3.87(m)3.67–3.70(m) 61.4CH2
(CH2)4′-8′ 1.27–1.30(m) 29.2–29.9CH2 – – –
9′ 1.23–1.25(m) 22.7CH2 – – –
10′ 0.81(t,J=6.5) 14.1CH3 – – –
Plantmaterial
TheaerialpartsofPlectranthusmontanusBenth.(syn. Plectran-thuscylindraceusHoechst.Ex.Benth.),Lamiaceae,werecollectedin March2011fromAbha,SaudiArabia.Theplantwasidentifiedby Dr.MohamedYousef,Prof.ofPharmacognosy,CollegeofPharmacy, KingSaudUniversity,SaudiArabia.Avoucherspecimen(P-5-2011) wasdepositedattheherbariumoftheDepartment.
Extractionandisolation
Theair-driedpowderedaerialparts(1kg)wasextractedwith MeOH (4×3l). The combined MeOH extract was concentrated under reduced pressure to yield a dark green viscous residue (19.2g).ThelatterwassubjectedtoVLCusinghexane,EtOAc,and MeOH.Eachfractionwasconcentratedtogivehexane(2.9g),EtOAc (5.6g),andMeOH(9.8g)fractions.TheEtOAcfraction(5.6g)was subjectedtoVLCusinghexane:EtOAcgradienttoaffordten subfrac-tions:P-1toP-10.SubfractionP-3(718mg)waschromatographed overSiO2 column(50g,50×3cm)usinghexane:EtOAcgradient togivestigmasterol(82.1mg,colorlessneedles).SubfractionP-4 (560mg)wassimilarlytreatedassubfractionP-3togiveimpure plectraterpene (1). Purification of 1 wasachieved onRP18 col-umnchromatography(30g,50×2cm)usingMeOH:H2Ogradient toafford 1 (15.9mg,white amorphous powder).Subfraction P-5(610mg)wassubjectedtoSiO2 column(55g,50×3cm)using hexane:EtOAcgradienttoaffordsitosterylferulate(37.5mg,white amorphouspowder).SubfractionP-6 (380mg)wassubjectedto SiO2 column (35g, 50×2cm) using hexane:EtOAc gradient to affordimpuresitosterylferulatewhichwaspurifiedonLiChrolut
EN/RP18solidphaseextractiontubeusingH2O:acetonitrile gradi-enttoyieldcholest-5-en-3-O--d-glucopyranoside(2)(11.5mg, whiteamorphouspowder).SubfractionP-7(508mg)wassubjected toSiO2 column (45g,50×3cm)usingn-hexane:EtOAcgradient gavestigmasterol-3-O--d-glucopyranoside(89mg,white amor-phouspowder).
Plectraterpene(3-(decanoyloxy)-19-hydroxy-urs-12-ene;1): whiteamorphouspowder;[␣]D+39(c=0.1,MeOH);IR(KBr):3436, 2951,1729,1619,1248cm−1;1H(CDCl3,700MHz)and13CNMR data(CDCl3,176MHz)seeTable1;HR-ESI-MSm/z597.5242(calcd
for597.5247[M+H]+,C
40H69O3).
Cholest-5-en-3-O--d-glucopyranoside; 2: white amorphous
powder;[␣]D+56(c=0.5,MeOH);IR(KBr):3396,2945,1635cm−1; 1H (CDCl
3, 700MHz) and 13C NMR data (CDCl3, 176MHz)see
Table 1; HR-ESI-MS m/z549.4159 (calcd for 549.4155 [M+H]+,
C33H57O6).
Alkalinehydrolysisofcompound1
A solution of 1 (7mg) in 3% KOH/MeOH (5ml) was left to
standfor15minatroomtemperaturethenneutralizedwith1N HCl/MeOH.The solutionwasextractedwithCHCl3.The solvent
wasevaporatedandtheresidueobtainedwaschromatographed
onSiO2column,usinghexane:EtOAcgradienttofurnishthemethyl esterofdecanoicacid,whichwasidentifiedbyGC-MSusingClarus 500GC/MS(Perkin-Elmer,Shelton,CT)aspreviouslyoutlined(
Al-Musayeibetal.,2013;El-Shanawanyetal.,2015;Ibrahim etal.,
700 M.Aminaetal./RevistaBrasileiradeFarmacognosia27(2017)698–701
Resultsanddiscussion
Thedried-powderedaerialpartswereextractedwithMeOH.
TheconcentratedMeOHextractwassubjectedtovacuumliquid
chromatography(VLC)usinghexane,EtOAc,andMeOH.TheEtOAc
fractionwasseparatedonVLC,silicagel,andRp18CCtoyieldone new(1)andfourknowncompounds.
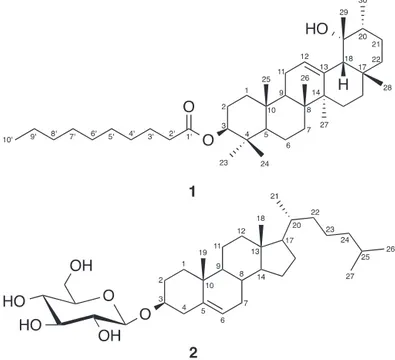
Compound1wasobtainedaswhiteamorphouspowder.Itgave apositiveLiebermann-Burchard’sreaction(Gołembiewskaetal., 2013), indicatingitstriterpenoidalnature.Itsmolecularformula
was determined as C40H68O3 based on the HRESIMS
pseudo-molecularionpeaksatm/z597.5242(calcdforC40H69O3,597.5247 [M+H]+),requiringsevendegreesofunsaturation.Thesedegrees ofunsaturationcanbeaccountedforfiveringssystem,oneolefinic doublebond,andoneestercarbonylgroup.ItsIRspectrumshowed characteristic absorptionbands for hydroxyl (3436cm−1), ester carbonyl(1729cm−1),anddoublebond(1619cm−1).The13Cand
HSQCspectrashowedthepresenceof40 carbons,consistingof
ninemethyls, seventeenmethylenes,six methinesoneof them
for oxymethine at ı(C) 79.0 C(3) and one for a tri-substituted olefinicdoublebondatı(C)124.4C(12),andeightquaternary car-bonsincludingonecarbonylatı(C)170.3C(1′)andoxygen-bonded quaternarycarbonı(C)72.9C(19).Detailed1Dand2DNMR anal-ysisof1suggestedthatitisanursanetypepentacyclictriterpene
(Ibrahimetal.,2012;Ibrahimetal.,2016a,b).The1HNMR
spec-trumof1exhibitedsixsingletmethylsatı(H)0.83H-C(23),0.89 H-C(24),1.02H-C(25),1.03H-C(26),1.14H-C(27),0.81 H-C(28), and1.15H-C(29),andonedoubletmethylgroupatı(H)0.98(d,
J=6.3Hz,H-C(30)),establishingtheursane-typecarbonframework
of1(Ibrahimetal.,2012;Khedretal.,2016).Theycorrelatedtothe
carbonsresonatingatı(C)28.2,15.9,16.9,17.6,23.5,28.4,26.6,and 21.4,respectivelyintheHSQCspectrum.Theirpositionswere con-firmedbytheobservedHMBCcrosspeaksofH-C(23)andH-C(24)to C(3),C(4),andC(5),H-C(25)toC(1),C(9),andC(10),H3-26toC(7), C(8),C(9),andC(14),H-C(27)toC(8),C(13),C(14),andC(15), H-C(28)toC(17),C(18),andC(22),H-C(29)toC(18),C(19),andC(20), andH-C(30)toC(19),C(20),andC(21)(Fig.1).Anoxymethine sig-nalwasobservedatı(H)4.31(dd,J=11.5,5.0Hz,H-C(3)),showing HSQCcrosspeaktothecarbonsignalatıC79.0C(3).This assign-mentwasestablishedbythe1H–1HCOSYcorrelationsofH-C(3)
withH-C(2)andproved bytheHMBCcrosspeaksofH-C(1),
H-C(23),andH-C(24) toC(3)(Fig.1).Furthermore,the1Hand13C NMRspectraof 1 revealedsignals atı(H) 5.14(d,J=3.5Hz, H-C(12))/ı(C)124.4C(12)and139.6C(13),indicatingthepresence ofatri-substitutedolefinicdoublebond(Table1).Itspresenceat
HO
OH
OH HO
HO
O
O
O O 5
Fig.1.Somekey1H–1HCOSY( )andHMBC(H
→C)correlationsof1and2.
C(12)/C(13)wassecuredbasedonthe3JHMBCcorrelationsof H-C(9)andH-C(18)toC(12)andH-C(27)toC(13).Theoxygen-bonded quaternarycarbonatıC72.9wasassignedtoC(19).Thisassignment wasestablishedbythecrosspeaksofH-C(18),H-C(20),H-C(21), andH-C(30)toC(19)observedinHMBC.Additionally,thesignalsfor primarymethylatı(H)0.81(t,J=6.5Hz,H-C(10′))/ı(C)14.1C(10′), aliphaticmethylenesclusteratı(H)1.27–1.30(m,H-C(4′-8′)/ı(C) 29.2–29.9C(4′-8′),andcarbonylatı(C)170.3C(1′)indicatedthe presenceoffattyacylmoietyin1(Mohamedetal.,2013;Mohamed,
2014;El-Shanawanyet al.,2015).Alkalinehydrolysis of1 gave
amethylesterofdecanoicacid,whichwasidentifiedbyGC–MS molecularionpeakatm/z186[M]+.Thiswasfurtherconfirmedby theESIMSfragmentionpeakatm/z442[M+H-OC10H19]+.Its con-nectivityatC(3)wasestablishedbasedontheHMBCcorrelation ofH-C(3)toC(1′)andconfirmedbythedownfieldshiftofH-C(3) (ı(H)4.31)(Al-Musayeibetal.,2013).Therelativestereochemistry atC-3andC-19wasassignedbasedonthecomparisonofthe cou-plingconstantvalues aswellas1Hand 13C chemicalshiftwith thoseofrelatedtriterpenes(MahatoandKundu,1994;Akbarand
Malik,2002;Kimetal.,2012).Onthebasisofthesefindingsandby
comparisonwiththeliterature,thestructureof1wasassignedas 3-(decanoyloxy)-19-hydroxy-urs-12-eneandnamed plectrater-pene.
Compound 2 was obtained as white amorphous powder.
It gave a positive Liebermann-Burchard’s test, suggesting its
steroidalnature(Gołembiewskaetal.,2013).ItsHRESIMS spec-trumshowedapseudo-molecularionpeakatm/z549.4159(calcd for549.4155[M+H]+),whichwascompatiblewiththemolecular formulaC33H56O6,representingsixdegreesofunsaturation.The IRspectrum of 2showed characteristic absorptionbands, indi-catingthepresenceofhydroxylgroup(3396cm−1),unsaturation (1635cm−1),andaliphaticC-H(2945cm−1).The1Hand13CNMR spectra exhibited two tertiary methyls [ı(H) 1.06 and 0.75 for H-C(18)/C(18)andH-C(19)/C(19),respectively],three secondary methyls[0.98(d,J=6.8Hz,H-C(21)),0.87(d,J=6.3Hz,H-C(26)), and0.86(d,J=6.3Hz,H-C(27))],adoublebondatı(H)5.40(brs, H-C(6))/ı(C) 121.4 C(6) characteristic for cholesteryl derivative
(Plouguerné etal., 2006).The placementof thedouble bondat
C(5)-C(6)wasassignedbasedontheobserved1H–1HCOSYand HMBCcorrelations(Fig.1).Thegeometryofthedoublebondwas deducedtobeZbasedonthecouplingconstantvalue(Plouguerné
M.Aminaetal./RevistaBrasileiradeFarmacognosia27(2017)698–701 701
andHSQCspectradisplayed33signals,27ofthemwereattributed tocholesterylmoietywitholefiniccarbonsatı(C)141.0C(5)and 121.4C(6)andtheremainingsixcarbonsforglucosemoiety.This wasconfirmedbytheobservedsignalsatı(H)4.40(d,J=7.0Hz, H-C(1′)/ı(C)101.0C(1′)characteristicforthe-glucopyranosemoiety
(Mohamedetal.,2014).IntheHMBC,thecrosspeakofH-C(1′)to
C(3)(ı(C)78.4)confirmedtheattachmentofglucosemoietyatC-3 (Fig.1).Onthebasisofthepreviousmentioneddata,2were iden-tifiedascholest-5-en-3-O--d-glucopyranosideandinagreement
withtheliterature(MustafaandAli,2011).
Theotherisolatedcompoundswereidentifiedasstigmasterol
(MohamedandIbrahim,2007;Ibrahimetal.,2016a,b),sitosteryl
ferulate (Jaiswal et al., 2015), and stigmasterol-3-O--d-gluco
(Ibrahim,2010)bytheanalysisofthespectroscopicdata(1D,2D
NMR,andMS)andcomparisonoftheirdatawiththoseinthe liter-ature.
Authors’contributions
MAandNAMdesignedthestudy,performedextraction, isola-tionandcharacterizationofisolatedconstituents.GAMandSRMI participatedNMRinterpretation,manuscriptpreparationandalso contributedtocriticalreadingofmanuscript.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgment
This research project was supported by a grant from the
‘ResearchCenterof theFemaleScientific andMedical Colleges’, DeanshipofScientificResearch,KingSaudUniversity.
References
Akbar,E.,Malik,A.,2002.AntimicrobialtriterpenesfromDebregeasiasalicifolia.Nat. Prod.Lett.16,339–344.
Alasbahi,R.H.,Melzig,M.F.,2010.Plectranthusbarbatus:areviewofphytochemistry, ethnobotanicalusesandpharmacology.PlantaMed.76,653–661.
Al-Musayeib,N.M.,Mohamed,G.A.,Ibrahim,S.R.M.,Ross,S.A.,2013. Lupeol-3-O-decanoate,anewtriterpeneesterfromCadabafarinosaForsk.growinginSaudi Arabia.Med.Chem.Res.22,5297–5302.
Asresa,K.,Tadesse,S.,Mazumder,A.,Bucar,F.,2013.EssentialoilofPlectranthus cylindraceusHochst.ex.Benth.fromEthiopia:chemicalcompositionand antimi-crobialactivity.J.Essent.OilBear.Plants16,136–143.
Elkhayat,E.S.,Ibrahim,S.R.M.,Mohamed,G.A.,Ross,S.A.,2015.TerrenolideS,anew anti-leishmanialbutenolidefromtheendophyticfungusAspergillusterreus.Nat. Prod.Res.30,814–820.
El-Shanawany,M.A.,Sayed,H.M.,Ibrahim,S.R.M.,Fayed,M.A.A.,2015.Stigmasterol tetracosanoate,anewstigmasterolesterfromtheEgyptianBlepharisciliaris. DrugRes.65,347–353.
Gołembiewska,E.,Skalicka-Wo ´zniak,K.,Głowniak,K.,2013.Methodsforthe isola-tionandidentificationoftriterpenesandsterolsinmedicinalplants.Curr.Issues Pharm.Med.Sci.26,26–32.
Grayer,R.J.,Eckert,M.R.,Lever,A.,Veitch,N.C.,Kite,G.C.,Paton,A.J.,2010. Distri-butionofexudatesflavonoidsinthegenusPlectranthus.Biochem.Syst.Ecol.38, 335–341.
Ibrahim,S.R.M.,2010.New2-(2-phenylethyl)chromonederivativesfromtheseeds ofCucumismeloLvar.reticulates.Nat.Prod.Commun.5,403–407.
Ibrahim,S.R.M.,Mohamed,G.A.,Shaala,L.A.,Banuls,L.M.Y.,VanGoietsenoven,G., Kiss,R.,Youssef,D.T.A.,2012.Newursane-typetriterpenesfromtherootbark ofCalotropisprocera.Phytochem.Lett.5,490–495.
Ibrahim,S.R.M.,Elkhayat,E.S.,Mohamed,G.A.,Khedr,A.I.M.,Fouad,M.A.,Kotb, M.H.R.,Ross,S.A.,2015.AspernolidesFandG,newbutyrolactonesfromthe endophyticfungusAspergillusterreus.Phytochem.Lett.14,84–90.
Ibrahim,S.R.M.,Mohamed,G.A.,Ross,S.A.,2016a.IntegracidesFandG:new tetra-cyclictriterpenoidsfromtheendophyticfungusFusariumsp.Phytochem.Lett. 15,125–130.
Ibrahim,S.R.M.,AlHaidari,R.A.,Mohamed,G.A.,Moustafa,M.A.A.,2016b.Cucumol A:acytotoxictriterpenoidfromCucumismeloseeds.Rev.Bras.Farmacogn.26, 701–704.
Jaiswal,S.G.,Pradhan,S.,Patel,M.,Naik,M.,Naik,S.,2015.Ricebranoildistillate,a choicefor[gamma]-oryzanol:separationandoxidativestabilitystudy.J.Food Res.4,36–43.
Khedr,A.I.M.,Ibrahim,S.R.M.,Mohamed,G.A.,Ahmed,H.E.A.,Ahmad,A.S.,Ramadan, M.A.,AbdEl-Baky,A.E.,Yamada,K.,Ross,S.A.,2016.Newursanetriterpenoids fromFicuspandurataandtheirbindingaffinityforhumancannabinoidand opi-oidreceptors.Arch.Pharm.Res.39,897–911.
Kim,K.H.,Choi,S.U.,Lee,K.R.,2012.CytotoxictriterpenoidsfromBerberiskoreana. PlantaMed.78,86–89.
Lukhoba,C.W.,Simmonds,M.S.J.,Paton,A.J.,2006.Plectranthus:areviewof ethanob-otanicaluses.J.Ethnopharmacol.103,1–24.
Mahato,S.B.,Kundu,A.P.,1994.13CNMRspectraofpentacyclictriterpenoids–a compilationandsomesalientfeatures.Phytochemistry37,1517–1575. Marwah,R.G.,Fatope,M.O.,Deadman,M.L.,Ochei,J.E.,Al-Saidi,S.H.,2007.
Antimi-crobialactivityandthemajorcomponentsoftheessentialoilofPlectranthus cylindraceus.J.Appl.Microbiol.103,1220–1226.
Mohamed,G.A.,2014.NewcytotoxiccycloartanetriterpenefromCassiaitalicaaerial parts.Nat.Prod.Res.28,976–983.
Mohamed,G.A.,Ibrahim,S.R.M.,2007.EucalyptoneG,anewphloroglucinol deriva-tive and other constituents from Eucalyptus globulus Labill. Arkivoc (xv), 281–291.
Mohamed,G.A.,Ibrahim,S.R.M.,Ross,S.A.,2013.Newceramidesandisoflavonefrom theEgyptianIrisgermanicaL.rhizomes.Phytochem.Lett.6,340–344. Mohamed,G.A.,Ibrahim,S.R.M.,Al-Musayeib,N.M.,Ross,S.A.,2014.New
anti-inflammatoryflavonoidsfromCadabaglandulosaForssk.Arch.Pharm.Res.37, 459–466.
Mustafa,A.,Ali,M.,2011.Newsteroidallactonesandhomomonoterpenicglucoside fromfruitsofMalvasylvestris.ActaPol.Pharm.68,393–401.
Orabi,K.Y.,Mossa,J.S.,Muhammed,I.,Alloush,M.H.,Galal,A.M.,El-Feraly,F.S., McPhai,A.T.,2000.NeweudesmanesesquiterpenesfromPlectranthus cylin-draceus.J.Nat.Prod.63,1665–1668.
Plouguerné,E.,Kikuchi,H.,Oshima,Y.,Deslandes,E.,Stiger-Pouvreau,V.,2006. Isola-tionofcholest-5-en-3-olformatefromtheredalgaGrateloupiaturuturuYamada anditschemotaxonomicsignificance.Biochem.Syst.Ecol.34,714–717. Rahman,M.A.,Mossa,J.S.,Al-Said,M.S.,Al-Yahya,M.A.,2004.Medicinalplant
diver-sityinthefloraofSaudiArabia1:areportonsevenplantfamilies.Fitoterapia 75,149–161.