w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Aqueous
extract
of
Baccharis
trimera
improves
redox
status
and
decreases
the
severity
of
alcoholic
hepatotoxicity
Ana
Carolina
S.
Rabelo
a,
Glaucy
R.
de
Araújo
a,
Karine
de
P.
Lúcio
a,
Carolina
M.
Araújo
a,
Pedro
H.
de
A.
Miranda
a,
Breno
de
M.
Silva
b,
Ana
Claudia
A.
Carneiro
b,
Érica
M.
de
C.
Ribeiro
b,
Wanderson
G.
de
Lima
c,
Gustavo
H.
B.
de
Souza
d,
Geraldo
C.
Brandão
d,
Daniela
C.
Costa
a,∗aLaboratóriodeBioquímicaMetabólica,DepartamentodeCiênciasBiológicas,ProgramadePós-graduac¸ãoemCiênciasBiológicas,UniversidadeFederaldeOuroPreto,OuroPreto,
MG,Brazil
bLaboratóriodeBiologiaeBiotecnologiadeMicro-organismos,DepartamentodeCiênciasBiológicas,ProgramadePós-graduac¸ãoemCiênciasBiológicas,UniversidadeFederalde
OuroPreto,OuroPreto,MG,Brazil
cLaboratóriodeMorfopatologia,DepartamentodeCiênciasBiológicas,ProgramadePós-graduac¸ãoemCiênciasBiológicas,UniversidadeFederaldeOuroPreto,OuroPreto,MG,Brazil dLaboratóriodeFarmacognosia,EscoladeFarmácia,ProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldeOuroPreto,OuroPreto,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23June2017 Accepted13September2017 Availableonline4November2017
Keywords:
Ethanol Redoximbalance
Baccharistrimera
Aqueousextract Hepatotoxicity
a
b
s
t
r
a
c
t
Themetabolismofethanoloccursmainlyintheliver,promotingincreaseofreactiveoxygenspeciesand nitrogen,leadingtoredoximbalance.Therefore,antioxidantscanbeseenasanalternativetoreestablish theoxidizing/reducingequilibrium.Theaimofthisstudywastoevaluatetheantioxidantand hepatopro-tectiveeffectofaqueousextractofBaccharistrimera(Less.)DC.,Asteraceae,inamodelofhepatotoxicity inducedbyethanol.TheextractwascharacterizedandinvitrotestswereconductedinHepG2cells.Itwas evaluatedthecellsviabilityexposedtoaqueousextractfor24h,abilitytoscavengingtheradicalDPPH, besidestheproductionofreactiveoxygenspeciesandnitricoxide,andtheinfluenceonthe transcrip-tionalactivityoftranscriptionfactorNrf2(12and24h)afterexposureto200mMethanol.Theresults showedthataqueousextractwasnon-cytotoxicinanyconcentrationtested;moreover,itwasobserved adecreaseinROSandNOproduction,alsopromotingthetranscriptionalactivityofNrf2.Invivo,we pre-treatmentmaleratsFisherwith600mg/kgofaqueousextractand1hlater5ml/kgofabsoluteethanol wasadministrated.Aftertwodaysoftreatment,theanimalswereeuthanizedandlipidprofile,hepatic andrenalfunctions,antioxidantstatusandoxidativedamagewereevaluated.Thetreatmentwithextract improvedliverfunctionandlipidprofile,reflectingthereductionoflipidmicrovesiculesintheliver.It alsopromotedanincreaseofglutathioneperoxidaseactivity,decreaseofoxidativedamageand MMP-2activity.Theseresults,analyzedtogether,suggestthehepatoprotectiveeffectofB.trimeraaqueous extract.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Ethanolisthemostusedalcoholinalcoholicbeveragesandits
abusiveconsumptionisassociatedwithvarioushealthproblems
worldwide(LíveroandAcco,2016).Themetabolizationofethanol occursmainlyintheliver,whereitisconvertedtoacetaldehyde byalcoholdehydrogenaseandsubsequentlyoxidizedtoacetateby
acetaldehydedehydrogenase(Cederbaum,2012).Theseenzymes
useNAD+ as cofactor and generate NADH, thus decreasingthe
∗ Correspondingauthor.
E-mail:daniela.costa@iceb.ufop.br(D.C.Costa).
NAD+/NADH ratio,affecting severalmetabolic pathways(Smith
et al., 2007), besides promoting the increase of acetaldehyde
adductsandreactiveoxygenspecies(ROS),leadingtooxidative
stress(Luetal.,2012;Hanetal.,2016).Insomecircumstances, themicrosomaloxidationsystemofethanol(CYP2E1)canbe
acti-vated,whichcontributesevenmoretotheROSformation(Smith
etal.,2007;Cenietal.,2014;Hernándezetal.,2015).
Alcoholicfattyliverdisease(AFLD)isthefirstresponseofthe livertoethanoluseandischaracterizedbyaccumulationoflipidsin hepatocytes(Cenietal.,2014).Anoptimalpharmacological
treat-mentforAFLDwouldreduceinflammatoryparameters,oxidative
stressandlipidaccumulation,andavoidfibroticevents.However,
thedevelopmentofadrugthatiscapableofactingonsomany
differentpathwaysisextremelydifficult.Forthisreason,asingle
https://doi.org/10.1016/j.bjp.2017.09.003
drugtherapyhasnotbeendeveloped,butcombinedtherapiesin anattempttoreversehepatocyteinjury(LíveroandAcco,2016).
Duetothegreatimportanceofoxidativestressinthe pathogen-esisofAFLD,severalstudieshavefocusedontheuseofantioxidants
to preventoxidative damage and improve liver function (Ceni
etal.,2014;Hernándezetal.,2015;LíveroandAcco,2016).ROS
canbeneutralizedbyenzymatic antioxidants,suchas
superox-idedismutase(SOD),catalase,glutathioneperoxidase(GPx)and
glutathionereductase(GR)andnonenzymaticasglutathione, vita-minsanddietaryantioxidants(Chenetal.,2015;Hanetal.,2016).
The antioxidant enzymes are mainly regulatedby thefactor-2
nuclear-erythroidfactor(Nrf-2)(Lushchak,2014;Chenetal.,2015; González et al., 2015). This factor is usually found in the cell cytoplasmassociatedwiththeECH-associatedcalcite-likeprotein (Keap-1),whichallowsitslabelinganddegradationNrf2via pro-teasome(Chenetal.,2015).However,inthepresenceofoxidative stressthefactormigrates tothenucleus,where it bindstothe
antioxidantresponse element(ARE), promotingtheantioxidant
enzymesexpression(Lushchak,2014;Chenetal.,2015;González
etal.,2015;KimandKeum,2016).
In addition to the enzymatic antioxidants, the inclusion of
antioxidantsinthedietisofgreatimportanceandthe consump-tionisrelatedtothereductionoftheriskofthedevelopmentof diseasesassociatedwiththeaccumulationoffreeradicals,since
inthesecompoundssubstancesthatactinsynergisminthe
pro-tectionof cells andtissues canbefound(Bianchi and Antunes,
1999). In fact, some studies have demonstrated the beneficial effectofnatural dietaryantioxidants(Al-Sayedetal.,2014; Al-Sayedetal.,2015;Páduaetal.,2010;Líveroetal.,2016a,b;Fahmy etal.,2017).Thereby,medicinalplantshaveattractedthe atten-tionofresearchersaspotentialagentsagainstalcoholicliverinjury becauseoftheirantioxidantpotentialandthefewsideeffects(Ding et al.,2012).However, mostplant species areonly empirically used,andtherearefewstudiesthatprovetheirtherapeuticefficacy (Foglioetal.,2006).
Inthissense,Baccharistrimera(Less.)DC.,Asteraceae,isa medic-inalplantusedinpopularcultureandwidelydistributedinSouth America(Bonaetal.,2005).InBrazil,thisplantispopularlyknown ascarquejaandusedasgastricprotector(Líveroetal.,2016a,b),
hypoglycemic (Oliveira et al., 2011), anti-inflammatory (Karam
etal.,2013)andantioxidant(Páduaetal.,2013;deAraújoetal., 2016).Somestudies arefocusedin theuseof medicinalplants
inethanol-inducedintoxication,however moststudiesuseonly
alcoholic/hydroethanolicextractsanditisknownthatthesolvent usedfortheextractionofthesecondarymetabolitesisinvolvedin thebiologicalactivityoftheseplants(Rates,2001).Basedonthese evidencesandonthefactthattherearenostudieswithaqueous extractofB.trimerainethanol-inducedhepatotoxicityavailable, ourgoalwastocharacterizethisextractandverifyitseffecton pro-tectionagainstalcoholichepatotoxicityinvitroandinvivomodel.
Materialsandmethods
Plantmaterial
TheaerialpartsofBaccharistrimera(Less.)DC.,Asteraceae,were collectedinOuroPretocity,inMinasGeraisstate,Brazil.The
spec-imenswereauthenticatedand depositedat theHerbarium José
Badini(UFOP),OUPR22.127.Afteridentification,theaerialparts weredriedinaventilatedoven(30◦C),pulverizedandstoredin plasticbottles.Toobtaintheaqueousextract,approximately100g oftheplantwasextractedwith1lofwaterfor24h,bymaceration. Thesolidswereremovedbyvacuumfiltrationandthesolventwas removedbyarotaryevaporatorat40◦C(Páduaetal.,2010).
RP-UPLC-DAD-ESI-MSanalyses
Theaqueous extract wasanalyzed in theUltraPerformance
LiquidChromatography coupledtodiode arrangementdetector
andmass spectrometry.Inthis assay,20mg ofthesample was
appliedanddilutedwith4mlofMeOH/H2O(9:1).Theeluatewas driedandresuspendedinasolutionofmethanolandthenfiltered on Chromafil®
PDVF syringe filters (polyvinylidene dilfluoride, 0.20mm)inavolumesufficienttoobtain2mg/mlconcentrationof thesample.FortheanalysisinHPLC-DAD-EM,20lofthesample
wereinjectedintotheliquidchromatograph,inthesame
condi-tionsdescribedbydeAraújoetal.(2016).
Invitrotests
DPPHradical-scavengingactivity
The percentage of antioxidant activity of each substance
was assessed by DPPH free radical assay, according to Araújo
etal. (2015).In brief,aqueousextractwasdiluted inmethanol
80%and dilutionswereperformedtoobtaintheconcentrations
(25–500g/ml).Thestandardcurvewasperformedwiththe
ref-erenceantioxidantTrolox
(6-hydroxy-2,5,7,8-tetramethylchrome-2-carboxylic acid). As blankmethanol (80%) wasused and the
antioxidant activity was determined by the decrease in the
DPPH absorbance and the percent inhibition was calculated
usingthefollowingequation:%antioxidantactivity=(1−ASample
515/AControl515)×100.
Cellculture
Hepatocellularcarcinomacellline(HepG2)wasacquiredfrom
theCellBankfromtheFederalUniversityofRiodeJaneiro.Thecells
wereplacedinsterile75cm2growthvialscontainingtheDMEM
culture medium and supplemented with antibiotic
(Penicillin-Streptomycin)and10%(v/v)fetalbovineserum.Thebottleswere
incubatedin anovenat37◦C humidifiedwith5%carbon
diox-ide(CO2).Cellswereusedforassayswhentheconfluencereached
about80%,sothemediumwasaspiratedandthemonolayerwashed
withbufferedsaline(PBS).Afterthis,2mloftrypsinandEDTA solu-tion(0.20%and0.02%,respectively)wereused.Subsequently,the cellswerecentrifugedandthesupernatantwasdiscardedandthe
cellpelletwasresuspendedin1mlofDMEMmedium.Thecells
werethencountedwithTrypanBlue0.3%intheNeubauerchamber. Foreachexperimenttriplicatewasused,withbiologicalduplicate (totalingn=6pergroup).
Cellviabilityassay
CellviabilitywasdeterminedusingcolorimetricMTT
(3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay
as described previously (Fotakis and Timbrell, 2005). In brief,
HepG2 cells (1×105) were cultured in 96 well-plates with or
without different aqueous extract concentrations of B. trimera
(5–600g/ml),whichwasdilutedinDMEMmedium,andethanol
(5–800mM) for 24h. After incubation, medium was removed
and MTT solution(5mg/ml) was added and incubated for
fur-ther 1h at 37◦C. Subsequently, dimethyl sulfoxide was added
todissolveformazancrystalsandtheabsorbancewasmeasured
at 570nm. The cell viability percentage was calculated based
in the formula below, where the control was assigned 100%
viability.
% ofcellviability= absorbanceabsorbanceoftreatedcontrolcells×100
ROSandNOproduction
For the determination of reactiveoxygen species and nitric
twodifferentaqueousextractofB.trimeraconcentrations(10and
50g/ml),dilutedinDMEMmedium.Afteranincubationof3h,
themediumwasremovedand200mMofethanolwasaddedwith
50Mofcarboxi-H2DCFDA(forROSproduction)or10Mof
DAF-FM(for NOproduction). Theplate wasincubated for24hand,
then,HANKSwasadded.Thereadingwasobtainedinmicroplate
reader, using 485nm for excitation and 535nm for emission
microwave.
Luciferasereporterassay
To evaluate the effect of aqueous extract of B. trimera on
transcriptionalactivityofNrf2,LuciferaseassaySystemwasused, accordingtoSilvaetal.(2011),withsomemodifications.Forthis,a kitDualLuciferaseassaySystemwasused.Briefly,1.5×105HepG2
cellswereplatedin24-wellplatesandincubatedfor24h.Then,
mediumwithoutSFB wasaddedandincubatedforfurther24h.
Afterthistime,transfectionwasperformedwith100lperwellof mix(500ngoflipofectamine,100ngofpRL-TK,400ngofpPGL37
andmediumHGtocompletethevolume).Six-hourincubationwas
carriedoutand,then,10and50g/ml ofaqueousextractwere
added.After3-hourincubation,200mMofethanolwasaddedand
anewincubationwasperformedfor12or24h.Afterthis,100l
oflysis buffer(providedbythekit)wasadded andcentrifuged
for4minat10,000rpm.Then, 15lofsupernatantand35lof LuciferaseIIreagent(LAR-II)werereadinluminometer(580nm), providingthe“NetA”value.After,50lofStopandGlo®
were added,providingthe“NetB”value.ForcalculationstheNetA/NetB ratiowasused.
Invivotests
Animals
MaleFisherrats(220–250g),obtainedfromtheLaboratoryof ExperimentalNutritionfromtheFederalUniversityofOuroPreto, werekeptoncollectivecages,ina12hlight/darkcycleatroom temperatureandwerefasted12hwithwateradlibitumbeforethe
experiment.Allanimalswereused accordingtotheCommittee
guidelinesonCareandUseofAnimalfromFederalUniversityof
OuroPreto,Brazil(No.2016/01).
Theexperimentalprotocol
Theanimalsweredividedintothreegroups:
-Controlgroup(C)(n=7):received1mlofwater;
-Ethanolgroup(E) (n=5): received1mlofwater and 1hlater 5ml/kgofabsoluteethanol(El-Naga,2015).
-AqueousextractofB.trimera(Aq)(n=7):received600mg/kgof extractand 1hlater 5ml/kgofabsoluteethanol (Páduaetal., 2010,2013,2014).
Alltreatmentswereadministratedbygavage,totalingavolume of1mlofsolution.Theanimalsweretreatedfortwoconsecutive daysand24hafterthelastethanoldose,theywereeuthanizedby deepanesthesiainducedbyisoflurane.Theyweremaintainedona 12hfasting.
Analysisofbiochemicalserumparameters
Theserumwasusedfordeterminingtheurea,creatinine,ALT, AST,proteintotal,glucose,totalcholesterol,HDL,fractionnon-HDL,
triacylglycerides.Allmeasurementswereperformedby
commer-ciallaboratorieskitsLabtest®
(LagoaSanta,MG,Brazil)andBioclin® (BeloHorizonte,MG,Brazil).
Determinationofantioxidantsystem
TheantioxidantsystemwasevaluatedbySOD,catalase,
glu-tathione peroxidaseand reductaseactivity, beyondglutathione
total (oxidized and reduced). The assay for determination of
indirectSOD-activity is based onSOD competition with
super-oxide radical, formed by self-oxidation of pyrogallol, which is
responsibleforMTTreductionandformationofformazancrystals
(MarklundandMarklund,1974).Catalaseactivitywasdetermined
based on its ability to convert hydrogen peroxide (H2O2)into
water and molecular oxygen (Aebi, 1984). Glutathione system
wasdetermined by kit (Sigma–Aldrich, St. Louis,MO, USA).To
determine catalase and superoxide dismutase activity, 100mg
of liver tissue washomogenized in phosphate buffer(pH 7.4).
Total glutathione and reduced/oxidize glutathione
concentra-tions were determined by the homogenization of 100mg of
tissue in 5% sulfosalicylicbuffer. For correctionof thedosages,
theprotein wasmeasured bythe Lowrymethod(Lowry etal.,
1951). After homogenization, the samples were centrifuged at
10,000×g for 10min, at 4◦C. The supernatant was collected
and used as the sample and all dosages were according to
Bandeiraetal.(2017).
Determinationofoxidativestressmarkers
Inordertoevaluateoxidativedamagethiobarbituricacid reac-tivesubstances(TBARS)andcarbonylproteinsuchasmarkerswere
used.TheTBARSconcentrationwasdeterminedbasedon
thiobar-bituricacid(TBA)bindingtooxidizedlipids,accordingtoBuegeand Aust(1978).Inthemethodfordeterminingcarbonylatedprotein, itwasused2,4-dinitrophenylhydrazine(DNPH),whichreactswith carbonylgroupstogeneratethecorrespondinghydrazonethatcan beanalyzedspectrophotometrically,asdescribedbyLevineetal. (1994).Forcorrectionofthedosages,theproteinwasmeasuredby theLowrymethod(Lowryetal.,1951).
Gelatinzymography
MMP-2activitywasdetectedusinggelatinzymography.Briefly,
50mgoftissuewerehomogenizedin200ofRIPAbuffer(150mM
NaCl,50mMTris,1%IGEPAL,0.5%sodiumdeoxycholate,0.1%SDS, 1l/mlproteaseinhibitor atpH8.0).Afterhomogenization,the sampleswerecentrifugedat10,000×gfor10min,at4◦Candthe supernatantwascollectedandusedasthesample.Theactivitywas measuredaccordingtoAraújoetal.(2015).
Histologicalevaluation
For microscopic analysis, a portion of the liver from each
animal of experimental groups was fixed in 10% formalin and
immersedinparaffin.Sectionsof4mwereobtainedandtheslides
werestained withhematoxylin andeosin (H&E). The
photomi-crographswereobtainedat40×magnification(LeicaApplication
Suite,Germany).Liverhistologywasexaminedusingelevenimages obtainedatrandomfromthetissueandclassifiedforthedegreeof
microvesicularsteatosis.Theimageswereexaminedsemi
quan-titatively, considering that the degree of lipid infiltration was gradedreflectingthepercentage ofhepatocytescontaininglipid droplets.It wasgiventhevalues0–3accordingtothesteatosis,
where 0:none;1:1–33%; 2:33–66%; 3:>66%,as describedby
Bruntetal.(1999).
Statisticalanalysis
The data were analyzed by Kolmogorov–Smirnov test for
normality, and all data showed a normal distribution. All
values are expressed as the mean±standard error of the
mean (SEM). Statistical analysis was performed using
one-way analysis of variance (ANOVA), with Bonferroni posttest.
Prism 5.0 (GraphPad, La Jolla, CA, USA) was used to
per-formtheanalysis.Differenceswereconsideredsignificantwhen
20.0
15.0
10.0
5.0
0.0 1,2,3
4,5 7
6
11 10 9 8
121314
Time
AU
1.60 1.80 2.00 2.20 2.40 2.60 2.80 3.00 3.20 3.40 3.60 3.80 4.00
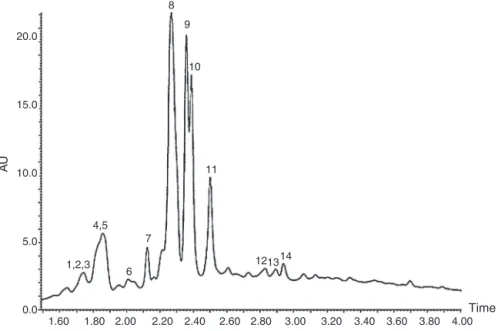
Fig.1.RP-UPLC-DADfingerprintsectionofaqueousextractofBaccharistrimera.1:3-O-feruloylquinicacid;2:4-O-caffeinoylquinicacid;3:5-O-caffeinoylquinicacid;4:3-O -caffeoyylquinicacid;5:4-O-feruloylquinicacid;6:5-O-feruloylquinicacid;7:apigenin-6,8-di-C-glucopyranosidium;8:3-O-isoferuloylquinicacid;9:5-O-isoferuloylquinic acid;10:6(8)-C-furanosyl-8(6)-C-hexosylflavone;11:6(8)-C-hexosyl-8(6)-C-furanosylflavone;12:3,4-di-O-caffeinylquinicacid;13:3,5-di-O-caffeoyylquinicacid;14: 4,5-di-O-caffeinoylquinicacid;15:4-O-isoferuloylquinicacid.
Table1
SubstancesidentifiedintheaqueousextractofBaccharistrimerabyLC-DAD-ESI-MS.
Peak Compound RT(min) UV(nm) LC–MS[M−H]−(m/z)
(fragmentationm/m)
1 3-O-feruloylquinicacid 1.73 321.1 367.29
(193.0;155.2;148.9;134.0)
2 4-O-caffeinoylquinicacid 1.74 320.1 353.38
(191.0;178.9;135.1)
3 5-O-caffeinoylquinicacid 1.75 323.1 353.38
(191.2;179.1;135.1)
4 3-O-caffeoyylquinicacid 1.86 323.1 353.38
(190.8;179.0;137.0)
5 4-O-feruloylquinicacid 1.88 323.1 367.36
(193.2;172.8;149.1;134.0)
6 5-O-feruloylquinicacid 1.96 320.1 367.38
(192.7;172.7;149.2;134.2)
7 Apigenin-6,8-di-C-glucopyranosidium 2.12 269.1;321.0 593.42
(547.1;503.0;472.9;431.1)
8 3-O-isoferuloylquinicacid 2.25 318.1 367.59
(193.1;172.8;149.1;133.8)
9 5-O-isoferuloylquinicacid 2.47 323.1 367.17
(191.2;172.8;149.1;134.1)
10 6(8)-C-furanosyl-8(6)-C-hexosylflavone 2.36 271.1;331.1 563.48
(515.1;473.1;443.0;383.1)
11 6(8)-C-hexosyl-8(6)-C-furanosylflavone 2.50 271.3;333.2 563.20
(515.1;472.9;442.9;383.2)
12 3,4-di-O-caffeinylquinicacid 2.81 282.1;320.5 515.20
(190.0;162.9;134.7)
13 3,5-di-O-caffeoyylquinicacid 2.93 283.1;321.2 515.17
(191.0;163.0;135.0)
14 4,5-di-O-caffeinoylquinicacid 2.95 286.1;320.3 515.40
(190.7;163.0;143.2;127.0)
15 4-O-isoferuloylquinicacid 3.46 322.1 367.22
(190.6;163.0;148.0)
Results
RP-UPLC-DAD-ESI-MSanalysisofaqueousextract
Twelvephenolicacidsandthreeflavonoidswereidentifiedin
theaqueousextractofB.trimera,byRP-UPLC-DAD-ESI-MS.The
RP-UPLC-DADfingerprintisshowninFig.1(Table1).
AntioxidantactivityinvitroofBaccharistrimera
Table2
Evaluationof Baccharistrimeraability to scavengingDPPHradical. The high-est concentrationofaqueous extract ofBaccharistrimera wasable to inhibit DPPHbyapproximately68%,whilethereferenceantioxidant(Trolox)required a concentrationof 300 timesgreater to inhibit thesame percentage. DPPH: 2,2-diphenyl-1picrylhydrazyl; Trolox: 6-hydroxy-2,5,7,8-tetra-trhylchroman-2-carboxylicacid.Thevaluesareexpressedasthemean±SD.
DPPHradicalscavengingactivity Inhibition%
AqueousextractofBaccharistrimera(g/ml)
500 68.1±11.1
250 30.5±1.38
100 8.10±0.90
50 3.30±0.88
25 0.59±0.27
Trolox(g/ml)
200232 95.9±0.19
175203 83.3±3.05
150174 72.0±2.82
125145 60.4±4.79
100116 47.3±1.24
75087 32.9±1.53
50058 22.0±0.77
25029 10.5±1.06
concentrationofapproximately300timeslessthantroloxisable
toinhibitthesamepercentageofDPPH(Table2).
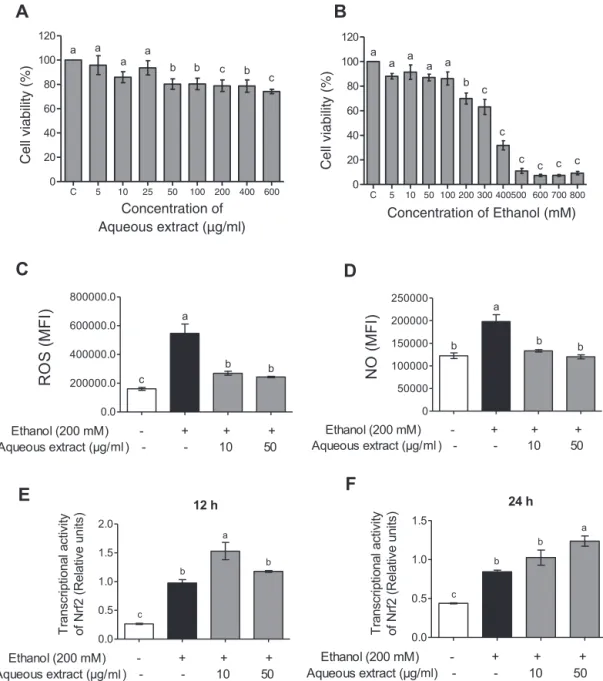
Baccharistrimeraaqueousextractdoesnotshowcytotoxicityin HepG2cells
TheresultsofFig.2(panelA)showedthattherewasno sig-nificantdifferenceintheHepG2cellsviabilityatconcentrationsof 5–25g/mlofaqueousextractofB.trimera,withviability
main-tained above85%. In theconcentrations of50–600g/ml there
wasasignificantreductioninviabilityinrelationtothecontrol, butmaintainedabove75%.InpanelBasignificantreductionwas
observed in cell viability from theconcentration of 200M of
ethanol.
Baccharistrimeraaqueousextractdecreasedareactivespecies productioninHepG2cellsincubatedwithethanol
ItcanbeobservedinFig.2thatethanolpromotedtheincrease
ofROS(panelC)and NO(panelD)inHepG2cells.Therewasa
reductionintheseparameterswhenthecellswerepretreatedwith aqueousextractinbothconcentrations(10and50g/ml),reaching similarlevelstonegativecontrol(cellsnotincubatedwithethanol).
BaccharistrimeraaqueousextractmodulatesNrf2 transcriptionalactivityinHepG2cells
ItcanbeobservedinFig.2thattheethanolisabletoinducethe Nrf2transcriptionalactivityin12h(panelE)and24h(panelF)of incubation,whencomparedtothecontrol.TheNrf2transcriptional activitywasincreasedwhencellswerepretreatedwith10g/ml ofaqueousextractofB.trimera,in12h,whencompared tothe ethanolgroupwithouttreatment.Whereasregardingthe24h,the concentrationof50g/mlofaqueousextractwasabletoinduce theincreasetheNrf2transcriptionalactivity.
EvaluationoftheeffectofBaccharistrimeraonbiochemical parametersinalcoholichepatotoxicity
Renalfunctionwasevaluatedbasedontheparameters
creati-nineandurea.Inordertoevaluateliverfunction,theparameters ALT,ASTandtotalproteinwereused.Totalcholesterol,HDL,
frac-tionnon-HDLandtriacylglycerollevelsweredeterminedforthe
lipidprofile.Table3showedtheincreaseincreatininelevels,total
Table3
Biochemicalmarkerlevelsinserumandplasmaofrats.Animalsreceivedabsolute ethanol(E);pretreatmentwithaqueousextract(Aq)and1hafterreceivedabsolute ethanol.Control(C)receivedwater.Differentletters(a,b,c,d)indicatesignificant differencefromeachotheratp<0.05,whilesamelettersindicatenosignificant difference(p>0.05).
Biochemicalparameters Treatedgroups
C E Aq
Urea(mg/dl) 59.6±1.7 71.81±5.5 70.92±6.1
Creatinine(mg/dl) 0.375±0.09b 0.7361±0.087a 0.57±0.079a
ALT 18.99±1.4a 21.83±2.02a 12.0±0.65b
AST 37.02±3.8 37.76±3.5 27.5±2.69
Totalprotein(mg/dl) 5.97±0.65a 4.12±0.17b 4.62±0.4b
Glucose(mg/dl) 100.6±5.8 108.5±6.7 103.1±10.4
Totalcholesterol(mg/dl) 97.15±2.6c 230.0±28.24a 144.0±19.11b
HDL(mg/dl) 43.8±4.05 35.82±0.31 42.92±1.18
FractionnonHDL(mg/dl) 58.8±10.61c 189.1±20.95a 99.98±25.82b
Triacylglycerides(mg/dl) 63.95±12.72 53.86±6.49 53.22±12.78
cholesterolandnon-HDLfraction,besidesadecreaseintotal
pro-teininthegroupofanimalsthatreceivedethanol.Pretreatment
withaqueousextractofB.trimerapromotedadecreaseinALT
activ-ity,totalcholesterolandnon-HDLfractionandanincreaseintotal
protein,comparedwithethanolgroup.Itwasnotobserved
sig-nificantdifferencesinurealevels,ASTactivity,glucose,HDLand
triacylglycerideslevelsinanyoftheexperimentalgroups.
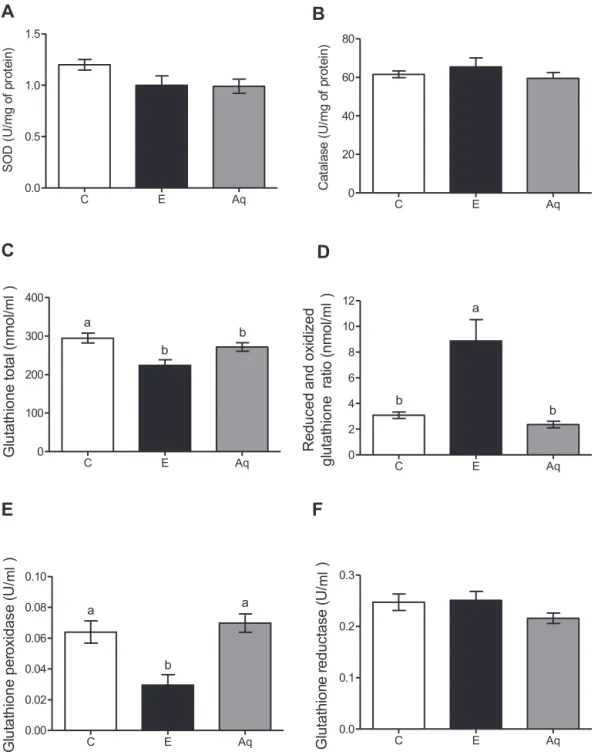
EffectofBaccharistrimeraontheantioxidantsysteminalcoholic hepatotoxicity
It couldbeobserved inFig. 3that theethanol consumption
didnotaltertheactivitiesofSOD(panelA)andcatalase(panel B)enzymes,comparedwithcontrolgroup.Theaqueousextractdid notaltertheseparameterseither.Regardingglutathionesystem, theresultsshowedasignificantdecreaseinglutathionetotal(panel C)and glutathioneperoxidase(GPx)activity(panelE),together withanincreaseinreduced/oxidizeglutathioneratio(panelD)in ethanolgroupwhencomparedwithcontrolgroup.B.trimera aque-ousextractpromotesanincreaseinGPxactivityandadecreasein reduced/oxidizeglutathioneratio.Glutathionereductaseactivity (panelF)didnotalterinanyoftheexperimentalgroups.
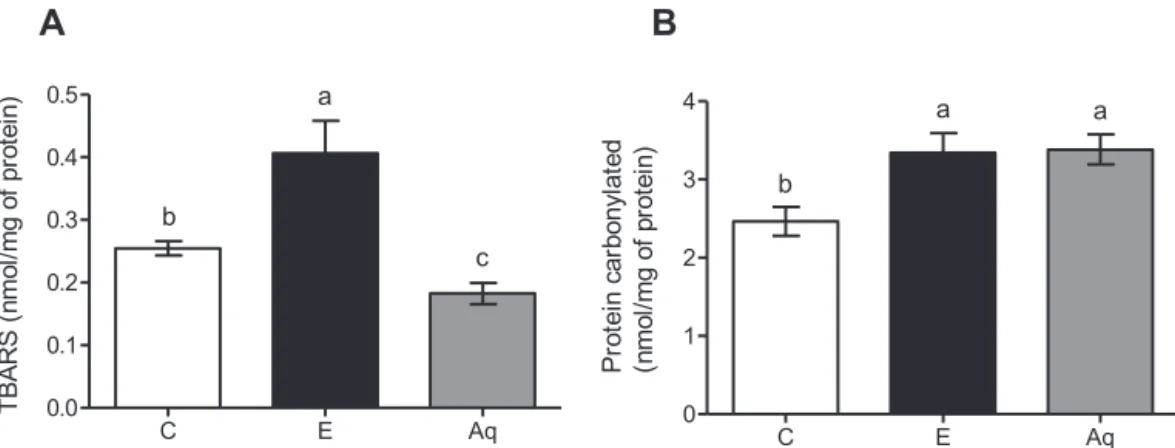
EvaluationoftheeffectofBaccharistrimeraonmarkersof oxidativestressinalcoholichepatotoxicity
InethanolgrouptherewasanincreaseinTBARS(Fig.4–panel A)andcarbonylatedprotein(panelB)levels,comparedtocontrol group.TheextractofB.trimerawasabletoreduceonlythelevelsof TBARS,noalterationswereobservedinthelevelsofcarbonylated protein.
BaccharistrimeradecreasestheMMP-2activityinalcoholic hepatotoxicity
ToevaluatetheMMP-2activitythezymographytechnicwas
used.Fig.5Arepresentsqualitativeimagesof geland5B repre-sentsthequantitativeactivity(banddensity).Then,itwasobserved
thatethanolpromotedanincreaseinMMP-2activity,when
com-paredtothecontrol.ThetreatmentwithB.trimerapromotesthe reductionofMMP-2activity,inrelationofethanolgroup.
Baccharistrimeraaqueousextractreducesmicro-steatosisin alcoholichepatotoxicity
Fig.2.CellviabilityofBaccharistrimeraaqueousextract(5–600g/ml)(A)andethanol(5–800mM)(B)for24hmeasuredbyMTTassay.EffectofBaccharistrimeraaqueous extractonethanol-inducedROS(C)andNO(D)production,expressionMFI(Mediumintensityoffluorescence).WealsoevaluatedtheeffectofBaccharistrimeraon ethanol-inducedNuclearfactorE2-relatedfactor2(Nrf2),vialuciferaseassay,for12h(E)and24h(F).Theresultswereexpressedasmean±S.D(n=6).Differentletters(a,b,c) indicatesignificantdifferencefromeachotheratp<0.05,whilesamelettersindicatenosignificantdifference(p>0.05).
withB.trimerawasabletoreducethedegreeofseverityofthe microvesicularsteatosis,mainlygrade1.
Discussion
Thepresentstudyinvestigatedthepotentialprotectiveeffects ofB. trimeraaqueousextract againsthepatotoxicity inducedby ethanol,aswellthecompoundspresentinthisextract.Invitro, abil-ityoftheextracttoscavengefreeradicalsandtheeffectofB.trimera onethanolmediatedROS,NOandtranscriptionalactivityofNrf2in HepG2cellswereexamined.Itwasprovidedforthefirsttimethat B.trimeraaqueousextractstimulatesthetranscriptionalactivityof Nrf2.Invivo,usingaratmodelofacuteintoxicationbyethanol, itwasdemonstratedthatB.trimeraalleviatedtheoxidative
dam-ages,improvingtheantioxidantdefenseandattenuatinghepatic
steatosis(Graphicabstract).Thisencouragestheadvancementof researchindicatingB.trimeraastherapeuticagentfor hepatopro-tection.
Flavonoids, caffeic acid derivatives and diterpenes have
been isolated from different extracts of B. trimera (Abad and
Bermejo, 2007; Verdi et al., 2005; Lívero et al., 2016a,b).
In our study, using LC-DAD-ESI-MS three flavonoids were
detectedinaqueousextract(apigenin-6,8-di-C-glucopyranosidium (7);6(8)-C-furanosyl-8(6)-C-hexosylflavone(10);6(8)-C -hexosyl-8(6)-C-furanosyl flavone (11)) and twelve phenolic acids (3-O-feruloylquinic acid (1); 4-O-caffeinoylquinic acid (2); 5-O-caffeinoylquinic acid (3); 3-O-caffeoyylquinic acid (4); 4-O-feruloylquinic acid (5); 5-O-feruloylquinic acid (6); 3-O -isoferuloylquinicacid(8);5-O-isoferuloylquinicacid(9); 3,4-di-O-caffeinylquinic acid (12); 3,5-di-O-caffeoyylquinic acid (13); 4,5-di-O-caffeinoylquinic acid (14); 4-O-isoferuloylquinic acid (15)).
TheabilityofaqueousextractofB.trimeratosequesterradicals wasevaluatedandtheresultsshowedthatalltestedconcentrations
showedanantioxidantcapacityinadose-dependentmanner.For
Fig.3.EffectofBaccharistrimeraaqueousextractonthelevelofSOD(A),catalase(B),glutathionetotal(C),reducedandoxidizedglutathioneratio(D),activityofglutathione peroxidase(E)andglutathionereductase(F),intheliversofrats.Control(C)receivedwater.Animalsreceivedabsoluteethanol(E);pretreatmentwithaqueousextract(Aq) and1hafterreceivedabsoluteethanol.Differentletters(a,b)indicatesignificantdifferencefromeachotheratp<0.05,whilesamelettersindicatenosignificantdifference (p<0.05).
intheotherinvitroassays,HepG2cellswereincubatedwith differ-entconcentrationsoftheaqueousextractandethanol.Theresults
showedthatB.trimeraaqueousextractwasnotcytotoxicatany
concentrationevaluated.Rodriguesetal.(2009)demonstratedthat
theaqueousB.trimeraextractwasnotcytotoxictobonemarrow
cellsatanyoftheconcentrationstested(500–2000g/ml). How-ever,Nogueiraetal.(2011)demonstratedtheaqueousextractwas cytotoxicin500g/mlinHTCandHEKcells(rathepatomacells andhumanembryokidneyepithelialcells,respectively) indicat-ingthatthetoxicitymaybetissue-specific.Inrelationtoethanol, therewasareductioninviabilityfrom200mM.Kumaretal.(2012) observedasignificantdecreaseinHepG2cellsviabilityexposedto ethanolfrom100mM.Basedonthis,non-cytotoxicconcentrations
ofB.trimerawereselected(10–50g/ml)andtheethanolcytotoxic
concentration(200mM)wereusedinthesubsequentexperiments.
WhenHepG2cellswereincubatedwithethanolthe
produc-tionofROSandNOwasincreased.Haorahetal.(2011)alsofound
increasedROSandNOinendothelialcellstreated withethanol.
Thisincreasecanbeexplainedbythefactthatthemainmetabolite
ofethanol,acetaldehyde,activatesNADPHoxidaseandinducible
nitricoxidesynthase(iNOS),whichleadstoanincreaseinthe
pro-ductionofEROandnitricoxide(NO),causingoxidativedamages
(Haorahetal.,2008;Rumpetal.,2010;Alikunjuetal.,2011).Gong andCederbaum(2006)observedthatinhepatocytesisolatedfrom ratstherewasanincreaseofNrf2,probablyduetotheinduction
Fig.4. EffectofBaccharistrimeraaqueousextractonthelevelofTBARS(A)andcarbonylatedprotein(B)intheliversofrats.Control(C)receivedwater.Animalsreceived absoluteethanol(E);pretreatmentwithaqueousextract(Aq)and1hafterreceivedabsoluteethanol.Differentletters(a,b)indicatesignificantdifferencefromeachother atp<0.05,whilesamelettersindicatenosignificantdifference(p<0.05).
Fig.5.EffectofBaccharistrimeraaqueousextractontheMMP-2activity,viagelatin zymography,intheliversofrats.A:Gelbands;B:banddensity.HT1080fibrosarcoma cellswereusedaspositivecontrol.Control(C)receivedwater.Animalsreceived absoluteethanol(E);pretreatmentwithaqueousextract(Aq)and1hafterreceived absoluteethanol.Differentletters(a,b)indicatesignificantdifferencefromeach otheratp<0.05,whilesamelettersindicatenosignificantdifference(p<0.05).
ROSproduction,withconsequentactivationofNrf2.Thesefindings areinagreementwithourresultsthatfoundanincreaseinNrf2 transcriptionalactivityincellsthatreceivedonlyethanol.Ethanol inducedNrf2transcriptionalactivityhasalsobeendemonstrated byothersauthors(Dongetal.,2011;Luetal.,2012).
ThepretreatmentwithB.trimerapromotedthereductionofROS andNO,returningtovaluessimilartothecontrol.Antioxidantscan actdirectlythroughtheeliminationofEROandERN,orindirectly, throughthemodulationofsignalingpathways(Paivaetal.,2015). Thus,thisstudyandothersshowedthatB.trimerahastheability tosequesterradicals,inferringthatthedecreaseinthesespecies canbeattributed,atleast,totheplant’sdirectactiondeOliveira etal.,2012;Páduaetal.,2013).Inaddition,itwasalsoinferred thatthisdecreasecanbeattributedtotheindirectaction,sinceB. trimeraaqueousextractpromotedanincreaseinNrf2 transcrip-tionalactivity,promotingantioxidantprotectionagainstthestress bytheethanol.Líveroetal.(2016a,b)havealreadydemonstrated thatthehydroethanolicextractofB.trimerapromotesanincrease expressionofNrf2,butnootherstudyhasshowntheeffectofB. trimeraovertheNrf2activity.Thus,thesedatainagreementwith thedecreaseofROSandNOshowthatB.trimeramaybeeffective inpreventingstressinducedbyethanol.
Fig.6.Representativehematoxylinandeosin-stainedhistologicalsectionsoflivers fromrats(A).Semi-quantificationwasdemonstratedinB.Severemicrovesicular steatosiswasobservedintheethanolgroup(E),butnotinthecontrolgroup(C).
Baccharistrimeraaqueousextract(Aq)attenuatedfataccumulationinhepatocytes. Theimageswerephotographedat400xmagnification.Scalebar=50m.Asterisk (*)indicatessignificantdifferencebetweentwogroups.
Toconfirmtheeffectsaninvivoexperimentwascarriedout,
wheretheanimalsreceivedonlyabsoluteethanol orwere
pre-treated with B. trimera aqueous extract. The results showed a
worsening kidney function, decrease in total protein, but ALT
and AST transaminases did not change in the ethanol group.
There are several situations in which there is loss of
correla-tionbetweenserumlevelsofliverenzymesandatissue injury,
sothatanincrease ofserumactivitiesof liverenzymemarkers
doesnotnecessarilyreflectonlivercelldeath(Contreras-Zentella andHernández-Mu˜noz,2016).However,acetaldehydeformed
dur-ing the metabolism of ethanol may form adducts with amino
acids,reflectingthegeneraldecreaseinproteinsynthesisandthe decreaseofplasmaproteinsecretion(Smithetal.,2007). Pretreat-mentwithB.trimera significantlydecreasedALTactivity.Lívero etal. (2016a,b)alsofounda decreased ALT activitywhenmice receivedhydroethanolicextract ofB.trimera.Theacute
glucoseconcentration,thisdifferencecanbeexplainedbythe nutri-tionalstatus at thetime alcohol isadministered (Steineretal., 2015).Probablebecauseouranimalsreceivedbalanced
commer-cialfeednochangeswerefoundinglucoseinanyexperimental
group.
Theresultsalsoshowedanincreaseintotalcholesterol,
non-HDL fraction, beyond hepatic micro-steatosis, but TAG did not
changeinethanolgroup.Afteracuteconsumptionofhighdosesof ethanoltheserumlevelsofTAGmayincrease,decreaseorremain normal,however,thetotal flow thatisabsorbed bytheliveris increasedduetothestimulatoryeffectsofethanolonliverblood
flow(BaraonaandLieber,1979),promotingtheaccumulationof
micro and/or macrovesicles lipids in hepatocytes (Baraona and
Lieber,1979;LíveroandAcco,2016).B.trimeraimprovedthelipid profileanddecreasedhepaticmicro-steatosis,protectingagainst ethanoldamage.Líveroetal.(2016a,b)showedthat hydroethano-licextractofB.trimeradecreasestheexpressionoftheScd1gene, whichisresponsibleforencodingthestearoyl-CoAdesaturase-1,
animportantenzymeinthebiosynthesisofthemainfattyacids
foundinTAG.Maybepartofthismechanism contributestothe
protectionmechanismbytheextract.
Theabilityofethanoltoinduceoxidativestressandantioxidant depletion,suchasglutathione,iswellrecognized(Luand Ceder-baum,2008; Hanetal.,2016).Our resultsshowedthat ethanol alterationsinglutathionemetabolismweremoresignificantthan alterationsin SODand CATactivities,sinceethanol-treatedrats exhibitedadecreaseinGPxactivityanddecreaseinoxidized glu-tathione,reflectinganincreaseintheGSH/GSSGglutathioneratio. ThedecreaseinGPxactivityinethanol-inducedintoxicationhas
also been demonstrated in other studies (Park et al., 2013; Li
etal.,2014; Yanet al.,2014).GPxisone oftheresponsiblefor thereductionofH2O2 towater, sinceadecreasein theactivity ofthisenzymewasobserved,itispossibletoinferthatinthese animalstherewasprobablyaccumulationofH2O2,contributingto oxidativestress.Thisinefficiencyintheantioxidantresponsemay justifytheincreaseintheTBARSandcarbonylatedproteinlevels observedinourstudy.Incarbontetrachloride-induced hepatotox-icitymodelsithasbeenshownthatthedecreaseofglutathioneis
relatedtotheincrease ofMDA(Azabetal.,2013;Al-Sayedand
Abdel-Daim,2014) B. trimera promotedincreased GPx activity,
contributingtoanadaptiveresponsewithaconsequentdecrease
in TBARS. In fact, themechanisms involved in the antioxidant
capacityofpolyphenolsincludethesuppressionofROSformation byinhibitionoftheenzymesinvolvedintheirproduction;direct eliminationofROS;orpositiveregulationinantioxidantdefense (Hussainetal.,2016).
Hepaticfibrosisisanimportanthistologicalfeatureassociated withtheprogression ofalcoholicliverdisease,characterizedby
increaseddepositionofextracellularmatrix components(ECM).
The key event in liver fibrogenesis is the activation of hepatic stellatecells(HSC),whichareamajorsourceofECMintheliver (Cenietal.,2014;Lasek,2016).Acetaldehydeisoneofthemain
mediators of alcohol-inducedfibrogenesis, as it may stimulate
the synthesis of fibrillar collagens and structural glycoproteins
ofECM.AcetaldehydemayfurtherpromoteECMremodelingby
upregulationofmetalloproteinase-2(MMP-2)(Cenietal.,2014).
In addition, H2O2 due to oxidative stress can activate
MMP-2 (Hopps et al., 2015). This fact can explain the increase in
MMP-2 activity found in the ethanol treated animals in our
experiment.However,thepretreatmentwithB.trimeradecreased
the MMP-2 activity. It has already been shown that the
caf-feinoylquinicacidshaveMMP-2inhibitoryactivity(Benedeketal., 2007).Since that in the characterization of ourextract it was
demonstrated the presence of caffeiolquinic acids, it is
possi-ble toinferthat this mechanismis involved withtheobserved
protection.
Thus,whenourresultswereanalyzedalltogethersuggestthat B.trimeraaqueousextractappearstobepromisingastherapeutic agentforhepatoprotection.
Authorcontributions
ACSRelaboratedandperformedallthework.GRA,GHBSGCB
contributed in collecting plant sample and identification,
con-fectionof herbarium,chromatographic analysis.KPL andPHAM
assisted in thepractical laboratorypart.CMA contributed with
the zymography technique. BMS, ACAC and EMCR contributed
withluciferaseassay.WGLcontributedwithhistologicalanalyses.
DCCdesignedthestudy,supervisedthelaboratoryworkand
con-tributedtocriticalreadingofthemanuscript.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbyFundac¸ãodeAmparoàPesquisado
EstadodeMinasGerais,CNPqandUniversidadeFederaldeOuro
Preto(PROPP/UFOP),Brazil.
References
Abad,M.J.,Bermejo,P.,2007.Baccharis(Compositae):areviewupdate.Arkivoc7, 76–96.
Aebi,H.,1984.Catalaseinvitro.MethodEnzymol.105,121–126.
Alikunju,S.,AbdulMuneer,P.M.,Zhang,Y.,Szlachetka,A.M.,Haorah,J.,2011.The inflammatoryfootprintsofalcohol-inducedoxidativedamageinneurovascular components.BrainBehav.Immun.25,129–136.
Al-Sayed,E.,Martiskainen,O.,Seifel-Din,S.H.,Sabra,A.N.,Hammam,O.A., El-Lakkany,N.M.,Abdel-Daim,M.M.,2014.Hepatoprotectiveandantioxidanteffect ofBauhiniahookeriextractagainstcarbontetrachloride-inducedhepatotoxicity inmiceandcharacterizationofitsbioactivecompoundsby HPLC-PDA-ESI-MS/MS.Biomed.Res.Int.,http://dx.doi.org/10.1155/2014/245171.
Al-Sayed, E., Abdel-Daim, M.M., Kilany, O.E., Karonen, M., Sinkkonen, J., 2015. Protective role of polyphenols from Bauhinia hookeri against car-bontetrachloride-inducedhepato-andnephrotoxicityinmice.Ren.Fail.37, 1198–1207.
Al-Sayed,E.,Abdel-Daim, M.M.,2014.Protective roleofcupressuflavonefrom Cupressus macrocarpa against carbon tetrachloride-induced hepato- and nephrotoxicityinmice.PlantaMed.80,1665–1671.
Araújo,C.M.,Lúcio,K.P.,EustáquioSilva,M.,Isoldi,M.C.,BiancodeSouza,G.H., Brandão,G.C.,Schulze,R.,Costa,D.C.,2015.Morusnigraleafextractimproves glycemicresponseandredoxprofileintheliverofdiabeticrats.FoodFunct.6, 3490–3499.
Azab,S.S.,Abdel-Daim,M.,Eldahshan,O.A.,2013.Phytochemical,cytotoxic, hepato-protectiveandantioxidantpropertiesofDelonixregialeavesextract.Med.Chem. Res.22,4269–4277.
Bandeira,A.C.B.,Silva,R.C.,RossoniJúnior,J.V.,Figueiredo,V.P.,Talvani,A.,Cangussú, S.D.,Bezerra,F.S.,Costa,D.C.,2017.Lycopenepretreatmentimproves hepatotox-icityinducedbyacetaminopheninC57BL/6mice.Bioorg.Med.Chem.Lett.25, 1057–1065.
Baraona,E.,Lieber,C.S.,1979.Effectsofethanolonlipidmetabolism.J.LipidRes.20, 289–315.
Benedek, B., Kopp,B., Melzig, M.F., 2007.Achillea millefoliums.l. is the anti-inflammatoryactivitymediatedbyproteaseinhibition?J.Ethnopharmacol.113, 312–317.
Bianchi,M.L.P.,Antunes,L.M.G.,1999.Radicaislivreseosprincipaisantioxidantes dadieta.Rev.Nutr.12,123–130.
Buege,J.Á.,Aust,S.D.,1978.Microsomallipidperoxidation.MethodEnzymol.52, 302–310.
Bona,C.M.,Biasi,L.A.,Zanette,F.,Nakashima,T.,2005.Estaquiadetrêsespéciesde Baccharis.Cienc.Rural.35,223–226.
Brunt,E.M.,Janney,C.G.,DiBisceglie,A.M.,Neuschwander-Tetri,B.A.,Bacon,B.R., 1999.Nonalcoholicsteatohepatitis:aproposalforgradingandstaging histolog-icallesions.Am.J.Gastroenterol.94,2467–2474.
Cederbaum,A.I.,2012.Alcoholmetabolism.Clin.LiverDis.16,667–685. Ceni,E.,Mello,T.,Galli,A.,2014.Pathogenesisofalcoholicliverdisease:roleof
oxidativemetabolism.WorldJ.Gastroenterol.20,17756–17772.
Contreras-Zentella,M.L.,Hernández-Mu˜noz,R.,2016.Isliverenzymereleasereally associatedwithcellnecrosisinducedbyoxidantstress?Oxid.Med.CellLongev., http://dx.doi.org/10.1155/2016/3529149.
deAraújo,G.R.,Rabelo,A.C.S.,Meira,J.S.,Rossoni-Júnior,J.V.,deCastro-Borges, W.,Guerra-Sá, R.,Batista,M.A., Silveira-Lemos,D.,Souza, G.H.B.,Brandão, G.C., Chaves, M.M., Costa, D.C., 2016. Baccharis trimera inhibits reactive oxygen species production through PKC and down-regulation p47phox phosphorylation of NADPH oxidase in SK Hep-1 cells. Exp. Biol. Med., http://dx.doi.org/10.1177/1535370216672749.
Ding,R.B.,Tian,K.,Huang,L.L.,He,C.W.,Jiang,Y.,Wang,Y.T.,Wan,J.B.,2012.Herbal medicinesforthepreventionofalcoholicliverdisease:areview.J. Ethnophar-macol.144,457–465.
Dong,J.,Yan,D.,Chen,S.,2011.StabilizationofNrf2proteinbyD3Tprovides pro-tectionagainstethanol-inducedapoptosisinPC12cells.PLoSONE6,e16845, http://dx.doi.org/10.1371/journal.pone.0016845.
El-Naga,R.,2015.Apocyninprotectsagainstethanol-inducedgastriculcerinratsby attenuatingtheupregulationofNADPHoxidases1and4.Chem.Biol.Interact. 242,317–326.
Foglio,M.A.,Queiroga,C.L.,Sousa,I.M.O.,Rodrigues,R.A.F.,2006.PlantasMedicinais comoFontedeRecursosTerapêuticos:UmModeloMultidisciplinar. Multiciên-cia,Availableat:http://www.multiciencia.unicamp.br/art047.htm[assessed 30.09.17].
Fahmy,N.M.,Al-Sayed,E.,Abdel-Daim,M.M.,Singab,A.N.,2017.Anti-inflammatory andanalgesicactivitiesofTerminaliamuelleriBenth.(Combretaceae).DrugDev. Res.78,146–154.
Fotakis,G.,Timbrell,J.A.,2005.Invitrocytotoxicityassays:comparisonofLDH, neu-tralred,MTTandproteinassayinhepatomacelllinesfollowingexposureto cadmiumchloride.Toxicol.Lett.160,171–177.
Gong,P.,Cederbaum,A.I.,2006.Nrf2isincreasedbyCYP2E1inrodentliverand HepG2cellsandprotectsagainstoxidativestresscausedbyCYP2E1.J.Hepatol. 43,144–153.
González,J.A.M.,Madrigal-Santillán,E.,Morales-González,A.,Bautista,M., Gayosso-Islas,E.,Sánchez-Moreno,C.,2015.Whatisknownregardingtheparticipation offactorNrf-2inliverregeneration?Cell4,169–177.
Han,K.H.,Hashimoto,N.,Fukushima,M.,2016.Relationshipsamongalcoholic liverdisease,antioxidants,andantioxidantenzymes.WorldJ.Gastroenterol. 7,37–49.
Haorah,J.,Ramirez,S.H.,Floreani,N.,Gorantla,S.,Morsey,B.,Persidsky,Y.,2008. Mechanismofalcohol-inducedoxidativestressandneuronalinjury.FreeRadic. Biol.Med.45,1542–1550.
Haorah,J.,Floreani,N.A.,Knipe,B.,Persidsky,Y.,2011.Stabilizationof superox-idedismutasebyacetyl-l-carnitineinhumanbrainendotheliumduringalcohol exposure:novelprotectiveapproach.FreeRadic.Biol.Med.51,1601–1609. Hernández,J.A.,López-Sánchez,R.C.,Rendón-Ramírez,A.,2015.Lipidsandoxidative
stressassociatedwithethanol-inducedneurologicaldamage.Oxid.Med.Cell Longev.,http://dx.doi.org/10.1155/2016/1543809.
Hopps,E.,LoPresti,R.,Montana,M.,Canino,B.,Calandrino,V.,Caimi,G.,2015. Analysisofthecorrelationsbetweenoxidativestress,gelatinasesandtheir tis-sueinhibitorsinthehumansubjectswithobstructivesleepapneasyndrome.J. Physiol.Pharmacol.66,803–810.
Hussain,T.,Tan,B.,Yin,Y.,Blachier,F.,Tossou,M.C.,Rahu,N.O.,2016.Oxidativestress andinflammation:whatpolyphenolscandoforus?Oxid.Med.CellLongev., http://dx.doi.org/10.1155/2016/7432797.
Karam,T.K.,Dalposso,L.M.,Casa,D.M.,deFreitas,G.B.L.,2013.Carqueja( Baccha-ristrimera):utilizac¸ãoterapêuticaebiossíntese.Rev.Bras.PlantasMed.15, 280–286.
Kim, J., Keum, Y.S., 2016. NRF2, a key regulator of antioxidants with two faces towards cancer. Oxid. Med. Cell Longev., http://dx.doi.org/ 10.1155/2016/2746457.
Kumar,S.K.J.,Liao,J.W.,Xiao,J.H.,Gokila-Vani,M.,Wang,S.Y.,2012. Hepatoprotec-tiveeffectoflucidoneagainstalcohol-inducedoxidativestressinhumanhepatic HepG2cellsthroughtheup-regulationofHO-1/Nrf-2antioxidantgenes.Toxicol. InVitro26,700–708.
Lasek,A.W.,2016.Effectsofethanolonbrainextracellularmatrix:implicationsfor alcoholusedisorder.AlcoholClin.Exp.Res.40,2030–2042.
Levine,R.L.,Williams,L.A.,Stadtman,E.R.,Shacter,E.,1994.Carbonylassaysfor determinationofoxidativelymodifiedproteins.MethodEnzymol.233,346–357. Li,M.,Lu,Y.,Hu,Y.,Zhai,X.,Xu,W.,Jing,H.,Tian,X.,Lin,Y.,Gao,D.,Yao,J., 2014.SalvianolicacidBprotectsagainstacuteethanol-inducedliverinjury through SIRT1-mediated deacetylation of p53 in rats. Toxicol. Lett. 228, 67–74.
Lívero,F.A.,Acco,A.,2016.Molecularbasisofalcoholicfattyliverdisease:from incidencetotreatment.Hepatol.Res.46,111–123.
Lívero,F.A.,Martins,G.G.,QueirozTelles,J.E.,Beltrame,O.C.,PetrisBiscaia,S.M., Cav-icchioloFranco,C.R.,OudeElferink,R.P.,Acco,A.,2016a.Hydroethanolicextract ofBaccharistrimeraamelioratesalcoholicfattyliverdiseaseinmice.Chem.Biol. Interact.260,22–32.
Lívero,F.A.R.,daSilva,L.M.,Ferreira,D.M.,Galuppo,L.F.,Borato,D.G.,Prando,T.B., Lourenc¸o,E.L.,Strapasson,R.L.,Stefanello,M.É.,Werner,M.F.,Acco,A.,2016b. HydroethanolicextractofBaccharistrimerapromotesgastroprotectionand heal-ingofacuteandchronicgastriculcersinducedbyethanolandaceticacid. Naunyn-Schmiedeberg’sArch.Pharmacol.389,985–998.
Lowry,O.H.,Rosenbrough,N.J.,Farr,A.L.,Randall,R.J.,1951.Proteinmeasurement withthefolinphenolreagent.J.Biol.Chem.193,265–275.
Lu,Y.,Zhang,X.H.,Cederbaum,A.I.,2012.EthanolinductionofCYP2A5:roleof CYP2E1-ROS-Nrf2pathway.Toxicol.Sci.128,427–438.
Lushchak,V.I.,2014.Freeradicals,reactiveoxygenspecies,oxidativestressandits classification.Chem.Biol.Interact.224,164–175.
Marklund,S.,Marklund,G.,1974.Involvementofthesuperoxideanionradicalinthe autoxidationofpyrogallolandaconvenientassayforsuperoxidedismutase.Eur. J.Biochem.47,469–474.
Nogueira,N.P.,Reis,P.A.,Laranja,G.A.,Pinto,A.C.,Aiub,C.A.,Felzenszwalb,I.,Paes, M.C.,Bastos,F.F.,Bastos,V.L.,Sabino,K.C.,Coelho,M.G.,2011.Invitroandinvivo toxicologicalevaluationofextractandfractionsfromBaccharistrimerawith anti-inflammatoryactivity.J.Ethnopharmacol.138,513–522.
Oliveira,A.C.P.,Endringer,D.C.,Amorim,L.A.S.,Brandão,M.G.L.,Coelho,M.M.,2011. EffectoftheextractsandfractionsofBaccharistrimeraandSyzygiumcuminion glycaemiaofdiabeticandnon-diabeticmice.J.Ethnopharmacol.102,465–469. deOliveira,C.B.,Comunello,L.N.,Lunardelli,A.,Amaral,R.H.,Pires,M.G.,daSilva, G.L.,Manfredini,V.,Vargas,C.R.,Gnoatto,S.C.,deOliveira,J.R.,Gosmann,G.,2012. PhenolicenrichedextractofBaccharistrimerapresentsanti-inflammatoryand antioxidantactivities.Mol.Cells17,1113–1123.
Pádua,B.C.,Silva,L.D.,RossoniJúnior,J.V.,Humberto,J.L.,Chaves,M.M.,Silva,M.E., Pedrosa,M.L.,Costa,D.C.,2010.AntioxidantpropertiesofBaccharistrimerain theneutrophilsofFisherrats.J.Ethnopharmacol.129,381–386.
Pádua,B.P.,RossoniJunior,J.V.,deBritoMagalhaes,C.L.,Seiberf,J.B.,Araujo,C.M., BiancodeSouza,G.H.,Chaves,M.M.,Silva,M.E.,Pedrosa,M.L.,Costa,D.C.,2013. BaccharistrimeraimprovestheantioxidantdefensesystemandinhibitsiNOS andNADPHoxidaseexpressioninaratmodelofinflammation.Curr.Pharm. Biotechnol.14,975–984.
Pádua,B.C.,RossoniJúnior,J.V.,Magalhães,C.L.B.,Chaves,M.M.,Silva,M.E.,Pedrosa, M.L.,BiancodeSouza,G.H.,Brandão,G.C.,Rodrigues,I.V.,Lima,W.G.,Costa, D.C.,2014.ProtectiveeffectofBaccharistrimeraextractonacutehepaticinjury inamodelofinflammationinducedbyacetaminophen.MediatorsInflamm., http://dx.doi.org/10.1155/2014/196598.
Paiva,F.A.,Bonomo,L.F.,Boasquivis,P.F.,Paula,I.T.B.R.,Guerra,J.,Leal,W.M.,Silva, M.E.,Pedrosa,M.L.,Oliveira,R.P.,2015.Carqueja(Baccharistrimera)protects againstoxidativestressandamyloid-inducedtoxicityinCaenorhabditis ele-gans.Oxid.Med.CellLongev.,http://dx.doi.org/10.1155/2015/740162. Park,H.Y.,Choi,H.D.,Eom,H.,Choi,I.,2013.Enzymaticmodificationenhancesthe
protectiveactivityofcitrusflavonoidsagainstalcohol-inducedliverdisease. FoodChem.139,231–240.
Rates,S.M.K.,2001.Plantsassourceofdrugs.Toxicon39,603–613.
Rodrigues,C.R.F.,Dias,J.H.,Semedo,J.G.,daSilva,J.,Ferraz,A.B.F.,Picada,J.N.,2009. MutagenicandgenotoxiceffectsofBaccharisdracunculifolia(D.C.).J. Ethnophar-macol.124,321–324.
Rump,T.J.,AbdulMuneer,P.M.,Szlachetka,A.M.,Lamb,A.,Haorei,C.,Alikunju, S.,Xiong,H.,Keblesh,J.,Liu,J.,Zimmerman,M.C.,Jones,J.,DonohueJr.,T.M., Persidsky,Y.,Haorah,J.,2010.Acetyl-L-carnitineprotectsneuronalfunction fromalcoholinducedoxidativedamageinthebrain.FreeRadic.Biol.Med.49, 1494–1504.
Silva,B.M.,Sousa,L.P.,Ruiz,A.C.G.,Leite,F.G.G.,Teixeira,M.M.,Fonseca,F.G.,Pimenta, P.F.P.,Ferreira,P.C.P.,Kroon,E.G.,Bonjardim,C.A.,2011.Thedenguevirus non-structuralprotein1(NS1)increasesNF-jBtranscriptionalactivityinHepG2cells. Arch.Virol.156,1275–1279.
Smith,C.,Smith,C.,Lieberman,M.,Marks,A.D.,2007.BioquímicaMédicaBásicade Marks,2nded.,pp.458–471.
Steiner,J.L.,Crowell,K.T.,Lang,C.H.,2015.Impactofalcoholonglycemiccontroland insulinaction.Biomol.Ther.5,2223–2246.
Verdi,L.G.,Brighente,I.M.C.,Pizzolatti,M.G.,2005.GêneroBaccharis(Asteraceae): aspectosquímicos,econômicosebiológicos.Quim.Nova28,85–94.
Lu,Y.,Cederbaum,A.I.,2008.CYP2E1andoxidativeliverinjurybyalcohol.FreeRadic. Biol.Med.44,723–738.