w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
A
validated
and
densitometric
HPTLC
method
for
the
simultaneous
quantification
of
reserpine
and
ajmalicine
in
Rauvolfia
serpentina
and
Rauvolfia
tetraphylla
Devendra
Kumar
Pandey
a,∗,
Radha
a,
Abhijit
Dey
baDepartmentofBiotechnology,LovelyProfessionalUniversity,Phagwara,Punjab,India
bDepartmentofBiologicalSciences,PresidencyUniversity(ErstwhilePresidencyCollege),Kolkata,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8December2015
Accepted11April2016
Availableonline28May2016
Keywords:
Highperformancethinlayer
chromatography Reserpine Ajmalicine
R.serpentinaandR.tetraphylla
a
b
s
t
r
a
c
t
Highperformancethinlayerchromatographicmethod(HPTLC)hasbeendevelopedforthequantification ofreserpineandajmalicineinrootpartoftwodifferentpopulationofRauvolfiaserpentina(L.)Benth. exKurzandRauvolfiatetraphyllaL.,Apocynaceae,collectedfromPunjabandUttarakhand.HPTLCof methanolicextractofrootcontainingindolealkaloids,i.e.,reserpineandajmalicine,wasperformedon TLCSilicagel60F254 (10cm×10cm)plateswithtoluene:ethylacetate:formicacid(7:2:1),asmobile phase.Quantificationofthereserpineandajmalicinewasperformedintheabsorption–reflectionmode at268nm.Therecoveryofreserpineandajmalicinewere99.3and98.7%respectively.Thecalibration curveswerelinearforboththereserpineandajmalicine,intherangeof200–1200ng.HPTLCdensitometry hasbeenperformedfortheestimationofreserpineandajmalicineinrootpartofR.serpentinaandR. tetraphyllaforthefirsttime.Themethodissimple,rapidandcosteffectiveandcanbeusedforroutine analysisofajmalicineandreserpineindifferentRauvolfiaspeciesaswellasforqualitycontrolofherbal drugscontainingRauvolfiaspecies.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Indole alkaloids are the bioactive compounds derived from plantswhichpossessanarrayofpharmacologicalpropertiessuch asanticancer(Wangetal.,2015;Zhu etal.,2015), antimalarial (Chierrito etal.,2014), antimicrobial(Cheenprachaet al.,2014) andcerebroprotective(Biradaretal.,2013)properties.Themajor bioactive alkaloids reported from various Rauvolfia species are reserpine,reserpiline, ajmaline,ajamalacine, rauvolfinine, serpi-nine,serpentine,serpentinine,yohimbine,vomilenine,picrinine, vinorine,norseredamine,seredamine(Stöckigtetal.,1981;Batista etal.,1996;Duezetal.,1986;DeBruynetal.,1989;Katoetal., 2002;Binduetal.,2014).Reserpineandajmalicineareconsidered astwomajorindolealkaloidsfromvariousRauvolfiaspecies. Reser-pineisacommonalkaloidknowntodepressthecentralnervous systemandtolowerbloodpressure(Faisaletal.,2005).Reserpine hasbeenreportedforantihypertensive(ShamonandPerez,2009), neuroprotective(Aryaetal.,2009)andanticancer(Abdelfatahand Efferth,2015)properties whereas ajmalicine hasbeenassessed
∗ Correspondingauthor.
E-mail:devendra.15673@lpu.co.in(D.K.Pandey).
for central depressant and adrenergic blocking (Bhargava and Borison,1957),antihypertensiveandcytotoxic(Fernández-Pérez etal.,2013)activities.
GenusRauvolfia,belongingtothe familyApocynaceae, com-prisesaround80specieswhicharedistributedintropicalclimatic conditions.Traditionally, R.serpentina(L.) Benth.ex Kurz, com-monly known as Sarpagandha, wasreported against snakebite, insomnia,melancholia,schizophreniaormoreviolentmental dis-orders,diarrhea,dysentery,choleraandcolic,scabies,malaria,eye inflammation,etc.(DeyandDe,2010,2012).R.serpentinahasan economicalimportanceandtherootpartoftheplantisusedin manyAyurvedicpolyherbalformationsi.rsarpagandhavati. Rau-volfiatetraphyllaL.popularlyknownas“devilpepper”or“bestill tree”isanendangeredwoodyshrubnativeintropicalAmericas (Faisaletal.,2013).EthnomedicinalimportanceofR.tetraphylla wasfoundintermsofitsuseagainstsnakebite,tostimulate uter-inecontractionandtofacilitatedifficultchildbirthcases(Sarma etal.,1999;DeyandDe,2012).Moreover,R.serpentinahasbeen reportedforpharmacologicalpropertiessuchasantbacterial, anti-inflammatoryandcytotoxicity(DeyandDe,2010).R.tetraphylla has also been reported to possess antipsychotic (Gupta et al., 2012a), antibacterialactivityandanti-inflammatory (GangaRao etal.,2012)properties.
http://dx.doi.org/10.1016/j.bjp.2016.04.002
0102-695X/©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
EarlierRauvolfiaalkaloidswereanalyzedbyquantitativethin layer chromatography (Habib and Court, 1971; Nguyen and Nikolova,1989).HPLCandTLCidentification,estimationand sep-arationofindolealkaloidfromR.serpentinaandR.vomitoriawas alsoachieved(Cieri,1983;Klyushnichenkoetal.,1994).Reserpine andrescinnamineinR.serpentinapreparationswasdetectedby liquidchromatographywithfluorescence(Cieri,1987).Recently, HPLC-UV and GC–MS were applied to determine indole alka-loidsandrelatedcompoundsinR.verticillata(Hongetal.,2013). In addition reserpine, ajmaline, and ajmalicine in R. serpentina weredeterminedbyreversed-phaseHPLC(Srivastavaetal.,2006). HPTLC,HPLC,anddensitometrywereusedfortheseparation of differentindolealkaloids fromR.serpentina roots (Guptaet al., 2006).Quantitativedensitometricdeterminationofreserpineand ajmalineby HPTLC wasperformed in R. vomitoria (Katiˇc et al., 1980).However,thereisnoHPTLCreportonsimultaneous quan-tificationofreserpineand ajmalicineindifferentpopulationsof Rauvolfiaspecies.Altitudinalandseasonalmodulationof stigmas-terolwasdeterminedin R.serpentinafollowing HPTLCmethods (Dey and Pandey, 2014a,b). In continuation to our studies on HPTLCprofilingofmedicinalplants (Pandeyet al.,2015,2016), here we report the simultaneous HPTLC determination of two indolealkaloids,ajmalicineandreserpineintwowildpopulationof R.serpentinaandR.tetraphylla.
Materialandmethods
Chemicalsandplantmaterials
Theanalyticalgradechemicalsusedinexperimentwere pur-chased fromE. Merck, India. TheHPTLC plate Silica gel60F254 (10cm×10cm)usedintheexperimentwerepurchasedfromE. Merck(Darmstadt, Germany). The standards, reserpine(A) and ajmalicine(B)werepurchasedfromHimedia,India.
TheplantsamplesofRauvolfiaspecieswascollectedfrom differ-entareasofPhagwara(Punjab)andDehradun(Uttarakhand)inthe monthsofSeptember–October2014.Theplantmaterialcollected fromdifferentplaceswasauthenticatedonthebasisof morpholog-icalcharactersbyTaxonomistintheDepartmentofBotany,Lovely ProfessionalUniversity,Phagwara(Punjab).Avoucherspecimens (VoucherNo.121214and151214ofR.serpentina(L.)Benth.exKurz andR.tetraphyllaL.,respectively)weredepositedatthe Depart-mentofBiotechnology,LovelyProfessionalUniversityfor future reference.
Preparationofsamplesolution
Theair dried (25–30◦C) rootparts of Rauvolfia species (1g)
were extracted thrice with 20ml of methanol for 45min by refluxmethodat70◦Cintemperaturecontrolledwaterbath.The
methanolicextractsweretransferredinconicalflaskandwere con-centratedandre-dissolvedin1mlofmethanol.
Preparationofstandardsolutions
Stocksolutionsofreserpineandajmalicine(1mgml−1)were preparedbydissolvingtheajmalicineandreserpineinmethanol respectively,and differentamounts(2,4,6, 8,10 and12l) of thesewereloadedtoaTLCplate,usinganLinomatapplicatorV forpreparingcalibrationcurves.
Chromatography
ACAMAGHPTLCscannerequippedwithanautomatic Linomat-V automatic sample applicator, TLC scanner III, and integrated
software WINCATS version:1.4.4.6337 wasused for the analy-sisofindolealkaloidsindifferentsamples.Thestationaryphase waspre-coatedsilicagel HPTLC60F254 (10cm×10cm)plateof 0.20mmlayerthicknessusedforthequantificationofreserpineand ajmalicineinRauvolfiaspecies.Theplantsamplesandthestandards wereloadedwiththehelpofLinomatapplicatorVontheTLCplate at8mmwidebandswithconstantapplicationrateof100nls−1 underaflowofN2gas.TheloadingofthesamplesontheTLCplate wasdonebykeepingspaceof15mmfromthebottomand15mm fromtheside,andthespacebetweentwospotswasmaintained 14.4mmoftheplate.
Detectionandestimationofreserpineandajmalicine
The TLC plate was kept in a Camag twin trough chamber (10cm×10cm),whichwaspre-saturatedwith25mlmobilephase withtoluene:ethylacetate:formicacid(7:2:1)for30min,atroom temperature(28±2◦C)and55±5%relativehumidity.Thelength
of the chromatogram run was 80mm from the base and the TLCplatewas driedby usingan air dryer,in a wooden cham-ber.Quantitative evaluation of theplate wasperformedin the absorption–reflectionmodeat268nm,slitwidth4mm×0.30mm, dataresolution100mstep−1andscanningspeed20mms−1.The radiationusedintheanalysiswasdeuteriumandtungstenlamp. Eachanalysiswascarriedoutintriplicate.
ValidationofHPTLCdensitometrymethod
Linearity
Stocksolutionsofreserpineandajmalicine werepreparedin methanolanddifferentamounts(2,4,6,8,10and12l)ofthese were loaded onto a TLC plate, using Linomat applicator V for preparingsixpointscalibrationcurves.Theregressionequationand correlationcoefficientwerefromcalibrationcurves,forreserpine, Y=538+431.15Xand0.991andforajmalicine,Y=−108.53+370X and0.994(Table1andFig.1).
Limitsofdetectionandquantification
Thelimitofdetection(LOD)andlimitofquantification(LOQ) wasdeterminedbyloadingtheblankmethanolontheTLCplate followingthemethodasexplainedbefore.Thesignal-to-noiseratio wasdetermined as 3:1 and 10:1considered for LOD and LOQ, respectively.TheLODandLOQforthereserpinewere60ngand 180ngrespectively.TheLODandLOQfortheajmalicinewere50ng and150ngrespectively.
Accuracy
Tothepre-analyzedsample,50and100geachofreserpineand ajmalicinewereaddedinthe100mgrootpowderofhighyieldingR. serpentinarootsamples(reserpine180gandajmalicine170g) andthemixturewasanalyzedbytheproposedmethod.The exper-imentwasconductedintriplicatetocheckrecoveryandaccuracy
Table1
Reserpineandajmalicinecontent(ngl−1)foundindifferentpopulationofplant
RauvolfiaserpentinaandR.tetraphyllabyHPTLCmethod(n=3) collectedfrom
Uttarakhand(RS-1andRT-1)andPunjab(RS-2andRT-2).
Sample Averagereserpine (%drywt.)
%CV Average ajmalicine
(%drywt.)CV
RS-1 0.18 1.46 0.17 1.55 RS-2 0.17 1.83 0.16 1.68 RT-1 0.16 2.42 0.15 2.67 RT-2 0.15 2.01 0.14 3.87
100.0
[AU]
80.0
70.0
60.0
50.0
40.0
30.0
20.0
10.0
0.0
200.0 300.0 400.0 500.0 [nm] 700.0
200.0 300.0 400.0 500.0 [nm] 700.0 100.0
[AU]
80.0
70.0
60.0
50.0
40.0
30.0
20.0
10.0
0.0
100.0
[AU]
80.0
70.0
60.0
50.0
40.0
30.0
20.0
10.0
0.0
100.0
[AU]
80.0
70.0
60.0
50.0
40.0
30.0
20.0
10.0
0.0
A
B
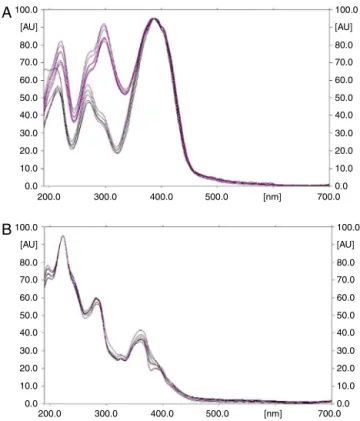
Fig.1. (A)OverlayspectraofReserpineinallthetrack.(B)Overlayspectraof ajmalicineinallthetrack.
ofthesystem.Therecoveryforreserpinewasfoundtobe99.3%and 99.6%andajmalicinewere99.2%and99.5%,respectively.
Precision
Sixsamplesofsameconcentrationseachfromthestock solu-tionsofreserpineandajmalicineandspottedontheHPTLCsilica gel60F254plateandanalyzedwiththeproposedmethodandwere
expressedin%CVforthesystemprecision.Formethodprecisionsix samplesofsameconcentrationswereappliedonaHPTLCplateand analyzedbytheproposedmethodtodeterminevariationexpressed in%CV.Theresultsforreserpineandajmalicinewerefoundtobe 1.28%and1.56%,respectively.
Resultsanddiscussion
Variouscompositionsofmobilephasesweretestedtogetbetter resolutionofreserpineandajmalicine.Theresolutionofreserpine
1 2 3 4 5 6
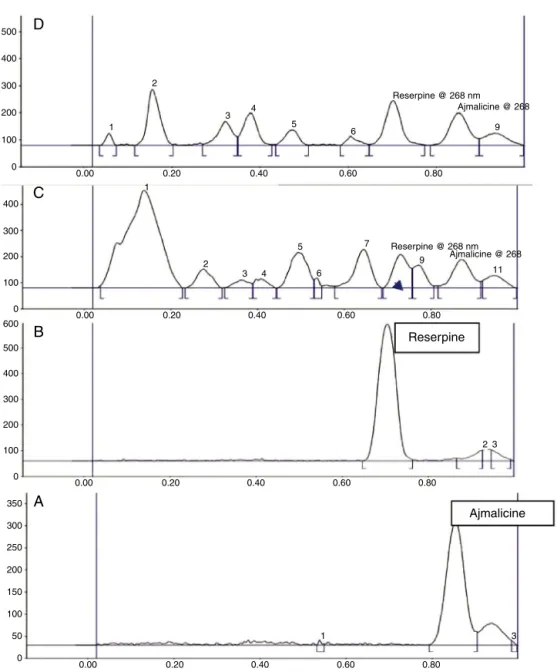
Fig.3.Track:1–6representsthefingerprintingchromatogram:firsttwoare Rau-volfiaserpentina,rootsamplescollectedfrom(1)Dehradun(Uttrakhand)and(2) (Punjab),thirdandfourthrepresentsthestandardofreserpine(3)andajmalicine (4),FifthandsixthshowingR.tetraphyllarootsamplescollectedfromtwostates Dehradun(5)andJalandhar(6).
and ajmalicine, with symmetricaland reproducible peaks, was achieved by using mobile phase consisting of toluene:ethyl acetate:formic acid (7:2:1). Peaks corresponding to reserpine and ajmalicine were recorded at Rf 0.69 and 0.85, respectively
(Figs. 2 and 3). The methanolic extract of Rauvolfia species, when subjected to HPTLC, showed the presence of reserpine and ajmalicine peaksin allthesamples.Comparisonof theUV spectral characteristic of the peaks for standards of reserpine andajmalicine,revealedtheidentityofreserpineandajmalicine presentinallsamples.Thecalibrationcurveswerelinearinthe range of 200, 400, 600, 800, 1000, 1200ng for reserpine and ajmalicine.Peakpuritytestsofreserpineandajmalicinewerealso conductedbycomparingspectraofstandardwithreserpineand ajmalicineinRauvolfiaspeciessampletrack(Figs.1,3and4).The highercontents(0.17%and0.18%ofajmalicineandreserpine)in roots ofR. serpentinaand R.tetraphylla (0.16%and0.15%) were recorded in roots of plant samples collected from Dehradun (Uttarakhand) than compared withtheplant samplescollected fromJalandhar(Punjab)ascomparedtoroots(Table1).
Earlier,hexane,chloroform,methanol,andwaterextractsofR. serpentinarootswerereportedlycontainedmarkerindolealkaloids, ajmaline,ajmalicine,andreserpine.Chloroformwascitedasthe mostpotentsolventforextractionofthesealkaloidswhereas defat-tingwithhexanewasindicatedforthelossofthealkaloids(Gupta
1000 [AU] 800 700 600 500 400 300 200 100 0 0.00
0.10 0.20
0.30
0.40 0.50 0.60
0.70 0.80
1.00 0 10 20
3040 50 60
7080
[Rf]
[mm] 100 Reserpine
Ajmalicine
500
400
300
200
100
0
400
300
200
100
0
0
0.00 0.20 0.40 0.60 0.80
0.00 0.20 0.40 0.60 0.80
0.00 0.20 0.40 0.60 0.80
0.00 0.20 0.40 0.60 0.80
50 100 150 200 250 300 350 100 200 300 400 500 600 0
1
2
3 4 5
6 7
9
11
2
1 3
3 2
3 4 5
6 9
Reserpine @ 268 nm
Reserpine @ 268 nm Ajmalicine @ 268
Ajmalicine @ 268
A
B
C
D
Reserpine
Ajmalicine 1
Fig.4.(A)StandardAjmalicine,(B)StandardReserpine,(C)ReserpineandAjmalicineinrootpartofRauvolfiatetraphyllacollectedfromDehradun,(D)ReserpineandAjmalicine
inrootpartofRauvolfiaserpentinacollectedfromDehradun.
etal.,2006).Extractionefficiencyofthetargetedindolealakaloids with“green”solventswasstudiedusingconventional, ultrasonica-tionandmicrowavetechniques(Guptaetal.,2012b).Inthepresent study we could observe the maximum contents of ajmalicine andreserpine in roots.Tissue specificmodulationof secondary metaboliteshasbeenreportedinvariousbotanicals(Williamsand Ellis,1989;HartmannandDierich,1998;Baranskaetal.,2006;Lim etal.,2010;Deyetal.,2016)which mightbeimplicatedtothe biosynthesisandaccumulationofspecificmetabolitesdepending onpresenceofspecificenzymes(Facchini,2001).
Conclusion
HPTLC densitometry is a rapid, reproducible, accurate, and selectivealternativetoHPLCfortheseparationoftheajmalicine andreserpineinR.serpentinaandR.tetraphylla.Further,rootsof R.serpentinaareenrichedinthedesiredconstituentsandcanbe usedforpreparationsofpolyherbalformulations.Itwasfoundthat thequantityofindolealkaloidsinRauvolfiatetraphyllawasatparto R.serpentinaandcanbeusedinthepreparationofherbal formula-tion.TheadvantageofHPTLCisthehighsamplethroughputwhich
resultsfromthesmallamountofsamplepreparationandlesseruse ofsolventinseparationofthecompoundsandthesimultaneous quantificationofseveralsamples.
Authors’contributions
Thecollectionandcultivationoftheplantsamplesweredoneby RadhawhiletheidentificationandHPTLCquantificationwasdone byDKPandAD.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
References
Arya,U.,Dwivedi,H.,Subramaniam,J.R.,2009.Reserpineamelioratesabetatoxicity
intheAlzheimer’sdiseasemodelinCaenorhabditiselegans.Exp.Gerontol.44, 462–466.
Abdelfatah,S.A.,Efferth,T.,2015. Cytotoxicityoftheindolealkaloidreserpine
fromRauwolfiaserpentinaagainstdrug-resistanttumorcells.Phytomedicine22, 308–318.
Baranska,M.,Baranski,R.,Schulz,H.,Nothnagel,T.,2006.Tissue-specific
accumula-tionofcarotenoidsincarrotroots.Planta224,1028–1037.
Batista,C.V.F.,Schripsema,J.,Verpoorte,R.,Rech,S.B.,Henriques,A.T.,1996.Indole
alkaloidsfromRauwolfiasellowii.Phytochemistry41,969–973.
Bhargava,K.P.,Borison,H.L.,1957.ComparativeeffectsofvariousRauwolfia
alka-loidsoncentrallyevokedvasopressorresponses.J.Pharmacol.Exp.Ther.119, 395–405.
Bindu,S.,Rameshkumar,K.B.,Kumar,B.,Singh,A.,Anilkumar,C.,2014.Distribution
ofreserpineinRauvolfiaspeciesfromIndia–HPTLCandLC–MSstudies.Ind. CropsProd.62,430–436.
Biradar,S.M.,Joshi,H.,Tarak,K.C.,2013.Cerebroprotectiveeffectofisolatedharmine
alkaloidsextractsofseedsofPeganumharmalaL.onsodiumnitrite-induced hypoxiaandethanol-inducedneurodegenerationinyoungmice.Pak.J.Biol.Sci. 16,1687–1697.
Chierrito,T.P.,Aguiar,A.C.,deAndrade,I.M.,Ceravolo, I.P.,Gonc¸alves,R.A.,de
Oliveira,A.J.,Krettli,A.U.,2014.Anti-malarialactivityofindolealkaloidsisolated
fromAspidospermaolivaceum.Malar.J.14,142.
Cheenpracha,S.,Raksat,A.,Ritthiwigrom,T.,Laphookhieo,S.,2014.Monoterpene
indolealkaloidsfrom thetwigsofKopsia arborea. Nat.Prod.Commun. 9, 1441–1443.
Cieri,U.R.,1983.IdentificationandestimationofthealkaloidsofRauvolfia
ser-pentinabyhigh-performanceliquidchromatography.J.Assoc.Off.Anal.Chem. 66,867–873.
Cieri,U.R.,1987.DeterminationofreserpineandrescinnamineinRauwolfia
ser-pentinapreparationsbyliquidchromatographywithfluorescencedetection. J.Assoc.Off.Anal.Chem.70,540–546.
DeBruyn,A.,Zhang,W.,Budˇeˇsinsk ´y,M.,1989.NMRstudyofthree
heteroyohim-binederivativesfromRauwolfiaserpentina:stereochemicalaspectsofthetwo isomersofreserpilinehydrochloride.Magn.Reson.Chem.27,935–940.
Dey,A.,Pandey,D.K.,2014a.HPTLCdetectionofaltitudinalvariationofthepotential
antiveninstigmasterolindifferentpopulationsofthetropicalethnicantidote Rauvolfiaserpentina.AsianPac.J.Trop.Med.7S1,S540–S545.
Dey,A.,Pandey,D.K.,2014b.HPTLCMethodforQuantitativeEvaluationofSeasonal
VariationofStigmasterolinRauvolfiaserpentina(L).Benth.exKurz.J.Biol.Act. Prod.Natl.4,254–261.
Dey,A.,Mukherjee,S.,De,A.,Pandey,D.K.,2016.Astigmasterolcontainingn-hexane
fractionofRauvolfiaserpentina(L).Benth.exKurz.(Apocynaceae)methanolic extractshowstissuespecificvariationofbiocidalactivityandantioxidation. J.HerbsSpicesMed.Plants22,81–91.
Dey,A.,De,J.N.,2010.Rauvolfiaserpentina(L).Benth.exKurz.–areview.Asian
J.PlantSci.9,285.
Dey,A.,De,J.N.,2012.Anti-snakevenombotanicalsusedbytheethnicgroupsof
PuruliaDistrict,WestBengal,India.J.HerbsSpicesMed.Plants18,152–165.
Duez,P.,Chamart,S.,Vanhaelen,M.,Vanhaelen-Fastré,R.,Hanocq, M.,Molle,
L.,1986.Comparisonbetweenhigh-performancethin-layer
chromatography-densitometryandhigh-performanceliquidchromatographyforthe determina-tionofajmaline,reserpineandrescinnamineinRauwolfiavomitoriarootbark. J.Chromatogr.A356,334–340.
Faisal,M.,Ahmad,N.,Anis,M.,2005.ShootmultiplicationinRauvolfiatetraphyllaL.
usingthidiazuron.PlantCellTiss.Org.80,187–190.
Faisal,M.,Alatar,A.A.,Hegazy,A.K.,2013.Molecularandbiochemical
characteri-zationinRauvolfiatetraphyllaplantletsgrownfromsyntheticseedsfollowing invitrocoldstorage.Appl.Biochem.Biotechnol.169,408–417.
Facchini,P.J.,2001.Alkaloidbiosynthesisinplants:biochemistry,cellbiology,
molecularregulation,andmetabolicengineeringapplications.Annu.Rev.Plant Physiol.PlantMol.Biol.52,29–66.
Fernández-Pérez,F.,Almagro,L.,Pedre ˜no,M.A.,GómezRos,L.V.,2013.Synergistic
andcytotoxicactionofindolealkaloidsproducedfromelicitedcellculturesof Catharanthusroseus.Pharm.Biol.51,304–310.
GangaRao,B.,UmamaheswaraRao,P.,SambasivaRao,E.,MallikarjunaRao,T.,
Praneeth,D.V.S.,2012.Evaluationofin-vitroantibacterialactivityand
anti-inflammatoryactivityfordifferentextractsofRauvolfiatetraphyllaL.rootbark. AsianPac.J.Trop.Biomed.2,818–821.
Gupta,S.,Khanna,V.K.,Maurya,A.,Bawankule,D.U.,Shukla,R.K.,Pal,A.,Srivastava,
S.K.,2012a.Bioactivityguidedisolationofantipsychoticconstituentsfromthe
leavesofRauwolfiatetraphyllaL.Fitoterapia83,1092–1099.
Gupta,S.,Shanker,K.,Srivastava,S.K.,2012b.HPTLCmethodforthe
simulta-neousdeterminationoffourindolealkaloidsinRauwolfiatetraphylla:astudyof organic/greensolventandcontinuous/pulsesonication.J.Pharm.Biomed.Anal. 66,33–39.
Gupta,M.,Srivastava,A.,Tripathi,A.,Misra,H.,Verma,R.,2006.UseofHPTLC,HPLC,
anddensitometryforqualitativeseparationofindolealkaloidsfromRauvolfia serpentinaroots.J.PlanarChromatogr.-Mod.TLC19,282–287.
Habib,M.S.,Court,W.E.,1971.EstimationofRauvolfiaalkaloidsbyquantitativethin
layerchromatography.J.Pharm.Pharmacol.2–3(Suppl.),2305.
Hartmann,T.,Dierich,B.,1998.Chemicaldiversityandvariationofpyrrolizidine
alkaloidsofthesenecioninetype:biologicalneedorcoincidence?Planta206, 443–451.
Hong,B.,Li,W.,Song,A.,Zhao,C.,2013.Determinationofindolealkaloidsand
highlyvolatilecompoundsinRauvolfiaverticillatabyHPLC-UVandGC–MS.J. Chromatogr.Sci.51,926–930.
Katiˇc,M.,Kuˇsan,E.,Proˇsek,M.,Bano,M.,1980.Quantitativedensitometric
determi-nationofreserpineandajmalineinRauwolfiavomitoriabyHPTLC.J.HighResolut. Chromatogr.3,149–150.
Kato,L.,Braga,R.M.,Koch,I.,Kinoshita,L.S.,2002.IndolealkaloidsfromRauvolfia
bahiensisA.DC.(Apocynaceae).Phytochemistry60,315–320.
Klyushnichenko,V.Y.,Yakimov,S.A.,Bychkova,T.P.,Syagailo,Y.V.,Kuzovkiha,I.N.,
Bulfson,A.N., Miroshnikov, A.I.,1994. HPLCand TLC separation of indole
alkaloid from Rauvolfia serpentina and R. vomitoria. Khim. Farm. Zh. 28, 58–61.
Lim,W.H.,Goodger,J.Q.,Field,A.R.,Holtum,J.A.,Woodrow,I.E.,2010.Huperzine
alkaloidsfromAustralasianandsoutheastAsian.Pharm.Biol.48,1073–1078.
Nguyen,K.C.,Nikolova,I.G.,1989.Quantitativedeterminationofalkaloidinrootbark
ofsomespeciesofRauvolfiaL.bythinlayerchromatography.Rastit.Resur.25, 594–599.
Pandey,D.K.,Parida,S.,Dey,A.,2016.ComparativeHPTLCanalysisofbioactive
markerbarbaloinfrominvitroandnaturallygrownAloevera(L.)Burm.f.Rev. Bras.Farmacogn.26,161–167.
Pandey,D.K.,Shahnawaz,Dey,A.,2015.ComparativeHPTLCanalysisofantioxidant
compoundgallicacidfrominvitroandnaturallygrownSteviarebaudiana.J.Biol. Act.Prod.Natl.5,397–405.
Shamon,S.D.,Perez,M.I.,2009.Bloodpressureloweringefficacyofreserpinefor
primaryhypertension.CochraneDatabaseSyst.Rev.7,CD007655.
Sarma,D.,Sarma,S.,Baruah,A.,1999.Micropropagationandinvitrofloweringof
Rauvolfiatetraphylla;apotentsourceofanti-hypertensiondrugs.PlantaMed. 65,277–278.
Srivastava,A.,Tripathi,A.K.,Pandey,R.,Verma,R.K.,Gupta,M.M.,2006.Quantitative
determinationofreserpine,ajmaline,andajmalicineinRauvolfiaserpentinaby reversed-phasehigh-performanceliquidchromatography.J.Chromatogr.Sci. 44,557–560.
Stöckigt,J.,Pfitzner,A.,Firl,J.,1981.Indolealkaloidsfromcellsuspensioncultures
ofRauwolfiaserpentinaBenth.PlantCellRep.1,36–39.
Wang, H.Y.,Wang, R.X.,Zhao, Y.X.,Liu, K., Wang, F.L.,Sun,J.Y., 2015. Three
newisomericindolealkaloidsfromNaucleaofficinalis.Chem.Biodivers.12, 1256–1262.
Williams,R.D.,Ellis,B.E.,1989.AgeandtissuedistributionofalkaloidsinPapaver
somniferum.Phytochemistry28,2085–2088.
Zhu,W.,Yang,B.,Komatsu,S.,Lu,X.,Li,X.,Tian,J.,2015.Binarystressinducesan