w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anatomical
and
histochemical
analysis
of
Dysphania
ambrosioides
supported
by
light
and
electron
microscopy
Rafaela
D.
Sá
a,
Asaph
S.C.O.
Santana
a,
Flávia
C.L.
Silva
b,
Luiz
Alberto
L.
Soares
a,
Karina
P.
Randau
a,∗aLaboratóriodeFarmacognosia,DepartamentodeCiênciasFarmacêuticas,UniversidadeFederaldePernambuco,Recife,PE,Brazil bDepartamentodeBiologia,UniversidadeFederalRuraldePernambuco,Recife,PE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23November2015 Accepted27May2016 Availableonline25June2016
Keywords:
Anatomy Amaranthaceae
Dysphaniaambrosioides
Histochemistry Mastruz
Microscopicanalysis
a
b
s
t
r
a
c
t
Dysphaniaambrosioides(L.)Mosyakin&Clemants(syn:ChenopodiumambrosoidesL.),Amaranthaceae, popularlyknownas“mastruz”,isanherbwidelyusedinBrazilasanthelmintic.Tocontributetothe knowledgeaboutmedicinalplants,amicroscopic analysiswas accomplishedtodescribethemain anatomicalcharactersofroot,stem,petioleandleafbladeofD.ambrosioidesandhistochemicaltests wereperformedontheleafblade.Cross-sectionswereobtained,byhand,formicroscopicanalysisof root,stem,petioleandleafblade;totheleafbladewerestillmadeparadermalsections,scanningelectron microscopyanalysis,macerationandhistochemicaltests.Themaincharactersusefulintheidentification oftheplantwere:anomaloussecondarythickeningintherootandstem;presenceofidioblasts contain-ingcrystalsandintheroot,stem,petioleandleafblade;inthesetherearealsoidioblastswithdruses; presenceofnon-glandularandglandulartrichomesinthestem,petioleandleafblade;stomataonthe stem,petioleandleafblade,identifiedintheseasanomocyticandanisocytic;dorsiventralmesophylland collateralvascularbundles.Macerationrevealedthatthevesselelementsarehelicaltype.Throughthe histochemicaltests,itwasevidencedthepresenceoflipophilicsubstances,essentialoils,oleoresins, phe-noliccompounds,starch,ligninandcalciumoxalatecrystals.Thisworkprovidessupporttothequality controlofthespecies.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
ThefamilyChenopodiaceaewascomprisedabout102genera and1400speciesdistributedworldwide(Joly,2002).Recent molec-ular phylogenetic studies include representatives of the family ChenopodiaceaeinthefamilyAmaranthaceae.Somespeciesofthe genusChenopodiumweretransferredtothegenusDysphania,as
ChenopodiumambrosioidesL.,whichiscurrentlyknownas Dyspha-niaambrosioides(L.)Mosyakin&Clemants(Fuentes-Bazanetal.,
2012a,b;Senna,2016).
D. ambrosioidesis popularlyknownas epazote,Mexicantea, American wormseed, paico, mastruz and erva-de-Santa-Maria
(Kliks, 1985;Albuquerqueet al.,2009).The speciesis nativeto
CentralandSouthAmerica,originated,probably,fromMexico.It hasspontaneousgrowthintropicalandsubtropicalregions(mainly AmericaandAfrica)andalsointemperatezones(fromthe Mediter-raneantoCentralEurope)(Kismann,1991).InBrazil,itsdistribution
∗ Correspondingauthor.
E-mail:kprandau@ufpe.br(K.P.Randau).
isextensive,occurringinalmostalltheterritory(Sousaetal.,2004;
Limaetal.,2006).
Itisanherbthatreachesupto1mhigh,beinghighlybranched. Theleavesarealternate,elongated,withjaggededges,acuteapex, hairyandhavedifferentsizes,wherethesmallerarelocatedon topoftheplantandaresessile;thelargeststandatthebottom andhaveshortpetiole.Theyhaveastrongandcharacteristicsmell. Theinflorescenceistheracemosatype,presentingsmallflowers greencolored.Theseedsarenumerous,sphericalandhaveblack color(Cruz,1995;LorenziandMatos,2002;Matos,2007;Lorenzi,
2008).
Theplantis consideredbytheWorldHealthOrganizationas oneofthemostusedamongtraditionalmedicines intheworld
(LorenziandMatos,2002).Theleavesarethepartoftheplantmost
oftenusedinfolkmedicineasanthelminticandalsoasantifungal
(Taylor,2005;Fenneretal.,2006;Neivaetal.,2011),fordigestive
disorders,musclepainsandbonefractures(Santayanaetal.,2005;
Garciaetal.,2010).IntheNortheastofBrazil,wherethespecies
iswidelyused,theleavesaremixedinablenderwithmilkforflu treatments(Moraisetal.,2005).Inendemicareasof leishmania-sis,thepopulationoftenusesitsleavesinthetopicaltreatmentof ulcerscausedbythedisease(Franc¸aetal.,1996).
http://dx.doi.org/10.1016/j.bjp.2016.05.010
Phytochemical studies have identified polyphenols and ter-penesasthemainconstituentsofD.ambrosioides(Jorgeetal.,1986;
Jainetal.,1990;Paréetal.,1993;Kiuchietal.,2002;Hegazyand
Farrag,2007;Hallalaetal.,2010;Jardimetal.,2010;Neivaetal.,
2011;Okhaleetal.,2012;Barrosetal.,2013;Sá,2013).Theessential
oiloftheleavesismainlycomposedofmonoterpenes,butthe lit-eratureshowsthatthereiswidevariationbothinitscomposition andinitspercentageofconstituents(Onochaetal.,1999;Gupta
etal.,2002;Cavallietal.,2004;Jardimetal.,2010;Sáetal.,2014).
SeveralbiologicalactivitieshavebeenreportedforD. ambro-sioides (Sá et al., 2015), such as antitumor (Nascimento et al.,
2006;Barrosetal.,2013),antipyretic,analgesic,anti-inflammatory,
antinociceptive(Hallalaetal.,2010;TrivellatoGrassietal.,2013), antifungal (Jardim et al., 2010), anthelmintic(Guimaraeset al.,
2001;Neivaetal.,2011)andantiprotozoal(Monzoteetal.,2007;
Patrícioetal.,2008;Monzoteetal.,2014).
Given the recognized popular use and the pharmacological studies that have demonstrated the therapeutic potential, D. ambrosioidesisoneof71speciesofplantsthatarousetheBrazilian government’sinterestfortheproductionofphytotherapics,being presentintheNationalRelationMedicinalPlantsofInterestto Uni-fiedHealthSystem(MS,2009).
Consideringthattheknowledgeofthemicroscopic character-istics is fundamental for the standardization of plants used as medicines,thisstudyaimstodescribethemainanatomical charac-tersoftheroot,stem,petioleandleafbladeofD.ambrosioidesand performhistochemicaltestsontheleafblade.
Materialsandmethods
Plantmaterial
Several adult specimens of Dysphania ambrosioides (L.) Mosyakin&Clemants(syn:ChenopodiumambrosioidesL.), Ama-ranthaceae, cultivated under full sun, were collected in the
garden of the Laboratório de Fitoterapia, in the company PernambucoParticipac¸õeseInvestimentosS/A,locatedinRecife at the Pernambuco State in Brazil. The voucher specimen was deposited in the Herbarium UFP-Geraldo Mariz, of the Uni-versidade Federal de Pernambuco, Brazil, under registration number69718.
Anatomicalcharacterization
Fortheirstructuralcharacterization,variouscross-sectionsat themiddleregionoftheroot,stem,petioleandleafbladefixedin
FAA50%(Johansen,1940)wereobtainedbyhand,usinga
com-monrazorblade.Forleafbladewerealsoperformedparadermal sectionsontheadaxialandabaxialfaces.Allsectionswere clar-ified in sodiumhypochlorite solution (50%) (Kraus and Arduin, 1997). Posteriorly, cross-sections were stained with safranin and astra blue (Bukatsch,1972) and paradermal sectionswere stainedwithmethyleneblue(1%)(Krauter,1985).Subsequently, semipermanenthistologicalslideswerepreparedcontaining cross-sectionsandparadermalsectionsofbotanicalmaterial,following usual plant anatomy procedures (Johansen, 1940; Sass, 1951). Analyses were performed on images in software (Toup View Image),obtainedbydigitalcameracoupledtoalightmicroscope (Alltion).
Maceration
Themacerationwasperformedusingleafbladefragmentsthat weredisintegratedwiththemixtureof10%nitricacidand10% chromicacid(1:1),accordingtothemethodofJeffrey(Johansen, 1940).Analyseswerecarriedoutonimagesinsoftware(ToupView Image),obtainedbydigitalcameracoupledtoalightmicroscope (Alltion).
cx
pd
vb
cx pd
vb
C
D
B
A
id
am
Histochemicalcharacterization
Histochemical tests were made on cross-sections of fresh leaf blades obtained by the same method used in anatomical study. The following specific reagents were used to show the secretionsitesand/oraccumulationof substances:SudanIIIfor lipophilicsubstances(Sass,1951);Nadireagentforessentialoils and oleoresins (David and Carde, 1964);potassium dichromate (10%)forphenoliccompounds(Gabe,1968);Lugol’siodinereagent forstarch (Johansen,1940);phloroglucinolforlignin (Johansen,
1940);Dragendorff’sreagentfordetectingalkaloids(Farmacopeia
Brasileira,2010);antimonytrichloridefortriterpenesandsteroids
(Mace et al., 1974); vanillin chloridric for tannins (Mace and
Howell,1974)andhydrochloricacid(10%)toestablishthenature
ofthecrystals(Jensen,1962).
Controls were performed in parallel with the tests and semipermanenthistologicalslideswerepreparedcontainingthe cross-sections(Johansen,1940; Sass,1951).Analyses were per-formedonimagesin software (Toup ViewImage),obtainedby digitalcameracoupledtoalightmicroscope(Alltion).
tr
co
id
tr tr
id ep
co
end pa tr
id
vb ep
A
B
C
F
E
D
st
tr
vb
vb pa
ep
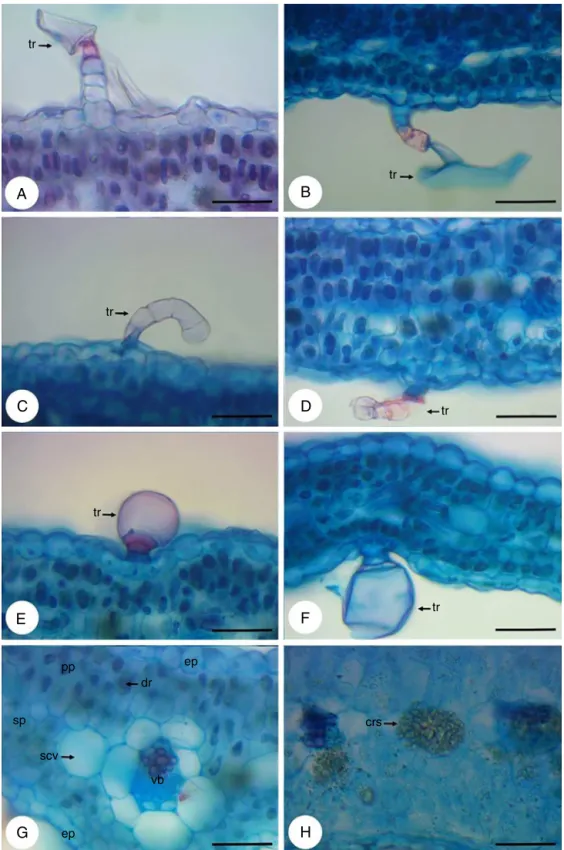
Fig.2.Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsofthestem.(A)Generalaspect,showingepidermis(ep),trichome(tr),collenchyma(co),parenchyma (pa),vascularbundles(vb)andidioblast(id)withcrystalsand;(B)Detailofnon-glandulartrichome(tr),multicellularanduniseriate,withenlargedcellsatthebaseandan elongatedapicalcell;(C)Detailofnon-capitateglandulartrichome(tr)withshortuniseriatestalkandasmallgloboidapexandbentstowardtheepidermis;(D)Detailof capitateglandulartrichome(tr)withashortpedicelandalargeunicellularhead,stomata(st)andepidermis(ep);(E)Detailofepidermis(ep),trichome(tr),collenchyma (co),parenchyma(pa),endodermis(end),vascularbundles(vb)andidioblast(id)withcrystalsand.(F)Detailofidioblast(id)withcrystalsand.Bars:A=500m;B,C,D,
F=50m;E=200m.
ep
co co co
co
co
co
tr st
ep
vb tr
tr
id vb
id pa
co ep
co tr
vb vb
vb id
A
B
C
D
H
G
F
E
pa
Scanningelectronmicroscopy(SEM)
Leafbladessampleswerefixedin2.5%glutaraldehyde(buffered with 0.1M sodium cacodylate) and post fixed in 2% osmium solution (buffered with 0.1M sodium cacodylate). After
dehydration in ethanol series, the material was submitted to critical point drying (Bal-Tec CPD 030). Suitable portions were mountedontoSEM stubsusingdouble-sidedadhesive tapeand sputter-coatedwithgold(LeicaEMSCD500)(Silveira,1989).Both adaxialandabaxialsurfaceswereexaminedwithaQUANTA200
st
ep
crs
A
B
C
D
E
F
H
G
ep
tr tr
tr
tr
tr dr
tr
st
FEGscanningelectron microscopeintheCentro deTecnologias EstratégicasdoNordeste(CETENE).
Results
Root
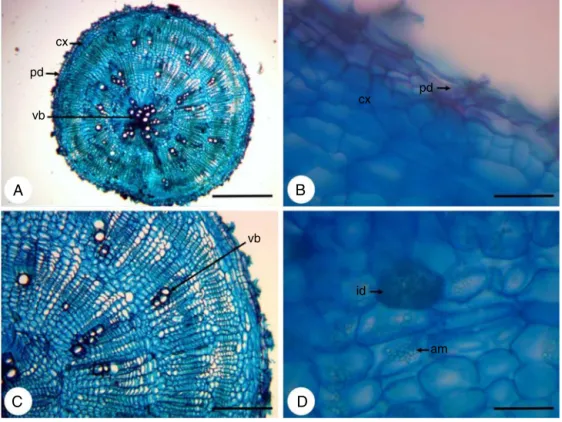
In cross-section the root hasa circular shape (Fig. 1A) and presentsperidermandaverysmallcorticalregion(Fig.1B),due tothedevelopmentofanomaloussecondarythickening, character-izedbyaconcentriczoneofcollateralvascularbundlesirregularly distributed, that arise from a succession of arcs of cambium (Fig.1C).Itisobservedinallrootseveralcellscontainingstarch andidioblastswithcrystalsand(Fig.1D).
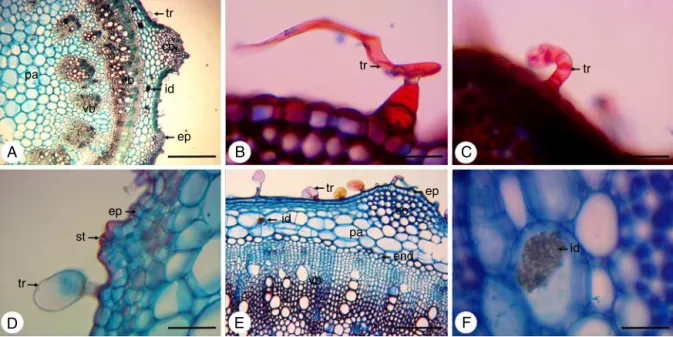
Stem
Incross-section,thestemhasapolygonalshape,withregions moreprominent(Fig.2A).Trichomesarelocatedthroughoutits extension(Fig.2AandD),whicharenon-glandulartrichome, mul-ticellularand uniseriate,withenlargedcells atthebaseandan elongatedapicalcell(Fig.2B)andalsoglandulartrichomes.These canbeoftwotypes:non-capitateglandulartrichomewithshort uniseriatestalkandasmallgloboidapexandbentstowardthe epi-dermis(Fig.2C),andthecapitateglandulartrichome,withashort pedicelandalargeunicellularhead(Fig.2D).
Theepidermisconsistsofasinglelayerofcellscoatedwitha thincuticle(Fig.2A,DandE).Stomataareinsertedabovethelevel oftheepidermalcells(Fig.2D).Inlessprominentregionsofthe stem,adjacenttotheepidermis,isfoundalayerofcellsthatmay bepartoftheepidermis,makingitmultilayered,ormayconstitute ahypodermis.Alreadyinmoreprominentregions,theangular col-lenchymaislocatedbeneaththeepidermis,beingcomposedoffour toninelayersofcells(Fig.2AandE).Inthecorticalparenchymaare presentidioblastswithcrystalsand(Fig.2A,EandF).The endoder-misisseenasalast corticallayer(Fig.2E).Aswellastheroot, thestemalsoexhibitsanomaloussecondarythickening.Itis dis-tinguishedtwodifferentzonesofvascularbundles:azonecloser totheendodermis,inwhichthecollateralbundlesaredistributed formingacontinuousring;andotherzoneclosesttothemedullary region,inwhichthecollateralbundlesaredistributed discontin-uously,separated fromeach otherby parenchyma(Fig.2A and E).
Petiole
Thepetiole,incross-section,hasconcave–convexshape,with two lateralextremities(Fig.3A).The epidermisis composedof a singlelayerofrounded cells and coveredwitha smooth and
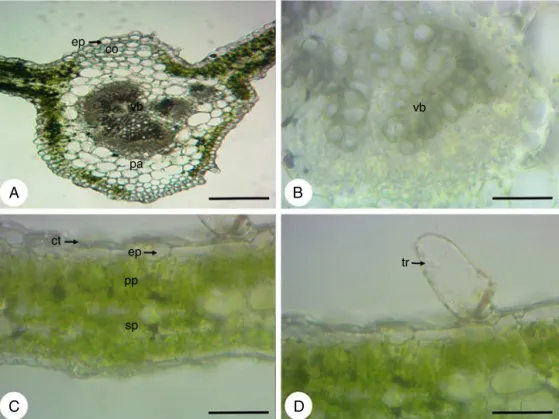
thin cuticle (Fig. 3A and B). Stomata are inserted in thesame levelofepidermal cells(Fig.3B).Asepidermalattachments,are presentnon-glandulartrichome,multicellularanduniseriate,with enlargedcellsatthebaseandanelongatedapicalcell(Fig.3C), besides non-capitate glandular trichome with short uniseriate stalkand asmallgloboidapex andbents towardtheepidermis (Fig.3D)andcapitateglandulartrichome,withashortpediceland a largeunicellularhead(Fig.3E).Thecollenchymaofthe angu-lartypeisobservedbelowtheepidermisinthecentralregionof thepetiole(Fig.3Aand F),beingformedbytwoorthreelayers ofcells.Thistissueisalsopresentinthelateralextremitiesand adjacenttophloem(Fig.3A,BandF).Thevascular bundlesare collateraland arearrangedin asemicircle inthecentralregion ofthepetiole(Fig.3Aand F).Smallervascular bundlesare dis-playedinthelateralextremities(Fig.3BandG).Intheparenchyma are observed idioblasts containing crystal sand (Fig. 3A, F andH).
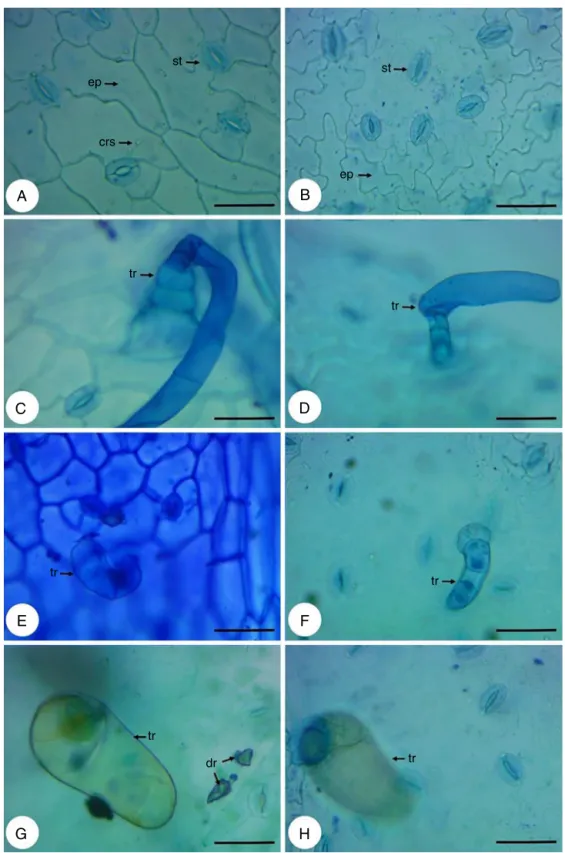
Leafblade
Theepidermis,infrontalview,consistsofcellswithstraightor slightlysinuouswallsontheadaxialface(Fig.4A)andofcellswith wallsmoresinuousontheabaxialface(Fig.4B).Thestomata,that areanomocyticandanisocytic,arepresentonbothsides(Fig.4A andB).Non-glandulartrichomes(Fig.4CandD)andglandular tri-chomes(Fig.4E–H)occurbothontheadaxialface(Fig.4EandG) asontheabaxialface(Fig.4FandH)andareofthesametype pre-viouslydescribedinthestemandpetiole.However,theglandular trichomesoccuringreaternumberontheadaxialface,amongthe twotypesofglandulartrichomesfound,predominatethecapitate glandulartrichome.Inthiscutisstillpossibletoviewidioblasts withcrystalsand(Fig.4A)anddruses(Fig.4G).
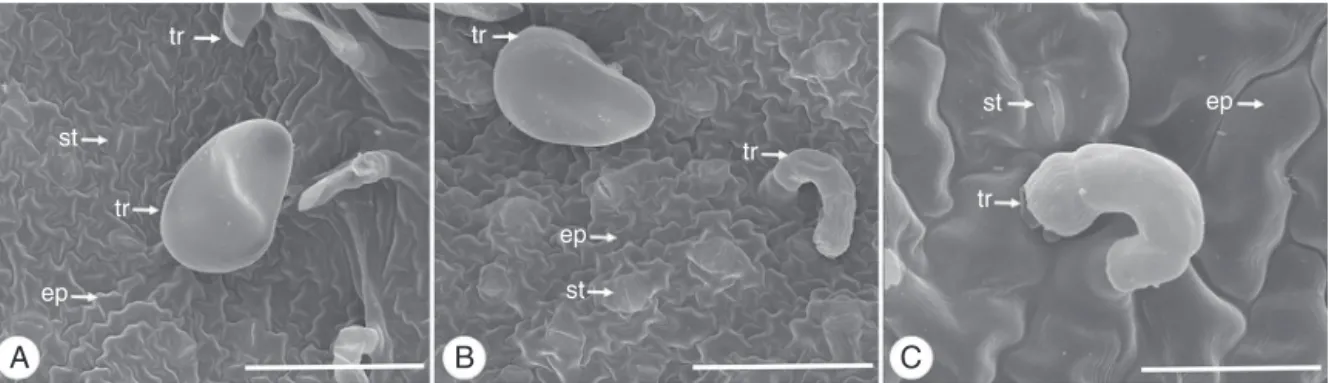
IntheleafbladeanalysisinSEMitwaspossibleobservedwith moredetailthelargestsinuosityoftheepidermalcellswallson theabaxialface(Fig.5AandB)andthatonthisfacethestomata aresituatedonthesamelevelorslightlyaboveofepidermalcells (Fig.5B),whileontheadaxialfacetheyarelocatedonthesamelevel ofepidermalcells(Fig.5C).Itwasalsoobservedthenon-glandular (Fig.5A)andglandulartrichomes(Fig.5A–C).
Incross-sectionoftheleafbladewereobservedallthetrichomes (Fig.6A–F)viewedintheparadermalcutandinSEM,alsoratifying thefactthattheglandulartrichomesarepredominantintheabaxial face.Theepidermisisuniseriate,coatedwithathincuticlelayerand ismadeofroundedcellsorslightlyelongated(Fig.6G).The meso-phyllhasorganizationdorsiventral.Itconsistsofoneortwolayers ofpalisadeparenchymaand twotofourlayersofdensespongy parenchyma(Fig.6G).Secretorycavities(Fig.6G)andidioblasts withcrystalsandanddruses(Fig.6GandH)arefoundinthe meso-phyll.
tr tr
st
tr
ep
st
tr
st
tr
ep
ep
A
B
C
tr
A
B
D
C
E
F
H
G
tr
tr tr
tr
tr
crs ep
dr pp
vb
ep scv sp
Fig.6. Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsoftheleafblade.(A)Detailofnon-glandulartrichome(tr),multicellularanduniseriate,withenlarged cellsatthebaseandanelongatedapicalcellontheadaxialface;(B)Detailofnon-glandulartrichome(tr),multicellularanduniseriate,withenlargedcellsatthebaseand anelongatedapicalcellontheabaxialface;(C)Detailofnon-capitateglandulartrichome(tr)withshortuniseriatestalkandasmallgloboidapexandbentstowardthe epidermisontheadaxialface;(D)Detailofnon-capitateglandulartrichome(tr)withshortuniseriatestalkandasmallgloboidapexandbentstowardtheepidermisonthe abaxialface;(E)Detailofcapitateglandulartrichome(tr)withashortpedicelandalargeunicellularheadontheadaxialface;(F)Detailofcapitateglandulartrichome(tr) withashortpedicelandalargeunicellularheadontheabaxialface;(G)Detailoftheepidermis(ep),palisadeparenchyma(pp),spongyparenchyma(sp),secretorycavity (scv),vascularbundle(vb)andidioblastwithdruse(dr);(H)Detailofidioblastwithcrystalsand(crs).Bars:A–H=50m.
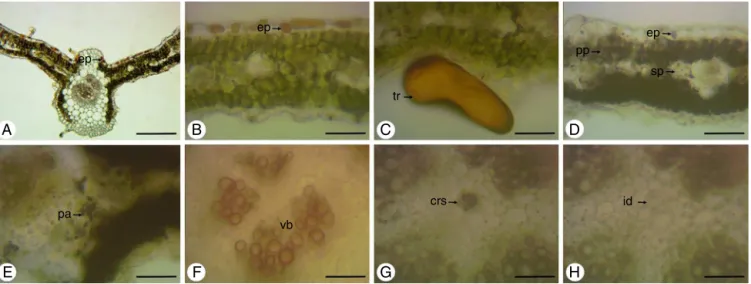
The midribhas biconvex cross-section (Fig. 7A). It presents similarepidermistowhichissituatedinthemesophyll(Fig.7B). Furthermore, all three types of trichomes visualized in the mesophyllarealsopresentinthemidrib(Fig.7C–E).Angular col-lenchymaappearsinsubepidermalposition,formedbytwotothree
tr
A
B
C
F
E
D
pa co
co co
ep
co
dr crs
tr
crs
dr
pa
vb
co tr
tr vb
Fig.7.Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsofmidri(B)(A)Generalaspect,showingcollenchyma(co),parenchyma(pa),vascularbundles(vb)and trichome(tr);(B)Detailoftheepidermis(ep),collenchyma(co)andidioblastswithcrystalsand(crs)anddruse(dr);(C)Detailofnon-glandulartrichome(tr),multicellular anduniseriate,withenlargedcellsatthebaseandanelongatedapicalcell;(D)Detailofnon-capitateglandulartrichome(tr)withshortuniseriatestalkandasmallgloboid apexandbentstowardtheepidermis;(E)Detailofcapitateglandulartrichome(tr)withashortpedicelandalargeunicellularhead;(F)Detailofthevascularbundles(vb) locatedintheparenchyma(pa),collenchyma(co)adjacenttophloemandidioblastswithcrystalsand(crs)anddruse(dr).Bars:A=500m;B,C,D,E=50m;F=200m.
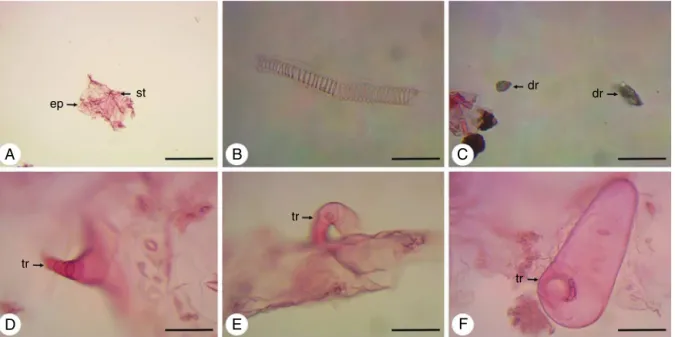
TheleafofD.ambrosioides,aftermaceration,presentsepidermal cells,stomata(Fig.8A),vesselelementsofthehelicaltype(Fig.8B) andidioblastswithdruses(Fig.8C).Thethreetypesoftrichomes viewedintransverseandparadermalsectionsandinSEMarealso foundinmaceratedleaf(Fig.8D–F).
Fig.9A–Dshowscross-sectionsoffreshleafbladeswithout addi-tionofreagent.
AfterusingSudanIII,lipophilicsubstanceswerefoundinthe cuticlecoveringtheadaxialandabaxialfaces(Fig.10A),andare also present within capitate glandular trichomes with unicel-lular head (Fig. 10B). In these trichomes,lipophilic substances maybethosewhichconstitutetheessentialoilandoleoresinsof
D.ambrosioides,consideringthat,withtheNadireagent,these com-poundsexhibitblueandpinkcolorations,respectively(Fig.10Cand D).Oleoresinswerealsofoundintheparenchymacellsofthemidrib (Fig.10E)andintheupperepidermiscellsnexttothemesophyll (Fig.10F).
Potassiumdichromate(10%)revealedthepresenceof pheno-liccompoundsintheadaxialepidermalcells(Fig.11AandB),as wellasinsidethecapitateglandulartrichomewithunicellularhead (Fig.11C).Starchispresentintheepidermalcellsandinthe meso-phyll(Fig.11D),asalsointheparenchymaofthemidrib(Fig.11E). Thephloroglucinolevidencedlignificationofxylematicvesselin midrib(Fig.11F).Fig.11GandHshow,respectively,thepresence
ep
A
B
C
F
E
D
st dr
tr tr
tr
dr
ep co
vb
pa
A
B
D
C
vb
tr ep
pp
sp ct
Fig.9.Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsoftheleafbladewithoutaddingreagents.(A)Midribshowingepidermis(ep),collenchyma(co), parenchyma(pa)andvascularbundles(vb);(B)Detailofvascularbundle(vb);(C)Detailsofcuticle(ct),epidermis(ep),palisadeparenchyma(pp)andspongyparenchyma (sp);(D)Detailofcapitateglandulartrichome(tr)withashortpedicelandalargeunicellularhead.Bars:A=200m;B–D=50m.
ct
ct
A
B
C
F
E
D
tr
tr
ep pa
tr
Fig.10.Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsoftheleafbladeafterreactionwithSudanIIIandNadireagent.(A)Lipophilicsubstancesinthe cuticle(ct)liningtheepidermis;(B)Lipophilicsubstancesinsidecapitateglandulartrichome(tr);(C)Essentialoilinsidecapitateglandulartrichome(tr);(D)Oleoresininside capitateglandulartrichome(tr);(E)Oleoresininparenchyma(pa);(F)Oleoresininepidermal(ep)cells.Bars:A–F=50m.
ofcrystalsinleafofD.ambrosioidesandtheirdissolutionwiththe testofhydrochloricacid(10%),confirmingthattheyareofcalcium oxalate.TestswithDragendorff’sreagent,antimonytrichlorideand vanillinchloridricwerenegative.
Discussion
ThetransitionofsomespeciesofthefamilyChenopodiaceaeto thefamilyAmaranthaceaeisrecent.Althoughthemodification,the
literaturestilltreatsD.ambrosioidesasC.ambrosioides.Forthis rea-son,thediscussionofthisworktookintoconsiderationtheaspects ofthefamilyChenopodiaceae.
AccordingtoMetcalfeandChalk(1972),thefamily Chenopodi-aceaehasmanyanatomicalpointsincommonwithotherfamilies, suchastheAmaranthaceae.Oneofthesepointsisthecambium variation,oranomaloussecondarythickening(Wilson,1924;Joshi,
1937;Balfour,1965).Theformationofsuccessivecambiaisknown
ep
ep
tr
pp
sp
id crs
vb pa
A
B
C
D
H
G
F
E
ep
Fig.11.Dysphaniaambrosioides(L.)Mosyakin&Clemants,cross-sectionsoftheleafbladeafterreactionwithpotassiumdichromate(10%),Lugol’siodinereagent, phloroglu-cinolandhydrochloricacid(10%).(A)Viewoftheleafshowingphenoliccompoundsinepidermal(ep)cells;(B)Detailofepidermal(ep)cellscontainingphenoliccompounds; (C)Detailofphenoliccompoundsinsidecapitateglandulartrichome(tr);(D)Starchinepidermal(ep)cells,palisadeparenchyma(pp)andspongyparenchyma(sp);(E)Detail ofstarchinparenchyma(pa);(F)Viewofvascularbundles(vb)withlignifiedwalls;(G)Detailofidioblastwithcrystalsand(crs)beforereactionwithhydrochloricacid(10%); (H)Detailofidioblast(id)afterreactionwithhydrochloricacid(10%).Bars:A=200m;B–H=50m.
occursmorefrequently(MetcalfeandChalk,1972;Carlquist,2007). InChenopodiaceae,thesecondarygrowthstartsfromacambiumin anormalposition.Subsequently,theothercambiaemergefurthest fromthefirst,producingxylemtoinsideandphloemtooutside
(Esau,1997).Thesuccessivecambiaandthevascularbundlesare
embeddedinparenchymaltissue(MetcalfeandChalk,1972). Withrespectyet tothesecondary growthof theplant,it is observedtheperidermformationinroots,butnotinstems.The absenceofperiderminstemwasalreadyobservedin12species ofChenopodium,includingD.ambrosioides(Bonzanietal.,2003),as wellasinotherspeciesofChenopodiaceae,suchasAtriplexhalimus,
Atriplexcristata,Atriplexoestophora(FahnandZimmermann,1982;
Jáureguietal.,2014);Allenrolfeapatagonica,Heterostachys
olivas-cens,Heterostachysritteriana(CuadraandHermann,2014);andin
Salsolakalisubsp.Ruthenica(BercuandBavaru,2004).
Thepolygonalshapeofthestemwiththepresenceofregions moreprominentformedbycollenchymatictissueiscommonin Chenopodiaceae (Fahn and Zimmermann, 1982; Bonzani et al.,
2003;BercuandBavaru,2004;Jáureguietal.,2014).Bonzanietal.
(2003)observedthatinChenopodiumburkartiiandChenopodium
retusumthecollenchymabecomeslignifiedwhenthesespeciesare insecondarygrowth.AlreadyinotherspeciesofChenopodium stud-ied,includingD.ambrosioides,thecollenchymaremainscomposed ofcellulosicwallsinsecondarygrowth.
Stomatawerefoundinthestem,petioleandleafbladeofthe plant.Amphistomaticleavesarecharacteristicsofthe Chenopo-diaceae family (Metcalfe and Chalk, 1972; Moris et al., 1996;
Bonzanietal.,2003;CuadraandHermann,2014;Jáureguietal.,
2014).Anomocyticstomata arethe mostfrequent,but arealso foundanisocytic stomata in Chenopodiumchilense, tetracytic in
Chenopodiummultifidum(Bonzanietal.,2003)andparacyticin Sal-solakalisubsp.ruthenica(BercuandBavaru,2004).Previousstudies withD. ambrosioides only affirmedthe presence ofanomocytic stomata,differentfromtheresultsfoundinthisstudy,whichare describedstomataanomocyticandanisocytic(Jorgeetal.,1986;
CostaandTavares,2006).
ThepresenceofdifferenttypesoftrichomesinChenopodiaceae wasreportedbysomeauthors(Holm,1923;MetcalfeandChalk,
1972;SilvaFilhoetal.,1992;Bonzanietal.,2003).Accordingto
MetcalfeandChalk (1972),in thefamilyare present trichomes
uniseriate, branched, stellate and capitate glandular trichome. InthegenusChenopodiumaremostcommonthenon-glandular
trichome uniseriate and the capitate glandular trichome. Both wereobservedinthestem,petioleandleafbladeofD.ambrosioides, butitwasalsofoundtheglandulartrichomeuniseriatewithbody bent toward epidermis and with secretory distal cell rounded. ThislattertypeoftrichomeisnotdescribedbyMetcalfeandChalk
(1972),however,inadetailedstudyofthetrichomesofthestem
andleavesofChenopodiumspecies,Bonzanietal.(2003)citedall kindsoftrichomesfoundinthiswork.
Thereisstillcontroversyregardingthepresenceofglandular trichomesonthefacesoftheleafbladeofD.ambrosioides.Asare seeninthisstudy,thepresenceonbothfaceswasalsoidentified
byHolm(1923),Jorgeetal.(1986)andBonzanietal.(2003)in
D.ambrosioides.Jorgeetal.(1986)affirmedthattheglandular tri-chomespredominateintheadaxialface,inagreementwiththe resultsdescribedinthispaperanddifferingfromCostaandTavares
(2006),whoreportedthesetrichomesrestrictedtoabaxialface.
Studiessuggestthatthedensityandthechangeinthe propor-tionofnon-glandularandglandulartrichomescanbeinfluenced byenvironmentalconditions,includingherbivoryandwater avail-ability(Rautioetal.,2002;Gonzalesetal.,2008).Sincethespecies isfoundintropical,subtropical andtemperate zones(Kismann, 1991),itcanbeexpectedthatthesevariationsoccurinD. ambro-sioides.
MostspeciesofChenopodiaceaehavedorsiventralmesophyll
(Metcalfeand Chalk,1972).Therearesomeexceptions,suchas
C.retusum,Chenopodiumoblanceolatum(Bonzanietal.,2003)and
Salsola sp. (Metcalfeand Chalk, 1972) that present isobilateral mesophyll.
Idioblastscontainingcrystalsandwereoftenfoundinallplant partsexamined.Onlyintheleafbladewereobserveddruses.The confirmation,bytestingwithhydrochloricacidthatthecrystals areofcalciumoxalatecorroboratestheresultofCostaandTavares
(2006).AccordingMetcalfeandChalk (1972),theoccurrenceof
thesecrystalsgivesimportantdiagnosticvalueforthespecies,since theyarerestrictedbetweendicotyledonous.
notbeingpossibleperformingsectionstotheanatomical study
(FarmacopeiaBrasileira,2010).
Theyweredetectedintheleafbladeslipophilicandhydrophilic substances,evidencing lipids,essentialoils,oleoresins and phe-nolic compounds. These data corroborate the findings in the literatureforthespecies,knownforproducingvariouscompounds ofmetabolismusedinthedefenseandadaptationofplantstothe environmentandalsoinmedicine(Sáetal.,2015).Mostplants storestarchasareserve.Thiscarbohydrate,aswellasbeingrelated asanenergysourceisalsoanadaptivestrategyofthespeciesto adverseenvironmentalconditions(OliveiraandMarquis, 2002). Theligninpresentinthewallofthexylemvesselsisresponsible forsustainingtheplantandalsoprovidesdefensefortheplant, becauseit is considered as a substance resistantto pathogens, hideringtheircolonization(Silvaetal.,2005).
Conclusion
Throughthedifferentmicroscopytechniquesitwaspossibleto establishanatomicalfeaturesthatareusefulintheidentificationof
D.ambrosioides,whichwere:anomaloussecondarythickeningin therootandstem;presenceofidioblastscontainingcrystalsand intheroot,stem,petioleandleafblade;inthesetherearealso idioblastswithdruses;presenceofnon-glandularandglandular trichomesinthestem,petioleandleafblade;stomataonthestem, petioleandleafblade,identifiedinthatasanomocyticand aniso-cytic;dorsiventralmesophyllandcollateralvascularbundles.The macerationshowedtobeausefultoolwhenitcannotmakecuts totheanatomicalstudy.Inadditiontocontributingsignificantly totheknowledgeoftheanatomy,itwasalsopossibletoseethe histolocalizationofsomegroupsofmetabolitespresentintheleaf bladeofthespecies.Thus,theworkprovidesqualityparametersfor thespeciesstudied,sinceitdoesnothaveappropriatemonograph incurrentofficialcodes.
Authors’contributions
RScontributed in collectingplant sampleand identification, confectionofherbarium,runningthelaboratorywork,analysisof thedataanddraftedthepaper.AScontributedinthepreparation ofsemi-permanentslides.FSandLScontributedtocriticalreading ofthemanuscript.KRdesignedthestudy,supervisedthe labora-toryworkand contributedtocriticalreadingofthemanuscript. Alltheauthorshavereadthefinalmanuscriptandapprovedthe submission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsaregratefultoCNPq(132722/2011-9)forfinancial supportin theformofgrantsandfellowship awards.Theyalso thanktoPERPARTforsupplyingtheplantmaterial.
References
Albuquerque,U.P.,Araújo,T.A.S.,Ramos,M.A.,Nascimento,V.T.,Lucena,R.F.P., Mon-teiro,J.M.,Alencar,N.L.,Araújo,E.L.,2009.Howethnobotanycanaidbiodiversity conservation:reflectionsoninvestigationsinthesemi-aridregionofNEBrazil. Biodivers.Conserv.18,127–150.
Anvisa,2014.Resoluc¸ãoRDCn◦26de13/05/14,Dispõesobreoregistrode
medica-mentosfitoterápicose oregistroe anotificac¸ão deprodutos tradicionais fitoterápicos.AgênciaNacionaldeVigilânciaSanitária,Brasilia,DF.
Balfour,E.,1965.AnomaloussecondarythickeninginChenopodiaceae, Nyctagi-naceaeandAmaranthaceae.Phytomorphology15,111–122.
Barros,L.,Pereira,E.,Calhelha,R.C.,Due˜nas,M.,Carvalho,A.M.,Santos-Buelga,C., Ferreira,I.C.F.R.,2013.Bioactivityandchemicalcharacterizationinhydrophilic andlipophiliccompoundsofChenopodiumambrosioidesL.J.Funct.Foods5, 1732–1740.
Bercu,R.,Bavaru, E.,2004. AnatomicalaspectsofSalsolakali subspruthenica (Chenopodiaceae).Phytol.Balc.10,227–232.
Bonzani, N.E., Barboza, G.E., Bugatti, M.A., Ariza Espinar, L., 2003. Morpho-histologicalstudiesinthearomaticspeciesofChenopodiumfromArgentina. Fitoterapia74,207–225.
Bukatsch,F.,1972.BemerkungenzurdoppelfärbungAstrablau-Safranin. Mikrokos-mos61,255.
Carlquist,S.,2007.Successivecambiarevisited:ontogeny,histology,diversity,and functionalsignificance.J.TorreyBot.Soc.134,301–332.
Cavalli,J.,Tomi,F.,Bernardini,A.,Casanova,J.,2004.Combinedanalysisofthe essentialoilofChenopodiumambrosioidesbyGC,GC–MSand13CNMR spec-troscopy:quantitativedeterminationofascaridoleaheat-sensitivecompound. Phytochem.Anal.15,275–279.
Costa,M.V.L.,Tavares,E.S.,2006.AnatomiafoliardeChenopodiumambrosioidesL. (Chenopodiaceae)–erva-de-Santa-Maria.Rev.Bras.PlantasMed.8,63–71. Cruz,G.L.,1995.DicionáriodasplantasúteisdoBrasil,5thed.EditoraBertrandBrasil,
RiodeJaneiro.
Cuadra,V.P.,Hermann,P.M.,2014.AnatomíafoliarycaulinardetresSalicornieae (Chenopodiaceae)halófilasargentinas.PhytonInt.J.Exp.Bot.83,369–377. David,R.,Carde,J.P.,1964.Colorationdifférentielledêsinclusionslipidiqueet
ter-peniquesdêspseudophyllesduPinmaritimeaumoyendureactifnadi.C.R. Acad.Sci.ParisSer.D258,1338–1340.
Esau,K.,1997.Anatomiadasplantascomsementes.EditoraEdgardBlücher,São Paulo.
Fahn,A.,Zimmermann,M.H.,1982.DevelopmentofthesuccessivecambiainAtriplex
halimus(Chenopodiaceae).Bot.Gaz.143,353–357.
2010.FarmacopeiaBrasileira, 5thed.AgênciaNacionaldeVigilânciaSanitária, Brasília.
Fenner,R.,Betti,A.H.,Mentz,L.A.,Rates,S.M.K.,2006.Plantasutilizadasnamedicina popularbrasileiracompotencialatividadeantifúngica.Rev.Bras.Cienc.Farm. 42,369–394.
Franc¸a,F.,Lago,E.L.,Marsden,P.D.,1996.Plantsusedinthetreatmentofleishmanial ulcersduetoLeishmania(viannia)braziliensisintheendemicareaofBahia,Brazil. Rev.Soc.Bras.Med.Trop.29,229–232.
Fuentes-Bazan,S.,Mansion,G.,Borsch,T.,2012a.Towardsaspeciesleveltreeof thegloballydiversegenusChenopodium(Chenopodiaceae).Mol.Phylogenetics Evol.62,359–374.
Fuentes-Bazan,S.,Uotila,P.,Borsch,T.,2012b.Anovelphylogeny-basedgeneric classification for Chenopodiumsensu lato, and a tribalrearrangement of Chenopodioideae(Chenopodiaceae).Willdenowia42,5–24.
Gabe,M.,1968.TechniquesHistologiques.Masson&Cie,Paris.
Garcia,D.,Domingues,M.V.,Rodrigues,E., 2010.Ethnopharmacologicalsurvey amongmigrantslivinginthesoutheastAtlanticforestofDiadema,SãoPaulo, Brazil.J.Ethnobiol.Ethnomed.6,1–19.
Gonzales,W.L.,Negritto,M.A.,Suárez,L.H.,Gianoli,E.,2008.Inductionof glandu-larandnon-glandulartrichomesbydamageinleavesofMadiasativaunder contrastingwaterregimes.ActaOecol.33,128–132.
Guimaraes,D.L.,Llanos,R.S.N.,Acevedo,J.H.R.,2001.Ascaridisis:comparisonofthe therapeuticefficacybetweenpaicoandalbendazoleinchildrenfromHuaraz. Rev.Gastroenterol.Perú21,212–219.
Gupta,D.,Charles,R.,Mehta,V.K.,Garg,S.N.,Kumar,S.,2002.Chemicalcomposition oftheessentialoilofChenopodiumambrosioidesL.fromthesouthernhillsof India.J.Essent.OilRes.14,93–94.
Hallala,A., Benalia,S.,Markouk,M., Bekkouchea,K., Larhsinia,M., Chaitb,A., Romanec,A.,Abbada,A.,ElAbdounid,M.K.,2010.Evaluationoftheanalgesic andantipyreticactivitiesofChenopodiumambrosioidesL.AsianJ.Exp.Biol.Sci. 1,894–897.
Hegazy,A.K.,Farrag,H.F.,2007.AllelopathicpotentialofChenopodiumambrosioides
ongerminationandseedlinggrowthofsomecultivatedandweedplants.Glob. J.Biotechnol.Biochem.2,1–9.
Holm,T.,1923.ChenopodiumambrosioidesL.amorphologicalstudy.Am.J.Sci.6, 157–167.
Jain,N.,SarwarAlam,M.,Kamil,M.,Ilyas,M.,Niwa,M.,Sakae,A.,1990.Twoflavonol glycosidesfromChenopodiumambrosioides.Phytochemistry29,3988–3991. Jardim,C.M.,Jham,G.N.,Dhingra,O.D.,Freire,M.M.,2010.Chemicalcompositionand
antifungalactivityofthehexaneextractoftheBrazilianChenopodium ambro-sioidesL.J.Braz.Chem.Soc.21,1814–1818.
Jáuregui,D.,Castro,M.,Ruiz-Zapata,T.,LAPP,M.,2014.Anatomíadelosórganos vegetativosdedosespeciesdeAtriplex(Chenopodiaceae)deVenezuela.Rev. Biol.Trop.62,1625–1636.
Jensen,W.A.,1962.BotanicalHistochemistry:PrinciplesandPractice.W.H.Freeman &Co.,SanFrancisco.
Johansen,D.A.,1940.PlantMicrotechnique.McGraw-Hill,NewYork.
Joly,A.B.,2002.Botânica:introduc¸ãoàtaxonomiavegetal,13thed.Companhia Edi-tora,SãoPaulo.
Jorge,L.I.F.,Ferro,V.O.,Koschtschak,M.R.W.,1986.Diagnosecomparativadas
espé-ciesChenopodiumambrosioidesL.(erva-de-santa-maria)eCoronopusdidymus
(L.)Sm(mastruc¸o):principaiscaracterísticasmorfo-histológicasequímicas.Rev. Bras.Farmacogn.1,143–153.
Kiuchi,F.,Itano,Y.,Uchiyama,N.,Honda,G.,Tsubouchi,A.,Nakajima-Shimada,J., Aoki,T.,2002.Monoterpenehydroperoxideswithtrypanocidalactivityfrom
Chenopodiumambrosioides.J.Nat.Prod.65,509–512.
Kliks,M.M.,1985.Studies onthetraditional herbalanthelminticChenopodium ambrosioidesL.:ethnopharmacologicalevaluationandclinicalfieldtrials.Soc. Sci.Med.21,879–886.
Kraus,J.E.,Arduin,M.,1997.Manualbásicodemétodosemmorfologiavegetal.EDUR, RiodeJaneiro.
Krauter, D.,1985. Erfahrungenmit Etzolds FSA-Färbung für Pflanzenschnitte. Mikrokosmos74,231–233.
Lima,J.L.S.,Furtado,D.A.,Pereira,J.P.G.,Baracuthy,J.G.V.,Xavier,H.S.,2006.Plantas medicinaisdeusocomumnoNordestedoBrasil.CampinaGrande.
Lorenzi,H.,Matos,F.J.A.,2002.PlantasmedicinaisnoBrasil:nativaseexóticas. Edi-toraInstitutoPlantarum,NovaOdessa.
Lorenzi,H.,2008.PlantasdaninhasdoBrasil:terrestres,aquáticas,parasitase tóxi-cas,4thed.EditoraInstitutoPlantarum,NovaOdessa.
Mace,M.E.,Bell,A.A.,Stipanovic,R.D.,1974.Histochemistryandisolationof gossy-polandrelatedterpenoidsinrootofcottonseedlings.Phytophatology64, 1297–1302.
Mace,M.Z.,Howell,C.R.,1974.Histochemistryandidentificationofcondensedtanin precursorsinrootsofcottonseedlings.Can.J.Bot.52,2423–2426.
Matos,F.J.A.,2007.Plantasmedicinais:guiadeselec¸ãoeempregodasplantasusadas emfitoterapianoNordestedoBrasil,3rded.ImprensaUniversitária,Fortaleza. Metcalfe,C.R.,Chalk,L.,1972.AnatomyoftheDicotyledons:Leaves,Stem,andWood inRelationtoTaxonomyWithNotesonEconomicUses.ClarendonPress,Oxford. Monzote,L.,Montalvo,A.M.,Scull,R.,Miranda,M.,Abreu,J.,2007.Activity,toxicity andanalysisofresistanceofessentialoilfromChenopodiumambrosioidesafter intraperitonealoralandintralesionaladministrationinBALB/cmiceinfected
withLeishmaniaamazonensis:apreliminarystudy.Biomed.Pharmacother.61,
148–153.
Monzote,L.,García,M.,Pastor,J.,Gil,L.,Scull,R.,Maes,L.,Cos,P.,Gille,L.,2014. EssentialoilfromChenopodiumambrosioidesandmaincomponents:activity againstLeishmaniatheirmitochondriaandothermicroorganisms.Exp.Parasitol. 136,20–26.
Morais,S.M.,Dantas,J.D.P.,Silva,A.R.A.,Magalhães,E.F.,2005.Plantasmedicinais usadaspelosíndiosTapebasdoCeará.Rev.Bras.Farmacogn.15,169–177. Moris,M.,Gonzalez,J.A.,Gallardo,M.,Prado,F.E.,1996.Anatomicaland
func-tionaldifferencesandnyctinasticleafmovementsinChenopodiumalbumL.and
ChenopodiumhircinumSchrad.(Chenopodiaceae).Bot.J.Linn.Soc.121,133–141. MS,2009.PlantasdeinteresseaoSUS.MinistériodaSaúde,Brasília,DF.
Nascimento,F.R.F.,Cruz,G.V.B.,Pereira,P.V.S.,Maciel,M.C.G.,Silva,L.A.,Azevedo, A.P.S.,Barroqueiro,E.S.B.,Guerra,R.N.M.,2006.AsciticandsolidEhrlichtumor inhibitionbyChenopodiumambrosioidesL.treatment.LifeSci.78,2650–2653. Neiva,V.A.,Ribeiro,M.N.S.,Cartágenes,M.S.S.,Moraes-Coutuinho,D.F.,Nascimento,
F.R.F.,Reys,A.S.,Amaral,F.M.M.,2011.Estudospré-clínicosdeatividade giardi-cidadeChenopodiumambrosioidesL.eapadronizac¸ãodosextratosnapesquisa edesenvolvimentodefitoterápicos.Rev.Ciên.Saúde13,155–165.
Okhale,S.E.,Egharevba,H.O.,Ona,E.C.,Kunle,O.F.,2012.Phytochemicaland prox-imateanalysesandthinlayerchromatographyfingerprintingoftheaerialpart
ofChenopodiumambrosioidesLinn.(Chenopodiaceae).J.Med.PlantsRes.6,
2289–2294.
Oliveira,P.S.,Marquis,R.J.,2002.TheCerradosofBrazil:EcologyandNaturalHistory ofaNeotropicalSavanna.ColumbiaUniversityPress,NewYork.
Onocha,P.A.,Ekundayo,O.,Eramo,T.,Laakso,I.,1999.Essentialoilconstituentsof
ChenopodiumambrosioidesL.leavesfromNigeria.J.Essent.OilRes.11,220–222. Paré,P.W.,Zajicek,J.,Ferracini,V.L.,Melo,I.S.,1993.Antifungalterpenoidsfrom
Chenopodiumambrosioides.Biochem.Syst.Ecol.21,649–653.
Patrício,F.J.,Costa,G.C.,Pereira,P.V.S.,Aragão-Filho,W.C.,Sousa,S.M.,Frazão,J.B., Pereira,W.S.,Maciel,M.C.G.,Silva,L.A.,Amaral,F.M.M.,Rebêlo,J.M.M.,Guerra, R.N.M.,Ribeiro,M.N.S.,Nascimento,F.R.F.,2008.Efficacyoftheintralesional treatmentwithChenopodiumambrosioidesinthemurineinfectionbyLeishmania
amazonensis.J.Ethnopharmacol.115,313–319.
Rautio,P.,Markkola,A.,Martel,J.,Tuomi,J.,Härmä,E.,Kuíkka,K.,Siitonen,A.,Riesco, I.L.,Roitto,M.,2002.Developmentalplasticityinbirchleaves:defoliationcauses ashiftfromglandulartononglandulartrichomes.Oikos98,437–446. Sá,R.D.,2013.EstudofarmacognósticodeChenopodiumambrosioidesL.
(Chenopo-diaceae).Recife,104f.Dissertac¸ãodeMestrado,ProgramadePós-graduac¸ãoem CiênciasFarmacêuticas.UniversidadeFederaldePernambuco.
Sá,R.D.,Galvão,M.A.M.,Ferreira,M.R.A.,Soares,L.A.L.,Randau,K.P.,2014. Chemi-calcompositionoftheessentialoilfromleavesofChenopodiumambrosioidesL. growninRecife-PE,Brazil.Rev.Bras.Farm.95,855–866.
Sá,R.D.,Soares,L.A.L.,Randau,K.P.,2015.ÓleoessencialdeChenopodium ambro-sioidesL.:estadodaarte.Rev.Ciênc.Farm.BásicaApl.36,267–276.
Santayana,M.P.,Blanco,E.,Morales,R.,2005.PlantsknownastéinSpain:an ethno-pharmaco-botanicalreview.J.Ethnopharmacol.98,1–19.
Sass,J.E.,1951.BotanicalMicrotechnique,2nded.IowaStateCollegePress,Ames. Senna,L.,2016.DysphaniainListadeEspéciesdaFloradoBrasil.JardimBotânico
doRiodeJaneiro,http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB139867 (accessedJanuary2016).
SilvaFilho,F.A.,Oliveira,P.L.,Mariath,J.E.A.,1992.TricomasdeChenopodiumretusum
Juss.exMoq.Insula21,43–58.
Silva,L.M.,Alquini,Y.,Cavllet,V.J.,2005.Inter-relac¸õesentreaanatomiavegetalea produc¸ãovegetal.ActaBot.Bras.19,183–194.
Silveira,M.,1989.Preparac¸ãodeamostrasbiológicasparamicroscopiaeletrônica devarredura.In:Manualsobretécnicasbásicasemmicroscopiaeletrônica.USP, SãoPaulo,pp.71–79.
Sousa,M.P.,Matos,M.E.O.,Matos,F.J.A.,Machado,M.I.L.,Craveiro,A.A.,2004. Con-stituintesquímicosativos epropriedadesbiológicasdeplantas medicinais brasileiras.EditoraUFC,Fortaleza.
Taylor,L.,2005.TheHealingPowerofRainforestHerbs:AGuidetoUnderstanding andUsingHerbalMedicinals.SquareOnePublishers,GardenCityPark,NY. TrivellatoGrassi,L.,Malheiros,A.,Meyre-Silva,C.,Buss,Z.S.,Monguilhott,E.D.,Fröde,
T.S.,Silva,K.A.B.S.,Souza,M.M.,2013.Frompopularusetopharmacological vali-dation:astudyoftheanti-inflammatory,anti-nociceptiveandhealingeffectsof
Chenopodiumambrosioidesextract.J.Ethnopharmacol.145,127–138.