w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Pulmonary
magnetic
resonance
imaging
is
similar
to
chest
tomography
in
detecting
inflammation
in
patients
with
systemic
sclerosis
Carolina
de
Souza
Müller
a,∗,
Danny
Warszawiak
b,
Eduardo
dos
Santos
Paiva
c,
Dante
Luiz
Escuissato
daUniversidadeFederaldoParaná(UFPR),HospitaldeClínicas,AmbulatóriodeEscleroseSistêmica,Curitiba,PR,Brazil
bUniversidadeFederaldoParaná(UFPR)eUniversidadePositivo,Curitiba,PR,Brazil
cUniversidadeFederaldoParaná(UFPR),DepartamentodeClínicaMédica,DisciplinadeReumatologia,Curitiba,PR,Brazil
dUniversidadeFederaldoParaná(UFPR),DepartamentodeClínicaMédica,DisciplinadeRadiologia,Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12February2015 Accepted22December2016 Availableonline18March2017
Keywords: Systemicsclerosis Magneticresonance Computedtomography
a
b
s
t
r
a
c
t
Interstitiallungdisease(ILD)andpulmonaryarterialhypertension(PAH)areprevalent com-plicationsofsystemicsclerosis(SSc)andarecurrentlytheleadingcausesofdeathrelatedto thedisease.Theaccuraterecognitionoftheseconditionsisthereforeofutmostimportance forpatientmanagement.
Astudywascarriedoutwith24SScpatientsbeingfollowedattheRheumatology Depart-mentoftheHospitaldeClínicasofUniversidadeFederaldoParaná(UFPR)and14healthy volunteers,withtheobjectiveofevaluating theusefulnessoflungmagneticresonance imaging(MRI)whenassessingILDinSSpatients.TheresultsobtainedwithlungMRIwere comparedtothoseobtainedbycomputedtomography(CT)ofthechest,currentlyconsidered theexaminationofchoicewheninvestigatingILDinSSpatients.
Theassessedpopulationwaspredominantlycomposedofwomenwithameanageof 50years,limitedcutaneousSS,andadiseasedurationofapproximately7years.Inmost cases,therewasagreementbetweenthefindingsonchestCTandlungMRI.Considering itisaradiation-freeexaminationandcapableofaccuratelyidentifyingareasoflungtissue inflammatoryinvolvement,lungMRIshowedtobeausefulexamination,andfurtherstudies areneededtoassesswhetherthereisanadvantageinusinglungMRIinsteadofchestCT whenassessingILDactivityinSSpatients.
©2017ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:carolinadesmuller@yahoo.com.br(C.S.Müller).
http://dx.doi.org/10.1016/j.rbre.2017.02.001
Ressonância
magnética
pulmonar
é
semelhante
à
tomografia
de
tórax
para
detectar
inflamac¸ão
em
pacientes
com
esclerose
sistêmica
Palavras-chave: Esclerosesistêmica Ressonânciamagnética Tomografiacomputadorizada
r
e
s
u
m
o
A doenc¸a intersticial pulmonar (DIP) e a hipertensão arterial pulmonar (HAP) são complicac¸õesprevalentesnaesclerosesistêmica(ES)econstituematualmenteas princi-paiscausasdemorterelacionadasàdoenc¸a.Oreconhecimentoprecisodessascondic¸õesé, portanto,defundamentalimportâncianomanejodospacientes.
Fez-seumestudocom24pacientescomESemacompanhamentonoservic¸ode reuma-tologiadoHospitaldeClínicasdaUniversidadeFederaldoParaná(UFPR)e14voluntários sadioscomobjetivodeavaliarautilidadedoexamederessonânciamagnética(RM)do pul-mãonaavaliac¸ãodaDIPempacientescomES.OsresultadosobtidoscomaRMpulmonar foramcomparadoscomosobtidosnatomografiacomputadorizada(TC)detórax,exame atualmenteconsideradodeeleic¸ãonainvestigac¸ãodaDIPempacientescomES.
Apopulac¸ãoavaliadaerapredominantementecompostapormulherescomidademédia de50anos,EScutânealimitadaetempodedoenc¸adeaproximadamenteseteanos.Na maioriadoscasos,houveconcordânciaentreosachadosnaTCdetóraxeRMdopulmão.Em setratandodeumexameisentoderadiac¸ãoecapazdeidentificarcomadequadaprecisão áreasdeacometimentoinflamatóriodotecidopulmonar,aRMdopulmãoderevelouum exameútil.SãonecessáriosmaisestudosparaavaliarsehávantagemdaRMdopulmão sobreaTCdetóraxnaavaliac¸ãodaatividadedaDIPempacientescomES.
©2017ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Systemic sclerosis (SSc) is an autoimmune disease of unknown origin and worldwide distribution that predomi-nantlyaffectswomeninthethirdorfourthdecadesoflife, which ischaracterized byfibrosis of the skin and internal organs,vasculopathyandimmunedysregulation.1,2
SSc has high morbidity and mortality, with pulmonary involvement in the form of interstitial lung disease (ILD) and/orpulmonaryarterialhypertension(PAH)beingthemain causeofdeathinthisdisease.3,4
ILDis a fairlycommon complicationof SSc,present in approximately50%ofpatientswithdiffusecutaneousSScand inuptoaquarterofpatientswithlimitedcutaneousSSc.5,6In
SSc,themostfrequentpatternofinterstitiallunginvolvement isnonspecificinterstitialpneumonia(NSIP),characterizedby the presence of ground-glass opacity, representing inflam-matory involvementoflungtissue(alveolitis), andtraction bronchiectasis and bronchiolectasis, which correspond to pulmonaryparenchymalfibrosis.InNSIP,pulmonary involve-mentpredominantlyaffectsthelowerlobes,isbilateraland symmetrical,andcommonlyshowsanadequateresponseto immunosuppressants.3,7
Therefore, as the treatment of ILD in SSc involves the use ofimmunosuppressants, theseshould be initiated earlyduringdiseaseevolution,i.e., inpre-fibrotic stages,in caseswithinflammatoryinvolvement(alveolitis)ofthelung parenchyma. Likewise, it is plausible to consider that the immunosuppressivetreatment,whichisnotfreeof compli-cationsandsideeffects,shouldonlybemaintainedaslongas thereisaninflammatorysubstratewhichitcanactupon.
SeveralmethodsareavailablefortheevaluationofILDin SSc,withchestCTbeingtheonemostcommonlyusedtest
(the“goldstandard”).Itisafast,widelyavailableexamination anditshighresolutionallowsanexcellentanalysisofthelung parenchymawhencompared,forinstance,toaplainchest X-ray.However,incomparisontothelatter,itinvolvesamuch higherdoseofradiation.8
Complementing thechest CTfindings, pulmonary func-tiontests,includingspirometry,lungvolumedetermination, carbon monoxide diffusioncapacity measurement and the 6-minute walkingtestare alsoperformedinthe ILD inves-tigation.
To identify areas of pulmonary inflammation, mag-netic resonance imaging (MRI) presents as a promising examination.9–11 Regarding theexaminationtechnique,one
canidentify,accordingtothetissuecharacteristics(hydrogen protonorganization,responsetothemagneticfieldandthe radiofrequencystimulus), relaxationtimesT1,T2and den-sityofhydrogenprotons(DP),withthesebeingtheparameters thatyieldimagebrightnessorsignal.Bychoosingthe param-etersofeachsequence,itispossibletoweighttheimagein T1,T2andothertypesofsequence,allowingthe differenti-ation betweennormaland pathologicaltissues.Commonly, thereisanincreaseinT2signalinpathologicalprocesses.12
InpulmonaryMRI,thelowdensityofprotons,whichgenerates alow-densitysignal,andmultipleair–tissueinterfaces (sus-ceptibilityartifact),aswellasmovementartifacts(respiratory, cardiac,andvascular),aregreatchallenges.13However,MRIis
anon-invasive,relativelyhigh-resolution,ionizing radiation-freeexamination.
However,inthefieldofSSc,fewstudieshavereportedon MRIusefortheevaluationofILD,whichismorecommonly usedtoanalyzecardiacinvolvementinthedisease.14The
inflammatorycellsinthelungs,resultinginanincreaseinthe numberofprotons,withaconsequentsignalincreaseinthe T2imagesattheMRIexamination.Insummary,weconsider thereisaroleforpulmonaryMRIwhendifferentiating pul-monaryparenchymaactivediseasewithground-glassopacity (increase in T2-weightedimages) from non-active disease, withfibrosisonly(absenceofsignalincreaseinT2images).
Therefore,theaimofthisstudyistoevaluatethe useful-nessofpulmonaryMRIintheidentificationofinflammation areasinthepulmonaryparenchymainpatientswithILDand SSc.
Material
and
methods
Across-sectionalstudywascarriedoutattheRheumatology DepartmentofHospitaldeClínicasofUFPR,whichassessed 24patientswithadiagnosisofSScand14healthyvolunteers (conveniencesampling)thatcomprisedthecontrolgroup.The inclusion criteria were:patients should be 18 years ofage orolderand haveadiagnosis ofSSc accordingtothe 1980 ACRcriteria.15Thefollowingwereexcludedfromthestudy:
patientswhowereunabletoundergopulmonaryMRI,suchas patientswithacardiacpacemakerorhearingaid;smokers; patientswithirregularappointmentand/orscheduled exam-inationattendance; patientswho refusedtosignthe study consentform.
Thepatientsweresubmittedtoroutinelaboratorytestsand evaluatedforSScactivityateachvisittotheoutpatientclinic (intervalsof3–5months),accordingtotheEUSTAR(Eular Scle-rodermaTrialsandResearch)criteria.Usingthelatter,scores are assigned accordingto parametersofcutaneous, vascu-lar,articular,and cardiopulmonaryinvolvement, aswell as complementary tests, and the disease is considered active ifthe sumofthe scoresis≥3.16 All patientswere
submit-tedtohigh-resolutionchestCT,pulmonaryfunctiontestsand echocardiographyeveryyear.Inadditiontotheusual outpa-tientfollow-up, thepatients inthe study werereferred for lungmagneticresonanceimaging(SiemensMagnetomAvanto 1,5T–Erlangen,Germany),inaspecializedmedicalcenter. T2-weightedimageswithrespiratorysynchronizationwereused. ThedateofthelungMRIwascoincidentwiththatofthelung CT.Bothtestswereanalyzedbythesameinvestigator(DW), whowasnotblindedtotheresultsofthecomparisonmethod. Theresearch project wasapproved bythe Research Ethics CommitteeandCONEP(CAAEnumber:03691412.4.0000.0096).
Statisticalanalysis
Dataareexpressedasmean±standarddeviationor percent-age(%).Toevaluatetheagreementbetweenthetests,Cohen’s Kappa,generalagreement,positiveandnegativeagreement indiceswere used.Statisticalanalysiswasperformedusing thestatisticalsoftwareJMP7.0®,SASInstitute,Inc.,Cary,NC.
Results
LungMRIwasperformedin24SScpatientsand14healthy vol-unteers,whoconstitutedthecontrolgroup(Table1).Patients were predominantly female (95.8%), with a mean age of
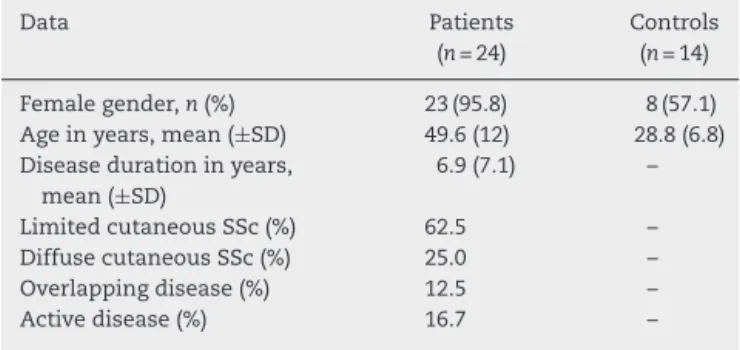
Table1–Clinicaldataofpatientsandcontrolgroup.
Data Patients
(n=24)
Controls (n=14)
Femalegender,n(%) 23(95.8) 8(57.1) Ageinyears,mean(±SD) 49.6(12) 28.8(6.8) Diseasedurationinyears,
mean(±SD)
6.9(7.1) –
LimitedcutaneousSSc(%) 62.5 –
DiffusecutaneousSSc(%) 25.0 –
Overlappingdisease(%) 12.5 –
Activedisease(%) 16.7 –
49.6±12yearsanddiseaseduration(estimatedfromthefirst symptom other than Raynaud’s phenomenon) of 6.9±7.1 years.ThemajorityhadlimitedcutaneousSSc(62.5%)andless thanonefifthofthepatientshadactivediseaseaccordingto theEUSTARactivityscore.Twopatientshadoverlapped dis-easewithrheumatoidarthritisandonepatientwithjuvenile idiopathicarthritis.
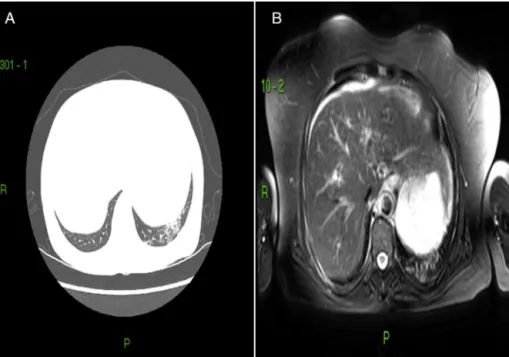
Whenassessingpatientsandcontrolssubmittedtochest CTandchestMRI,itwaspossibletoverifythatthefindings bybothtechniqueswereverysimilar.AmongtheSScpatients (n=24),79.2%(n=19)hadground-glassopacityareasonchest CT,ofwhich100%hadlungMRIwithincreasedT2-weighted signalsintheareascorrespondingtotheground-glassopacity findings.Oftheremaining5patientswithchestCTwithno evi-denceofground-glassopacity,3hadnormallungMRIresults and2patientshadMRIwithareasofincreasedT2-weighted signals(Figs.1and2).
Astheprimaryendpointofthestudy,weevaluatedthe per-formanceofchestMRIversuschestCT(the“goldstandard”)in theassessmentofILD.Inthisanalysis,restrictedtopatients, weobtainedforMRI:sensitivityof100%(82.35–100);Specificity of60%(14.66–94.73);Positivelikelihoodratioof2.50(0.85–7.31); Negativelikelihoodratioof0;Positivepredictivevalueof90.5% (69.62–98.83);Negative predictive valueof100%(29.24–100). TheresultsareshowninTable2.
In asecondary analysis, regardinghow lungMRI would behave in the control group, the presenceof ground-glass opacity was used as the “gold standard” in the chest CT, attaining 100%ofsensitivityforthe MRI(82.35–100); Speci-ficityof71.43%(41.90–91.00);Positivelikelihoodratioof3.50 (1.53–8.00);Negativelikelihood ratioof0;Positivepredictive valueof82.61%(61.22–95.05);Negativepredictivevalueof100% (69.15–100).
Inthe analysisofagreementbetweenthetests, Cohen’s Kappawas0.704(CI:0.328–1.0)andtheoverallagreementwas 91%.Thepositiveagreementwas100%andthenegative agree-mentwas60%.
Discussion
Fig.1–Respectively,chestCTandchestMRIofpatientwithSSc.A,showsground-glassattenuationareainthelowerlobe oftheleftlung;B,showsanareaofT2increaseinthecorrespondingarea.
Fig.2–Respectively,chestCTandchestMRIofpatientwithSSc.Ashowsanareaofreticularinfiltrateinthelowerlobeof therightlung,withtractionbronchiectasisandminimalground-glassattenuationareas;B,showsanincreaseinT2signal inthecorrespondingarea.
scleroderma,which allowedspecific treatmentand patient recovery.17
InthecaseofCollagenvasculardiseases,SScisthe pro-totype of the most characteristic pulmonary involvement.
Establishingthe usefulnessofchestMRIassessmentinthe detectionofILDinthesepatientsisofutmostimportance,as the examinationdoesnotrequireradiation useandcanbe repeatedwithminimalrisk.
Table2–ChestMRIperformancecomparedtochestCTintheassessmentofILD.
Data Sensitivity Specificity LR+ LR− PPV NPV
100% 60% 2.50 0 90.5% 100%
MRimagingisanextremelyusefulscreeningtoolfor dis-eases involving a variety of structures, such as the chest wall, pleura, heart, and mediastinum. In several situa-tions,itisshownasanequivalentorsuperiorexamination when compared to chest CT, as for instance, in the eval-uation of primary chest wall tumors, paraspinal masses andintheevaluationoflocaltumorextension. Itspositive points are the absence of ionizing radiation, an excellent contrastresolutionbetweenthenormalandpathological tis-sues,obtainingmultiplanarimagesand sensitivitytoblood flow.
When the use ofcontrast mediumfor MRI isrequired, gadolinium-basedagentsarecommonlyused,whichallows anexaminationwithgreatersensitivitywhen comparedto contrast-enhancedCTandhasalowerincidenceofadverse reactionsandcomplications.Whenassessingthepulmonary parenchyma, however, the MRI use is still restricted, due toseveralfactors,suchas thelower spatial resolutionand a longer time for image acquisition when compared to chestCT, movementartifacts causedbybreathingand car-diacmovements,lowamountofsignal-generatinghydrogen protons due to the presence of air in the airways and loss of signal induced by magnetic susceptibility at the border between the wall and the alveolar gas. In recent years, however, MRI assessment of the lungs has signifi-cantlyadvanced,withnewequipment,techniquesthatallow faster image detection and new contrast agents, such as inhaledhyperpolarizedgasesthatallowincreasedsignaland excellent spatial resolution of the pulmonary parenchyma images.9,13,18
Inthepresentstudy,weevaluatedtheusefulnessofchest MRI in identifying inflammation areas of the pulmonary parenchyma in the presence of ILD in SSc patients, com-paredwithchest CT findings. Theagreement betweenthe tests was high, which was corroborated by the kappa val-ues and agreement indices. We found that the lung MRI showed a sensitivity of 100% in the assessment of ILD and 100% in relation to chest CT. These are significant data,becausethis isaseveredisease,wherefalse-negative results willcause serious harm to the patient. The speci-ficity of 60% of the lung MRI compared to chest CT and of 71.43% in the assessment of ILD is reasonable. In the “false-positive” cases, in which chest CT did not disclose a ground-glass opacity pattern, but the lung MRI showed an increased T2-weightedsignal (n=2), it would be possi-bletoquestionwhether theMRIwassuperiortotheCT in detectingalveolitis inflammation inthe initialform. How-ever,theanalysisofthecontrolgroupdemonstratedahigher numberof“falsepositives”,whichdoesnotcorroboratethe hypothesis.
Asstudy limitations,we can mention the fact that the examinationswereperformedbyasingleexaminerandthe examinerwasnotblindedtotheresultsoftheexamination comparison(CTandMRI).Thesmallsamplesizeandthe sin-gleresearchcenterarealsoemphasized.Newstudieswitha largersamplesizeandspecializedcenterswillbeimportant tobroadenthedataanalysis.
InpatientswithSSc,chestMRIhasgoodsensitivitywhen comparedtochestCTandmayaddusefulinformationtothe assessmentofILDactivity.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.WalkerUA,TyndallA,CzirjakL,DentonC,Farge-BancelD, Kowal-BieleckaO,etal.Clinicalriskassessmentoforgan manifestationsinsystemicsclerosis:areportfromtheEULAR SclerodermaTrialsandResearchgroupdatabase.AnnRheum Dis.2007;66:754–63.
2.MullerCdeS,PaivaEdosS,AzevedoVF,RadominskiSC,Lima FilhoJH.Autoantibodyprofileandclinicalcorrelationina groupofpatientswithsystemicsclerosisinsouthernBrazil. RevBrasReumatol.2011;51:314–8,323–4.
3.CapobiancoJ,GrimbergA,ThompsonBM,AntunesVB, JasinowodolinskiD,MeirellesGS.Thoracicmanifestationsof collagenvasculardiseases.Radiographics.2012;32:
33–50.
4.LamblinC,BergoinC,SaelensT,WallaertB.Interstitiallung diseasesincollagenvasculardiseases.EurRespirJSuppl. 2001;32:69s–80s.
5.GilsonM,ZerkakD,WipffJ,DusserD,Dinh-XuanAT,Abitbol V,etal.Prognosticfactorsforlungfunctioninsystemic sclerosis:prospectivestudyof105cases.EurRespirJ. 2010;35:112–7.
6.FischerA,SwigrisJJ,GroshongSD,CoolCD,SahinH,Lynch DA,etal.Clinicallysignificantinterstitiallungdiseasein limitedscleroderma:histopathology,clinicalfeatures,and survival.Chest.2008;134:601–5.
7.KimEA,LeeKS,JohkohT,KimTS,SuhGY,KwonOJ,etal. Interstitiallungdiseasesassociatedwithcollagenvascular diseases:radiologicandhistopathologicfindings.
Radiographics.2002;22.SpecNo.:S151–65.
8.McColloughCH.Toscanornottoscan:considerationof medicalbenefitinthejustificationofCTscanning.Health Phys.2016;110:287–90.
9.HochheggerB,MarchioriE,IrionK,SouzaASJr,VolkartJ, RubinAS.Magneticresonanceofthelung:astepforwardin thestudyoflungdisease.JBrasPneumol.2012;38:105–15.
10.HopkinsSR,LevinDL,EmamiK,KadlecekS,YuJ,IshiiM,etal. Advancesinmagneticresonanceimagingoflungphysiology. JApplPhysiol(1985).2007;102:1244–54.
11.Ley-ZaporozhanJ,MolinariF,RisseF,PuderbachM,SchenkJP, Kopp-SchneiderA,etal.Repeatabilityandreproducibilityof quantitativewhole-lungperfusionmagneticresonance imaging.JThoracImaging.2011;26:230–9.
12.PooleyRA.AAPM/RSNAphysicstutorialforresidents: fundamentalphysicsofMRimaging.Radiographics. 2005;25:1087–99.
13.SantosMK,EliasJJr,MauadFM,MugliaVF,TradCS.Magnetic resonanceimagingofthechest:currentandnew
applications,withanemphasisonpulmonology.JBras Pneumol.2011;37:242–58.
14.HachullaAL,LaunayD,GaxotteV,deGrooteP,LamblinN, DevosP,etal.Cardiacmagneticresonanceimagingin systemicsclerosis:across-sectionalobservationalstudyof52 patients.AnnRheumDis.2009;68:1878–84.
15.Preliminarycriteriafortheclassificationofsystemicsclerosis (scleroderma).Subcommitteeforsclerodermacriteriaofthe AmericanRheumatismAssociationDiagnosticand TherapeuticCriteriaCommittee.ArthritisRheum. 1980;23:581–90.
diseaseactivitycriteriaforsystemicsclerosis.IV.Assessment ofskinthickeningbymodifiedRodnanskinscore.Ann RheumDis.2003;62:904–5.
17.PingitoreA,GuiducciS,ConfortiML,DeMarchiD,GarganiL, Moggi-PignoneA,etal.Earlydetectionofmyocardialand pulmonaryoedemawithMRIinanasymptomaticsystemic sclerosispatient:successfulrecoverywithpulsesteroid. Rheumatology(Oxford,England).2013;52:1920–1.
18.SemelkaRC,CemBalciN,WilberKP,FisherLL,BrownMA, Gomez-CamineroA,etal.Breath-hold3Dgradient-echoMR imagingofthelungparenchyma:evaluationof