w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Simultaneous
HPLC
analysis
of
crebanine,
dicentrine,
stephanine
and
tetrahydropalmatine
in
Stephania
venosa
Sumet
Kongkiatpaiboon
a,∗,
Nongnaphat
Duangdee
a,
Saisuree
Prateeptongkum
b,
Ngampuk
Tayana
a,
Wichayasith
Inthakusol
aaDrugDiscoveryandDevelopmentCenter,ThammasatUniversity,RangsitCampus,Pathumthani,Thailand
bDepartmentofChemistry,FacultyofScienceandTechnology,ThammasatUniversity,RangsitCampus,Pathumthani,Thailand
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5September2017 Accepted24October2017 Availableonline15November2017
Keywords: Alkaloids Standardization Menispermaceae HPLC
Quantitativeanalysis
a
b
s
t
r
a
c
t
Stephaniavenosa(Blume)Spreng.,Menispermaceae,hasbeentraditionallyusedastonicdrugand
treat-mentofvariousdiseasesinSouthEastAsiancountries.Inordertoevaluatethequalityandstandardization
ofS.venosaroots,theHPLCmethodforquantificationofthecontentofmajorcomponentsinS.venosa
wasdevelopedandvalidated.ThechromatographicseparationwasperformedonaHypersilBDSC18
columnusinggradientsystemof100mMammoniumacetateinwaterandmethanolwithflowrate
1ml/min.Detectionwavelengthwassetat210nmfortetrahydropalmatine,280nmfordicentrineand
crebanine,and270nmforstephanine.Thevalidatedmethodshowedgoodsensitivity,linearity,
pre-cision,andaccuracy.Thesuitablesolventthatyieldedhighestalkaloidscontentsfromthematrixwas
optimized.S.venosasamplescollectedfromvariouslocationswereanalyzed.Thepresentstudyprovided
comprehensiveoverviewofmajorcomponentsinS.venosa.Aremarkablevariationintheaccumulation
ofalkaloidsineachpopulationandthebetweenindividualinthesamepopulationcouldbeobserved.Our
resultsshowedtheheterogeneityofS.venosainThailandwhichwouldneedafurtherstudyforspecies
delimitations.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Stephaniavenosa(Blume)Spreng.,vernacularlynamedinThai as“Sa-Bu-Leud”,belongstotheMenispermaceaefamily(Forman, 1991).Thisplantisavineindigenousmedicinalherbdistributed inSouthEastAsiancountries.Theprominentredsapinitsstem isa characteristic keyfor thespeciesidentification.It hasbeen traditionallyusedasatonicdrug,fortreatmentofcancerand dia-betes,aphrodisiac,andforvariousindications(Ingkaninanetal., 2006;Kongkiatpaiboonetal.,2016).Importantbiological activi-tieshavebeenreportedincludingantimalarial(Likhitwitayawuid et al., 1999), cytotoxicity against cancer cell lines (Makarasen etal.,2011), antimicrobial(Makarasenetal.,2011),and acetyl-cholinesteraseinhibition(Ingkaninanetal.,2006;Kongkiatpaiboon etal.,2016).Somecellularmechanismshavebeenexplored. Cre-banine,amajorcomponentofS.venosa,exertsanti-proliferative
effects on human cancer cells through the induction of cell
cyclearrest attheG1phases andapoptosis (Wongsirisin etal.,
∗ Correspondingauthor.
E-mail:sumetk@tu.ac.th(S.Kongkiatpaiboon).
2012).Stephanine,thealkaloidfromS.venosa,couldinducethe reverseof mitoticexit,eventuallyleading tocelldeathby apo-ptosis(Leetal.,2017).l-Tetrahydropalmatine,thealkaloidfromS. venosawhichactsasadopaminereceptoragonist,couldattenuate
cocaineandmethamphetamineself-administrationand
cocaine-and methamphetamine-inducedreinstatement inrats (Mantsch
etal.,2007;Gongetal.,2016),modulatemethamphetaminereward behavior(Suetal.,2013),andinhibittheacquisitionof ketamine-inducedconditionedplacepreferencebyregulatingtheexpression ofextracellularsignal-regulatedkinases(ERK)andcAMPresponse element-bindingprotein(CREB)regulationinrats(Duetal.,2017). Dicentrine,aknownalpha1-adrenoceptorantagonist,couldhave atherapeuticpotentialtodevelopasantihypertensive, antihyper-lipidemicandothercardiovasculardrugs(Suetal.,1994;Yuetal., 1994a,b).
ThelocalwidespreadusesofStephaniaformedicalproperties hasledtoanincreasinginterestinthisplant.Thereporteddata showedthatalkaloidsarethemainphytochemicalconstituentsof thisgenus(Semwaletal.,2010).Although,isolationand elucida-tionofphytochemicalconstituentshavebeenextensivelydoneand someanalyticalmethodshavebeendevelopedinanother Stepha-niaspecies(Daryetal.,2017;Heetal.,2016),therearetheneeded
https://doi.org/10.1016/j.bjp.2017.10.004
DeionizedwaterwaspurifiedbyUltraClearTMsystem(Siemens
WaterTechnologiesCorp.). Ammoniumacetate andallreagents
wereofanalyticalgradeifnotstateotherwise.
Plantmaterials
SamplesrepresentingStephaniavenosa(Blume)Spreng.,
Menis-permaceae, were obtained from various location in Thailand
(Table 1). Identificationwas done based onthe key to species describedinFloraofThailand(Forman,1991).Voucherspecimens weredepositedatDrugDiscoveryandDevelopmentCenter, Tham-masatUniversity,Thailand.Eachsamplewasthoroughlycleaned bytapwater,cutintosmallpiecesanddriedinahotairovenat 50◦Cfor72h.Eachdriedsamplewasgroundintofinepowderand keptinanair-tightcontaineruntilused.
Extractionandisolationofmajorcomponents
Dicentrine(1),tetrahydropalmatine(2),andcrebanine(3)were isolatedinourpreviouswork(Kongkiatpaiboonetal.,2016).As described,thesamplewasmaceratedwithmethanolfor3×72h withoccasional shaking.Thecombinedextractwasfilteredand
concentrated using a rotary evaporator. The methanol crude
extractwasthenpartitionedwithdichloromethaneandwater.The lipophiliclayer,whichcontainsalkaloids,wasroughlyseparatedby
columnchromatography(CC)(Mercksilicagel60,70–230mesh)
withdichloromethane: EtOAc:MeOH (70:25:5, v/v/v)as mobile
phase. Fractions were monitored using TLC (silica gel 60 F254)
sprayedwithDragendorff’sreagent.Furtherpurificationwasmade byCC(Mercksilicagel60,230–400mesh).Thefinalcleaningup
wascarriedoutusingonaSephadexLH-20columnelutedusing
methanolaseluent.
data(Blanchfieldetal.,2003).
HPLCapparatusandconditions
HPLCwasperformedonanAgilent1260Series(Agilent
Tech-nologies)equippedwitha1260QuatpumpVLquaternarypump,
1260ALSautosampler,1260TCCcolumn thermostat,and1260
DADVLdiodearraydetector.Theseparationwasdoneona Hyper-silBDSC18column(4.6×100mmi.d.,3.5m)withaC18guard
column.Themobilephaseswere(A)100mMammoniumacetate
inwaterand(B)methanol.Gradientelutionwasusedfrom50%
Bto70%BinA for20min,100%Bfor10min.Thecolumn was
equilibratedwith50%BinAfor10minpriortoeachanalysis.The flowratewassetat1ml/minwithcontrolledtemperatureat25◦C. DADdetectorwassetatthewavelengthof210nmfordetection oftetrahydropalmatine,280nmfordicentrineandcrebanine,and 270nmforstephanine.Theinjectionvolumewas10lforevery
sampleandstandard.
Stockandworkingsolutionsstandardcompounds
Stockstandardsolutionoftetrahydropalmatine,dicentrine,
cre-banine,and stephanine withpuritymore than 90%determined
by HPLC was prepared by dissolving each standard compound
inmethanoltoobtaintheconcentrationof1000g/ml.Working
standardsolutionswereobtainedbyappropriatedilutionofthe stocksolutionswithmethanoltoobtainedthedesired concentra-tion.
InvestigationofsuitablesolventforStephaniavenosaextraction
ToobtainthehighestalkaloidscontentfromS.venosaextraction, varioussolvents, i.e. water, methanol,ethanol, acetonitrile,and
Table1
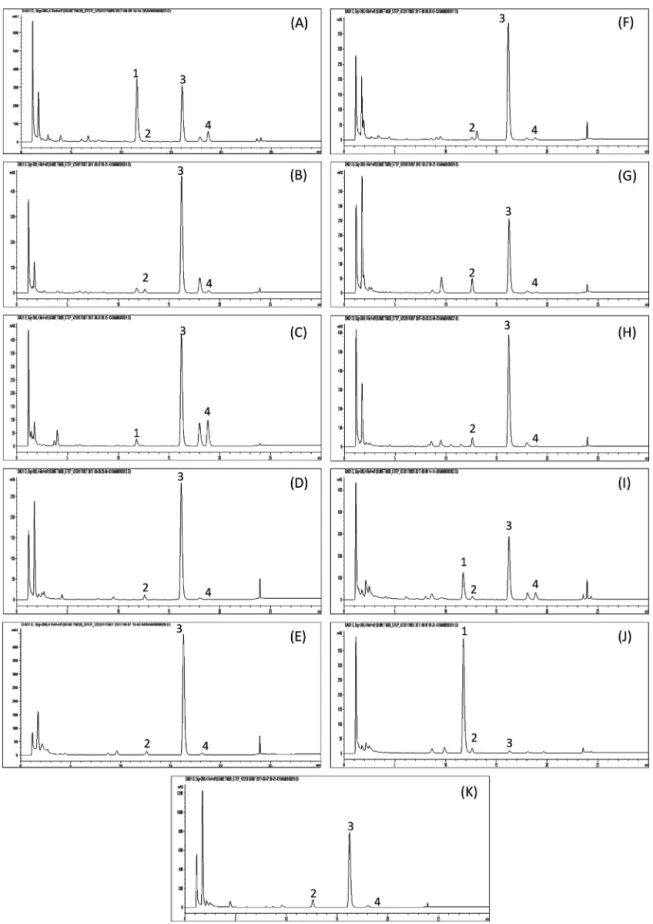
Percentageofdicentrine(1),tetrahydropalmatine(2),crebanine(3),andstephanine(4)inStephaniavenosacollectedfromvariouslocationsofThailand.
Locationa Contentb(mg/g)
Dicentrine(1) Tetrahydropalmatine(2) Crebanine(3) Stephanine(4)
ChiangMai,DoiAngKhang(N),Sample1 17.08±0.25 0.32±0.03 12.33±0.15 3.81±0.06 ChiangMai,DoiAngKhang(N),Sample2 – 1.48±0.03 18.30±0.21 0.49±0.01 ChiangMai,DoiAngKhang(N),Sample3 1.20±0.01 – 16.52±0.12 6.80±0.06
Lampang,Muang(N) – 1.31±0.11 12.00±0.65 <0.05
Uttaradit,Nampad(N) – 1.78±0.08 21.69±0.19 0.38±0.01
Kanchanaburi,Saiyok(SW) – 0.87±0.05 15.33±0.16 0.32±0.01
PrachuapKhiriKhan,Muang(SW) – 6.10±0.22 10.41±0.21 <0.05
Roi-Et,Muang(NE) – 5.47±0.04 23.37±0.14 0.05±0.02
Udonthani,Phen(NE) 3.20±0.04 0.61±0.02 5.73±0.05 1.00±0.01
Udonthani,NongWuaSo(NE) 20.38±0.72 1.61±0.06 0.16±0.02 – NakhonRatchasima,Wangnumkheo(E) – 9.19±0.30 30.27±0.90 <0.05
aFloristicregionsofThailand:N=northern,NE=northeastern,E=eastern,SW=southwestern. b Expressedasmean
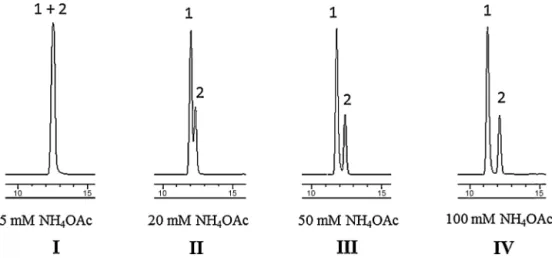
Fig.1.Influenceofammoniumacetateconcentrationsintheseparationofdicentrine(1)andtetrahydropalmatine(2)inreversed-phaseHPLC.Column:HypersilBDSC18
(4.6mmi.d.×10cm,3.5m).Mobilephase:(A)ammoniumacetateinwaterand(B)methanol.Gradientelution:50%BinAto70%BinAfor20min;then100%Bfor10min. Concentrationofammoniumacetatesolution:(I)5mMammoniumacetatesolution,(II)20mMammoniumacetatesolution,(III)50mMammoniumacetatesolution,and (IV)100mMammoniumacetatesolution;flowrate1ml/min,detectionat280nm.
mixturesofwaterandmethanolatdifferentratio,wereevaluated asextractingsolvents.CrudedrugpowderofS.venosa(50mg)was accuratelyweighedandseparatelyextractedwith5mlofthese sol-ventsbysonicationattheambienttemperature.Eachextractwas triplicatelypreparedandanalyzedbytheHPLC.Thesolvent yield-ingthehighestcontentofalkaloidsintheextractwaschosenasthe appropriatesolventforextraction.
Samplepreparation
Each powdered sample of S. venosa roots was accurately
weighedandextractedwithwater–methanol(30:70,v/v)at con-centrationof10mg/mlinanultrasonicbathforeach30min.Each samplewasdoneintriplicate.Priortoinjection,eachsolutionwas filteredthrougha0.2mnylonmembranefilterandanalyzedwith
HPLC.
Methodvalidation
Validationofthemethodwasdoneaccordingtothe Interna-tionalConferenceonHarmonizationguideline(ICH,1996/2005). Themethodwasvalidatedforlinearity,precision,accuracy,limit ofdetection(LOD),andlimitofquantitation(LOQ).
Linearity
Linearityofthemethodwasstudiedbyinjectingsevenknown concentrationsoftheanalytesintherangeof1.9–250g/mlin
trip-licate.Thecalibrationcurveswereobtainedbyplottingthepeak areaversustheamountofthestandard.
Precision
Themeasurementofintra-andinter-dayprecisionwasdone
byanalyzing50g/mlstandardsolution.Theintra-dayprecision
wasdeterminedbyanalyzingseventimeswithin1day,whilethe inter-dayprecisionwasexaminedforthreeconsecutivedaysbythe proposedmethod.Theprecisionwasexpressedaspercentrelative standarddeviation(%RSD).
Accuracy
Recovery wasused toevaluatethe accuracyof the method.
Standardadditionwasperformedwithpre-analyzedstandard solu-tion.Threedifferentlevelsofstandard mixtures wereaddedto thesampleextract.Spikesampleswerepreparedintriplicate.The
recoverywascalculatedasfollows:recovery(%)=100×(amount found−originalamount)/amountspiked.
Limitofdetectionandlimitofquantitation
Determinationofsignal-to-noiseratiowascalculatedunderthe proposedchromatographiccondition.LODwasconsideras3:1and LOQas10:1.
Results
HPLCmethoddevelopment
AHPLCmethodwasdevelopedforanalysisthecontentsofmajor alkaloids,dicentrine(1),tetrahydropalmatine (2),crebanine(3), andstephanine(4)inS.venosaroots.Optimizationofthemobile
phasecompositionswasdone.Reversed-phaseC-18columnwhich
is broadly usedin pharmaceutical separation, wasused in this study.Thecriticalseparationof1and2affectedbyammonium acetateconcentrationsareshowninFig.1.Fromvariousmobile phasestrialed,thesystemcontaininggradientsolventsystemusing
100mMammoniumacetateandwatergavethesymmetricpeaks
andprovidedthemostefficientseparationandspeed(Fig.1).The
wavelengthat210nmwhichgavehighabsorbancecapacitywas
usedfordetectingtetrahydropalmatine(2),at280nmfor dicen-trine(1)andcrebanine(3),and270nmforstephanine(4).
SuitablesolventforextractingalkaloidsfromStephaniavenosa
sol-Parameters Results
Dicentrine(1) Tetrahydropalmatine(2) Crebanine(3) Stephanine(4)
Regressionequationa Y=26.626X+46.607 Y=70.037X+146.33 Y=32.897X+65.959 Y=22.38+43.673
Correlationcoefficient(r2) 0.9998 0.9981 0.9998 0.9998
Linearrange,g/ml 1.9–250 1.9–125 1.9–250 1.9–250
LOD,g/ml 0.1 0.3 0.1 0.1
LOQ,g/ml 0.3 1 0.3 0.3
aXistheconcentrationofeachstandarding/ml;Yisthepeakareaat280nmfordicentrine(1)andcrebanine(3),210fortetrahydropalmatine(2),and270forstephanine
(4).
vents.Sonicationwaschosenasanextractionmethodduetoits simplicity,rapidityandcompatibilitywithvarioussolvents.After quantificationbyHPLC,thehighestalkaloidscontentwasfound inthewater-methanol(30:70,v/v)extractasshowninTable2. Although, tetrahydropalmatine (2) and crebanine (3) extracted frommixtureofwater–methanolatratioof50:50and30:70(v/v) werenotsignificantlydifferent.Mixtureofwater–methanolatratio of30:70(v/v)couldextractthehigheststephanine(4)content com-paredtotheothersolvents.Thus,mixtureofwater–methanolat ratioof30:70(v/v)waschosenasasuitablesolventforextraction.
Methodvalidation
TheHPLCmethodwasvalidatedforanalysisofthedicentrine (1),tetrahydropalmatine(2),crebanine(3),andstephanine(4)inS. venosaroots.Linearity,precision,accuracy,LOD,andLOQwere ana-lyzedformethodvalidationparameters(ICH,1996/2005).Linearity wasevaluatedbyusingstandardsolutionsdissolvedinmethanol at concentrations in the range of 1.9–250g/ml for dicentrine
(1), crebanine (3), and stephanine (4) while of 1.9–125g/ml
fortetrahydropalmatine(2).Eachconcentrationwasanalyzedin triplicate.The plotof thepeak areas versusthe concentrations
of all compounds provided a linear of this method with good
correlationcoefficient(Table3).Theinvestigationofintra-day pre-cisionbyseventimes injectionof125g/mlstandard solutions
withinonedayshowedtheresultthatthepercentageofrelative
standarddeviationwaslowerthan1%RSD.Whilethe
measure-mentofinter-day precisionbythreeconsecutivedays withthe samestandardprovided thepercentrelative standarddeviation lessthan3.8%(Table4).Theresultsgaveanacceptableprecision ofthemethod.Theaccuracyofthemethodwasdeterminedbythe recoveryvalues.Theresultsreportedtherecoveryofdicentrine(1), tetrahydropalmatine(2),crebanine(3),andstephanine(4)inthe rangedof96.30–98.82%(average97.78%),95.36–100.76%(average 98.17%),98.23–99.07%(average98.79%),and95.25–100.17% (aver-age98.42%),respectively,asshowninTable5.TheLODandLOQ, atsignaltonoiseratioas3:1forLOD,and10:1forLOQ,were0.1 and0.3,0.3and1,0.1and0.3,and0.1and0.3g/ml,for
dicen-trine(1),tetrahydropalmatine(2),crebanine(3),andstephanine (4),respectively(Table3).
Table4
Intradayandinterdayprecisionofdicentrine(1),tetrahydropalmatine(2),crebanine (3),andstephanine(4);resultsareshownas%RSD.
Compound Intra-day Inter-day
Day1 Day2 Day3
Dicentrine(1) 0.38 0.39 0.25 3.14
Tetrahydropalmatine(2) 0.54 0.40 0.18 3.56
Crebanine(3) 0.67 0.38 0.36 3.80
Stephanine(4) 0.33 0.41 0.27 3.71
Table5
Recoverystudyofdicentrine(1),tetrahydropalmatine(2),crebanine(3)and stepha-nine(4).
Level Compound Theoreticala
(g/ml)
Foundb(g/ml) Recoveryb (%)
1
Dicentrine(1) 46.32 45.49±0.53 98.22±1.15
Tetrahydropal-matine(2)
45.53 43.42±1.95 95.36±4.28
Crebanine(3) 87.96 86.40±0.79 98.23±0.90 Stephanine(4) 14.77 14.07±0.35 95.25±2.35
2
Dicentrine(1) 62.13 61.40±0.69 98.82±1.10
Tetrahydropal-matine(2)
65.98 64.92±1.76 98.39±2.40
Crebanine(3) 121.36 120.23±1.10 99.07±0.91 Stephanine(4) 21.13 21.17±0.17 100.17±0.81
3
Dicentrine(1) 80.88 77.89±0.70 96.30±0.87
Tetrahydropal-matine(2)
88.66 89.33±0.84 100.76±0.96
Crebanine(3) 160.63 158.70± 98.79±0.93 Stephanine(4) 24.63 24.60±0.14 99.84±0.80
Average
Dicentrine(1) 97.78
Tetrahydropal-matine(2)
98.17
Crebanine(3) 98.70
Stephanine(4) 98.42
aTheoreticalvalueistheamountcalculatedbyoriginalamountplusamount
spiked.
Standardizationofphytopharmaceuticalproductsaimsto
con-trol the consistency of the component product for safety and
biological activity for reproducible products quality. HPLC, a methodofchoiceforpharmaceuticalanalysis,isconsidered effi-cient and stringent for qualitative and quantitative analysis of
plantchemicalcompounds.Inthisstudy,theHPLCmethodwas
developedforanalysisthecontentsofmajoralkaloids,dicentrine (1),tetrahydropalmatine(2),crebanine(3),andstephanine(4)in
S.venosaroots. Optimizationofthemobilephase compositions
anddetection wavelengthweredonein ordertomaximizethe
efficiencyandsensitivityofthemethod.Validationhasbeen per-formedtoensurethelinearity,precision,accuracy,andsensitivity ofthemethodaccordingtotheICHguideline(ICH,1996/2005)and provedthatthemethodissuitableforitsintendeduse.
Criticalseparationof dicentrine(1)and tetrahydropalmatine (2) affected by ammoniumacetate concentrations (Fig. 1) rep-etitiouslyshowedtheinteraction ofalkaloids withtheresidual silanolgroupofthecolumnasdescribed inourpreviouspaper (Kongkiatpaiboon and Gritsanapan, 2012). It was probably due tothecompetitiveinteractionofthebuffercation withresidual silanolsasdescribedbytheLangmuirisotherm(Langmuir,1916; FliegerandCzajkowska-Zelazko,2011).However,slightlyvariation ofbatch-to-batchinHPLCcolumnproductionwasobserved. There-fore,inpracticalapplication,optimalconditionmayneeddifferent
ammoniumacetateconcentration.
Solventsused duringtheextractionarea crucial role inthe qualityandquantityofextractedcompounds.Sonication,which issimple,rapidandhasnolimitationonanysolventtype,was per-formedtodeterminethesuitablesolvent.Withvarioustypesof solventtrialed(Table2),themixtureofwater–methanol(30:70, v/v)wasthemostsuitablesolventthatyieldedhighestalkaloids contentsfromthematrix.Wealsoperformedthestudyeffectof solventpolaritytothedicentrine(1)extractingyields.Theresult
wasappearedinthesamemanner(datanotshown).Mixtureof
water–methanol(30:70, v/v) was also the efficient solvent for extractingdicentrine(1)comparedtotheothersolvents.Thus,it waschosenasextractingsolventinthesamplepreparation.
S.venosasamplescollectedfromvariouslocationswere
ana-lyzedusingthedevelopedHPLCmethodwhichcouldbeusedfor
routineanalysis.Besidesthereportedisolatedcomponentsineach individualS.venosastudy(Likhitwitayawuidetal.,1999; Ingkani-nanetal.,2006;Yodkeereeetal.,2013;Kitisripanyaetal.,2013;Le etal.,2017),thepresentstudyprovidedcomprehensiveoverview ofmajorcomponentsinS.venosa.Aremarkablevariationinthe accumulationofalkaloidsineachpopulationandthebetween indi-vidualinthesamepopulationcouldbeobserved.Duetoincomplete dataontheoccurrenceofthesealkaloids,nofurthergeographic segregationcanbededuced.Morphologicaldataoneach individ-ualhasbeen recordedand willbefindoutfor therelationship withtheirphytochemicalcomponentsinfurtherstudy.Ourresults
tine analysisofS.venosaraw materialsinpractical application.
The present study provided comprehensive overview of major
componentsinS.venosa.Aremarkablevariationinthe accumu-lationofalkaloidsineachpopulationandthebetweenindividual
in the same population could be observed. The heterogeneity
of S. venosa in Thailand suggests a further study for species delimitations.
Authors’contribution
SKcontributionincludedcollectingsamples,designingand per-forminglaboratorywork,analyzingtheresults,andpreparingthe paper.NTandWIcontributionincludedisolationandpurification ofthecompounds.NDandSPcontributionincludeddata interpre-tationandidentificationofthecompounds.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors gratefully acknowledge the financial support
provided by Thammasat University under Thai Government
Research Fund (Contract No. 56/2559). We thank Ms. Pajaree
Inthachub for discussion and identification of plant materials.
WealsoacknowledgeChulabhornResearchInstitutefortheNMR
measurement.
References
Blanchfield,J.T.,Sands,D.P.A.,Kennard,C.H.L.,Byriel,K.A.,Kitching,W.,2003. Char-acterisationofalkaloidsfromsomeAustralianStephania(Menispermaceae) species.Phytochemistry63,711–720.
Dary,C.,Bun,S.S.,Herbette,G.,Mabrouki,F.,Bun,H.,Kim,S.,Jabbour,F.,Hul,S., Baghdikian,B.,Ollivier,E.,2017.ChemicalprofilingofthetuberofStephania cambodicaGagnep.(Menispermaceae)andanalyticalcontrolbyUHPLC-DAD. Nat.Prod.Res.31,802–809.
Du,Y.,Du,L.,Cao,J.,Holscher,C.,Feng,Y.,Su,H.,Wang,Y.,Yun,K.M.,2017. Levo-tetrahydropalmatineinhibitstheacquisitionofketamine-inducedconditioned placepreferencebyregulatingtheexpressionofERKandCREBphosphorylation inrats.Behav.BrainRes.317,367–373.
Flieger,J.,Czajkowska-Zelazko,A.,2011.Comparisonofchaotropicsaltandionic lipidasmobilephaseadditivesinreversed-phasehigh-performanceliquid chro-matographyofbiogenicamine.J.Sci.Sep.34,733–739.
Forman,L.L.,1991.Menispermaceae.FloraThailand5,300–365.
Gong,X.,Yue,K.,Ma,B.,Xing,J.,Gan,Y.,Wang,D.,Jin,G.,Li,C.,2016. Levo-tetrahydropalmatine,anaturalmixeddopaminedreceptorantagonists,inhibits methamphetamineself-administrationandmethamphetamine-induced rein-statement.Pharmacol.Biochem.Behav.144,67–72.
ICH,1996/2005.InternationalConferenceonHarmonisationofTechnical Require-ments for Registration of Pharmaceuticals for Human Use. Validation of AnalyticalProcedures:TextandMethodology.ICH,Geneva.
Kitisripanya,T.,Komaikul,J.,Tawinkan,N.,Atsawinkowit,C.,Putalun,W.,2013. DicentrineproductionincallusandcellsuspensionculturesofStephaniavenosa. Nat.Prod.Commun.8,443–445.
Kongkiatpaiboon,S.,Gritsanapan,W.,2012.HPLCquantitativeanalysisof insecti-cidaldidehydrostemofolineandstemofolineinStemonacollinsiaerootextracts. Phytochem.Anal.23,554–558.
Kongkiatpaiboon,S.,Duangdee,N.,Prateeptongkum,S.,Chaijaroenkul,W.,2016. AcetylcholinesteraseinhibitoryactivityofalkaloidsisolatedfromStephania venosa.Nat.Prod.Commun.11,1805–1806.
Ingkaninan, K., Phengpa, P., Yuenyongsawad, S., Khorana, N., 2006. Acetyl-cholinesteraseinhibitorsfromStephaniavenosatuber.J.Pharm.Pharmacol.58, 695–700.
Langmuir,I.,1916.Theconstitutionandfundamentalpropertiesofsolidsandliquids. J.Am.Chem.Soc.38,2221–2295.
Le,P.M.,Srivastava,V.,Nguyen,T.T.,Pradines,B.,Madamet,M.,Mosnier,J.,Trinh,T.T., Lee,H.,2017.StephaninefromStephaniavenosa(Blume)Sprengshowed effec-tiveantiplasmodialandanticanceractivities,thelatterbyinducingapoptosis throughthereverseofmitoticexit.Phytother.Res.31,1357–1368.
Likhitwitayawuid,K.,Dej-adisai,S.,Jongbunprasert,V.,Krungkrai,J.,1999. Anti-malarialsfromStephaniavenosa,Prismatomerissessiliflora,Diospyrosmontana
andMurrayasiamensis.PlantaMed.65,754–756.
Makarasen,A.,Sirithana,W.,Mogkhuntod,S.,Khunnawutmanotham,N.,Chimnoi, N.,Techasakul,S.,2011.Cytotoxicandantimicrobialactivitiesofaporphine alkaloidsisolatedfromStephania venosa(Blume)Spreng. PlantaMed.77, 1519–1524.
Mantsch, J.R., Li, S.J., Risinger, R., Awad, S., Katz, E., Baker, D.A., Yang, Z., 2007.Levo-tetrahydropamaltineattenuates cocaineself-administrationand cocaine-induced reinstatement in rats. Psychopharmacology (Berlin) 192, 581–591.
Semwal,D.K.,Badoni,R.,Semwal,R.,Kothiyal,S.K.,Singh,G.J.P.,Rawat,U.,2010. ThegenusStephania(Menispermaceae):chemicalandpharmacological per-spectives.J.Ethnopharmacol.132,369–383.
Su,H.L.,Zhu,J.,Chen,Y.J.,Zhao,N.,Han,W.,Dang,Y.H.,Xu,M.,Chen,T.,2013.Rolesof levo-tetrahydropalmatineinmodulatingmethamphetaminerewardbehavior. Physiol.Behav.118,195–200.
Su,M.J.,Nieh,Y.C.,Huang,H.W.,Chen,C.C.,1994.Dicentrine,analpha-adrenoceptor antagonistwithsodiumandpotassiumchannelblockingactivities.Naunyn. SchmiedebergsArch.Pharmacol.349,42–49.
Wongsirisin,P.,Yodkeeree,S.,Pompimon,W.,Limtrakul,P.,2012.Inductionof G1arrestandapoptosisinhumancancercellsbycrebanine,analkaloidfrom
Stephanaivenosa.Chem.Pharm.Bull.60,1283–1289.
Yodkeeree,S.,Wongsirisin,P.,Pompimon,W.,Limtrakul,P.,2013.Anti-invasion effectofcrebanineandO-methylbulbocapninefromStephaniavenosavia down-regulatedmatrixmetalloproteinases andurokinase plasminogenactivator. Chem.Pharm.Bull.61,1156–1165.
Yu,S.M.,Ko,F.N.,Chueh,S.C.,Chen,J.,Chen,S.C.,Chen,C.C.,Teng,C.M.,1994a.Effect ofdicentrine,anovelalpha1-adrenoceptorantagonist,onhumanhyperplastic prostates.Eur.J.Pharmacol.252,29–34.